Cobalt-Nickel-Boron Supported over Polypyrrole-Derived Activated Carbon for Hydrolysis of Ammonia Borane
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
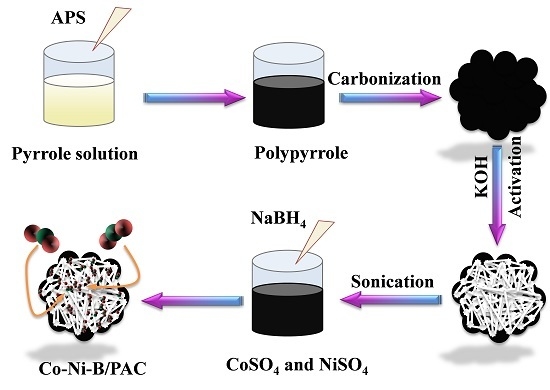
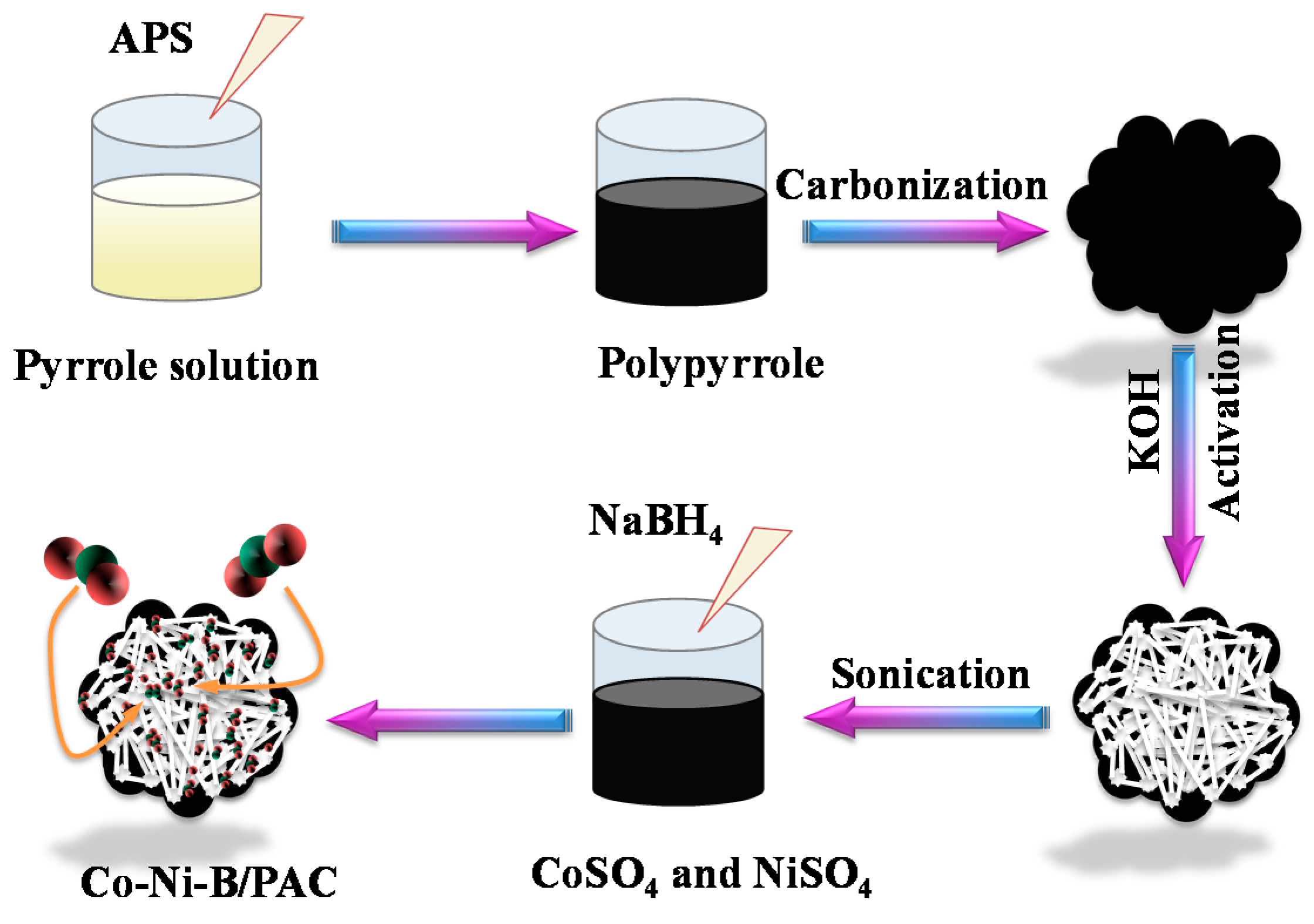
2.2. Synthesis of the Catalyst
2.3. Characterization of the Catalyst
2.4. Hydrogen Generation Measurement
3. Results and Discussion
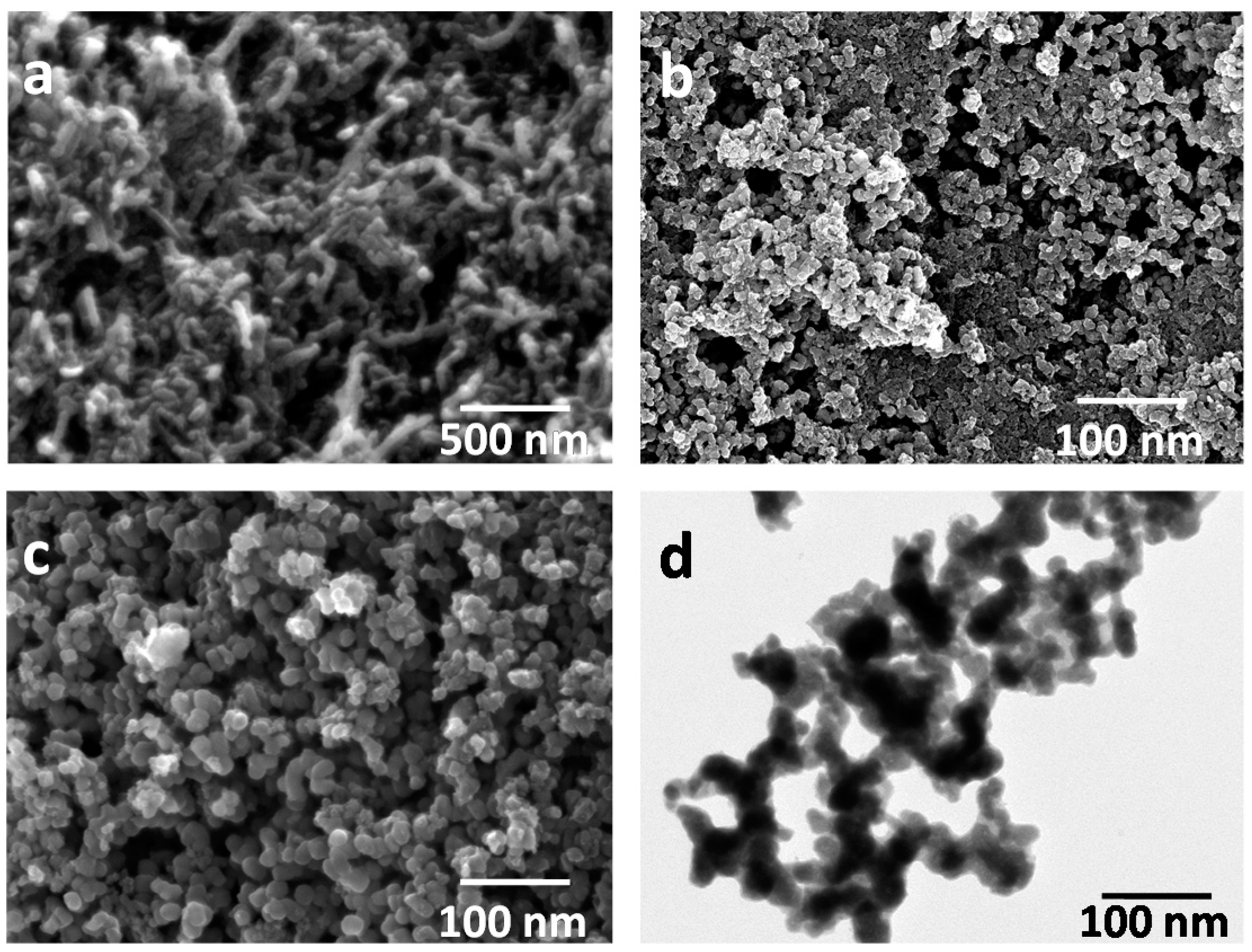
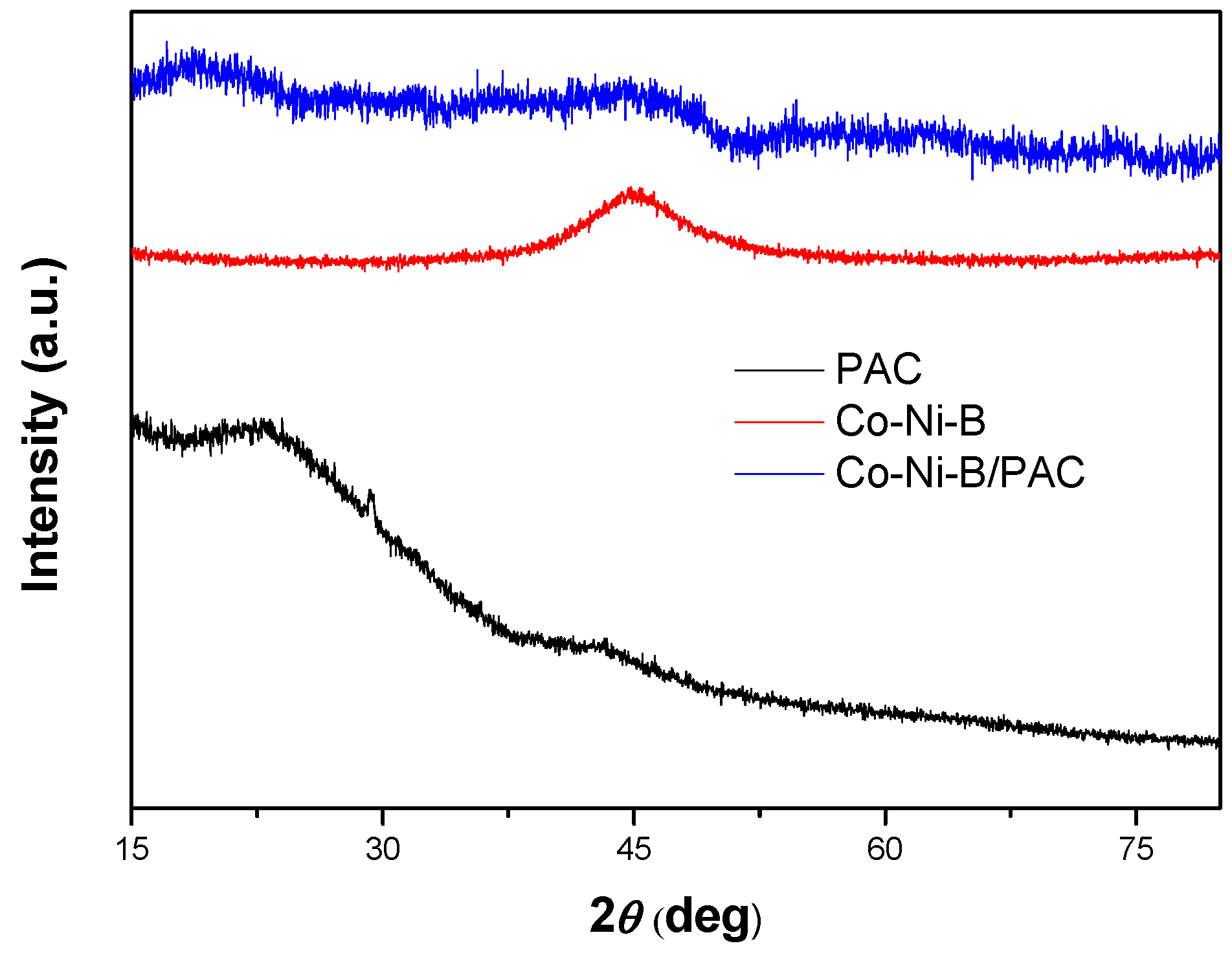
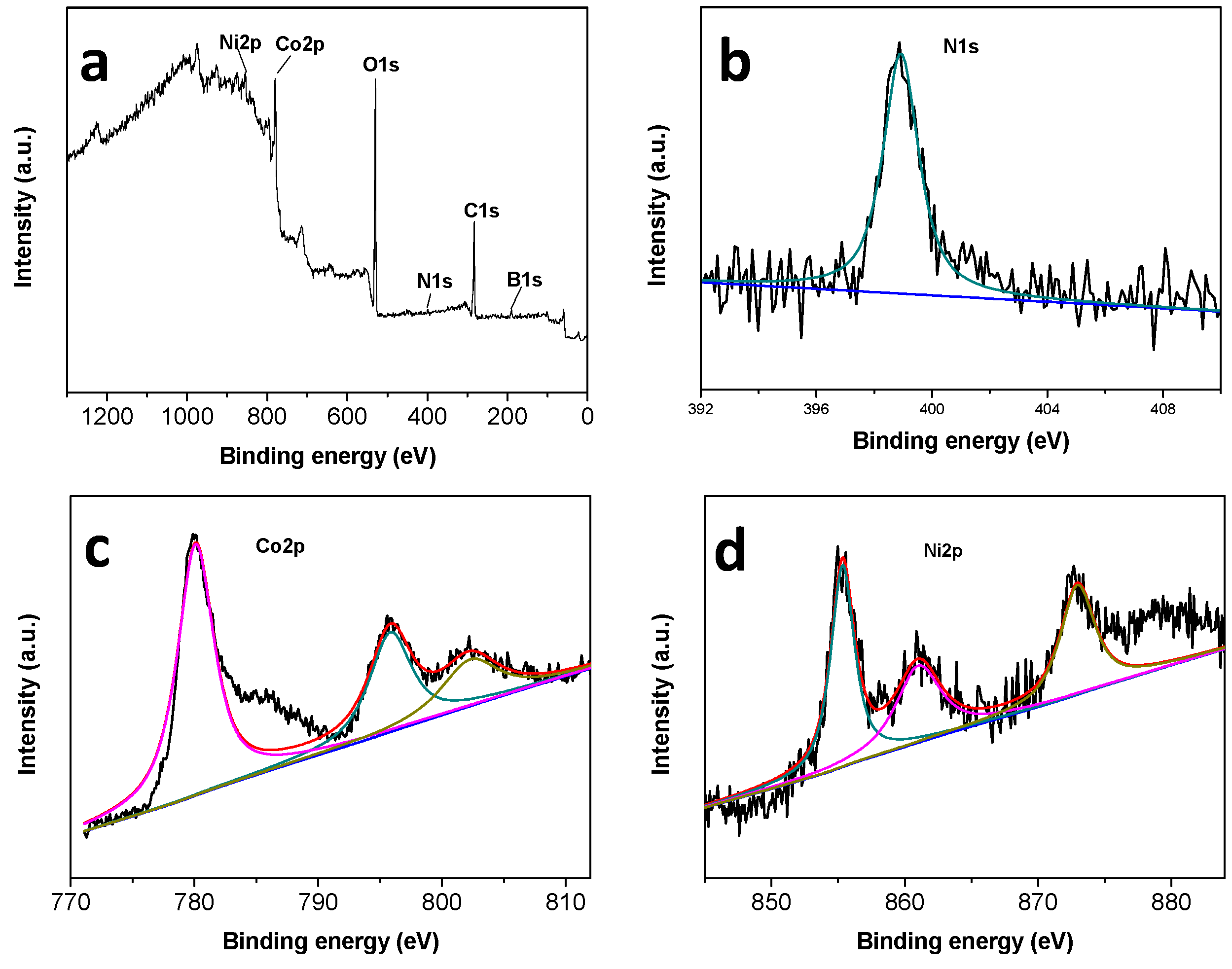
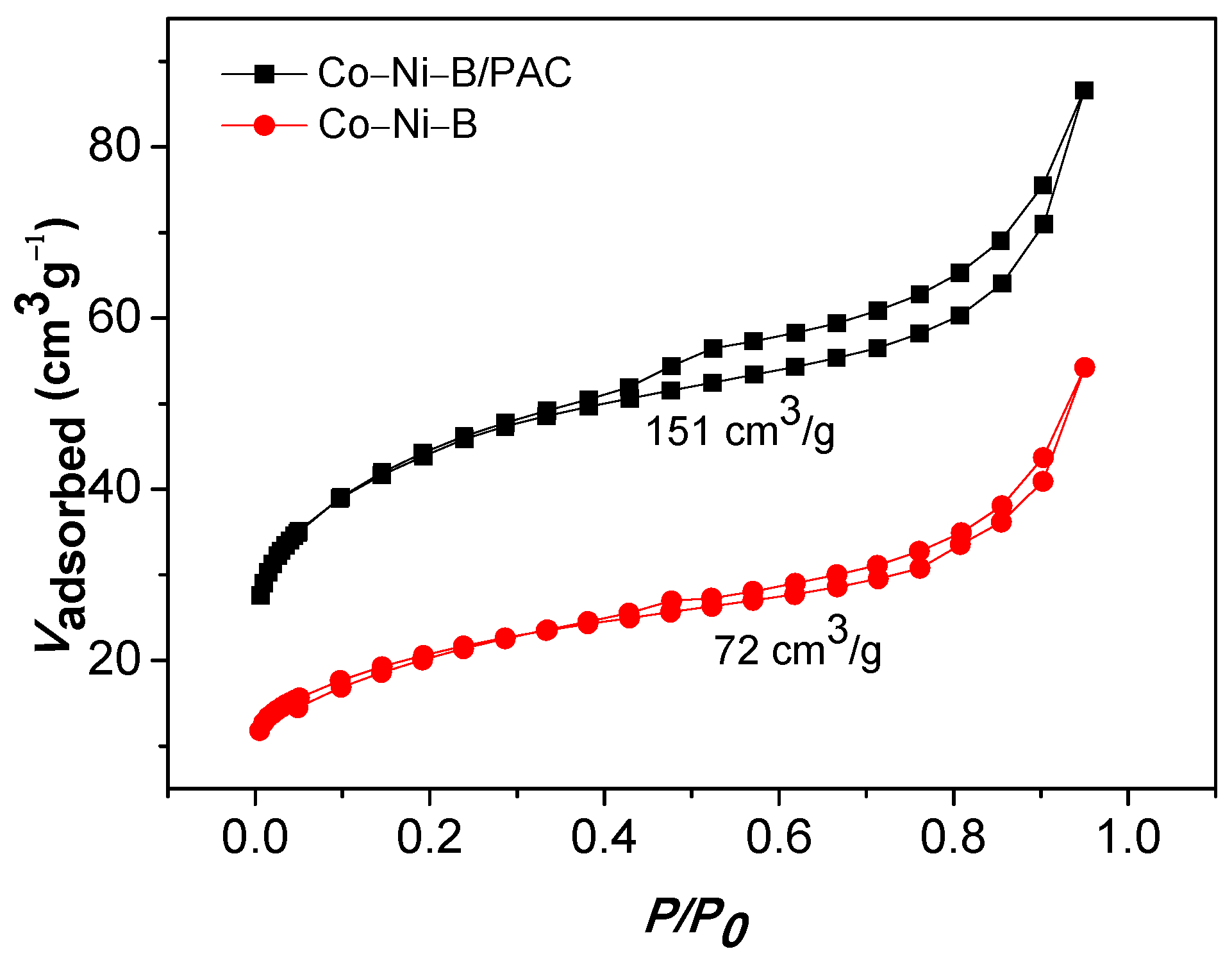
3.1. Characterization of the Catalysts
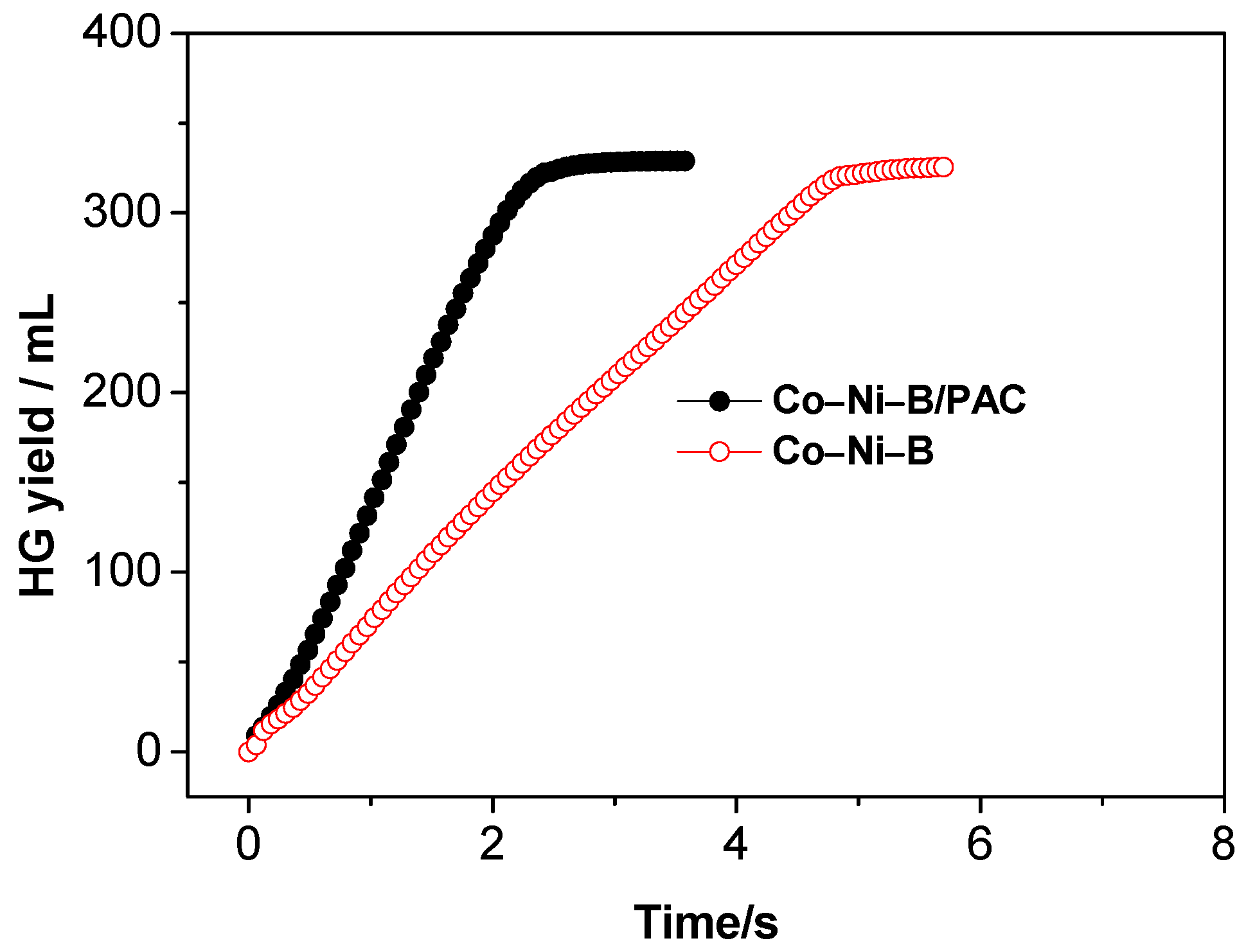
3.2. Effect of Different Types of Catalysts on Hydrogen Generation
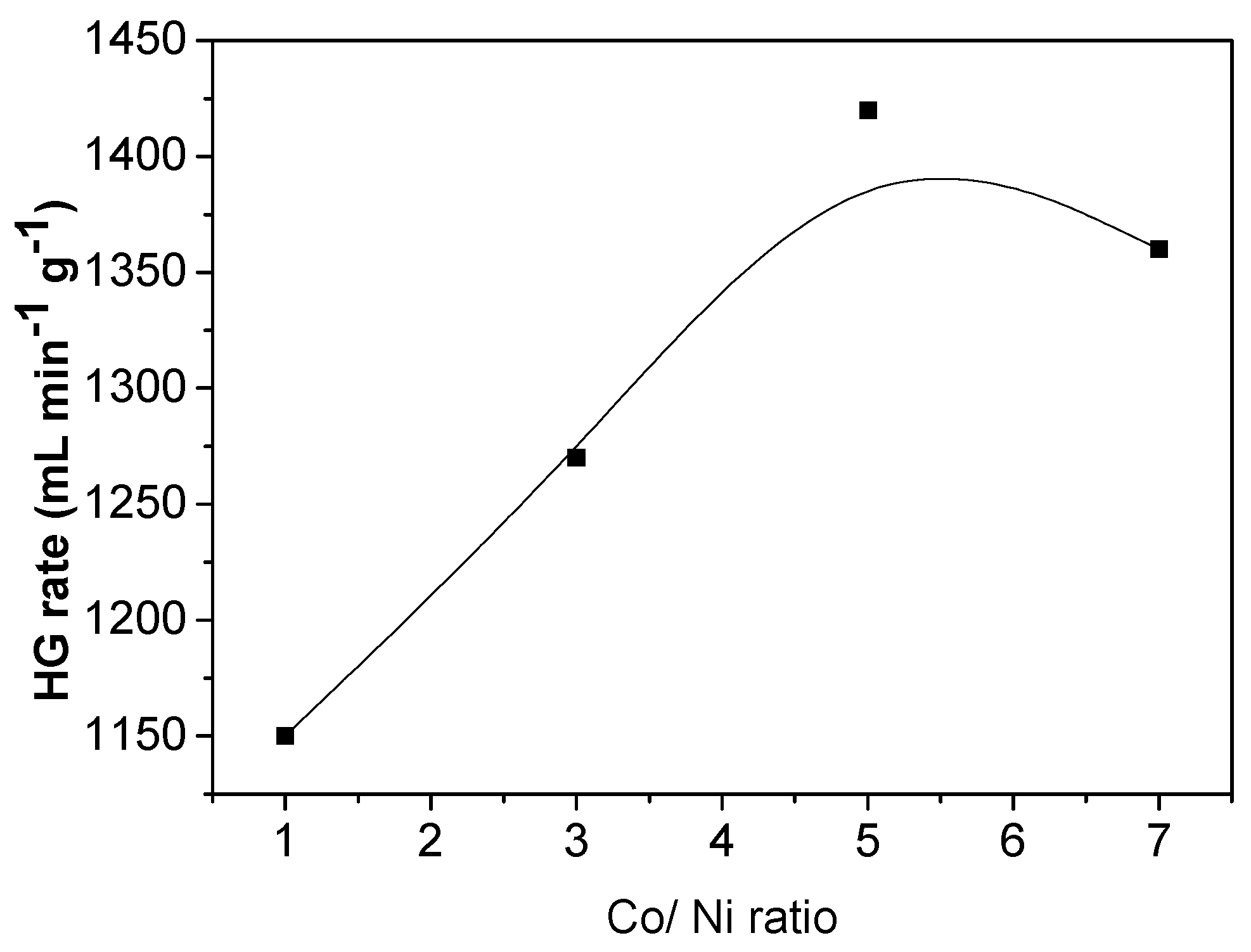
3.3. Effect of Co/Ni Molar Ratio on Hydrogen Generation
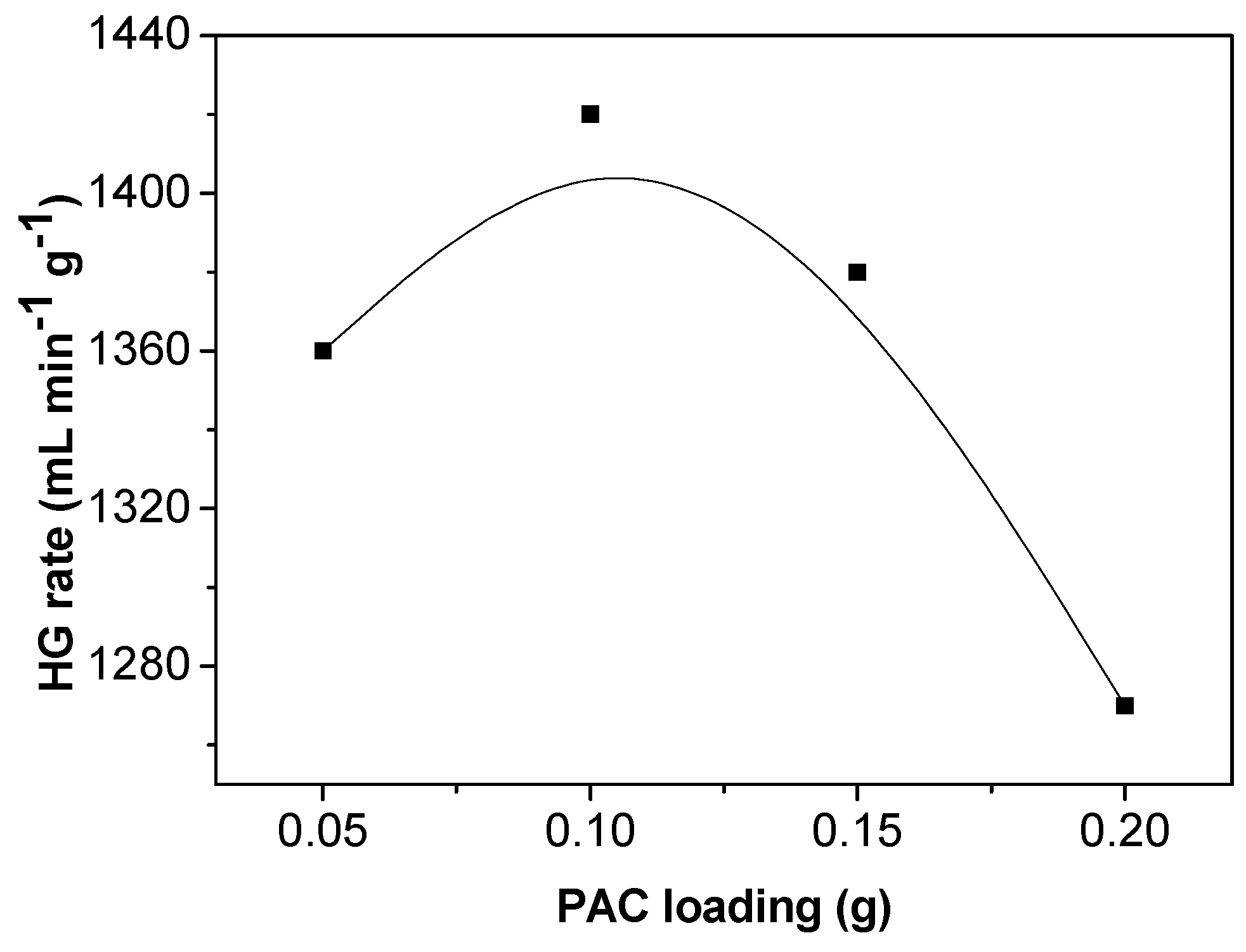
3.4. Effect of PAC Concentration on Hydrogen Generation
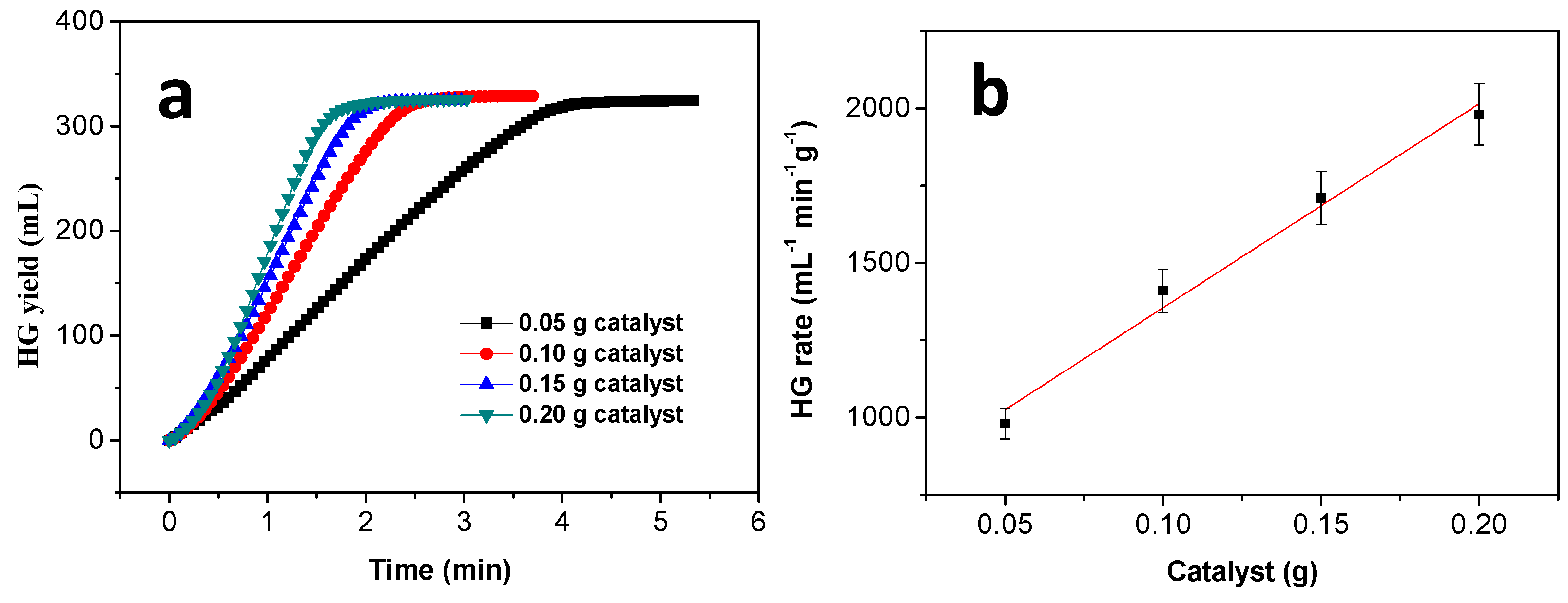
3.5. Effect of the Amount of Catalyst on Hydrogen Generation
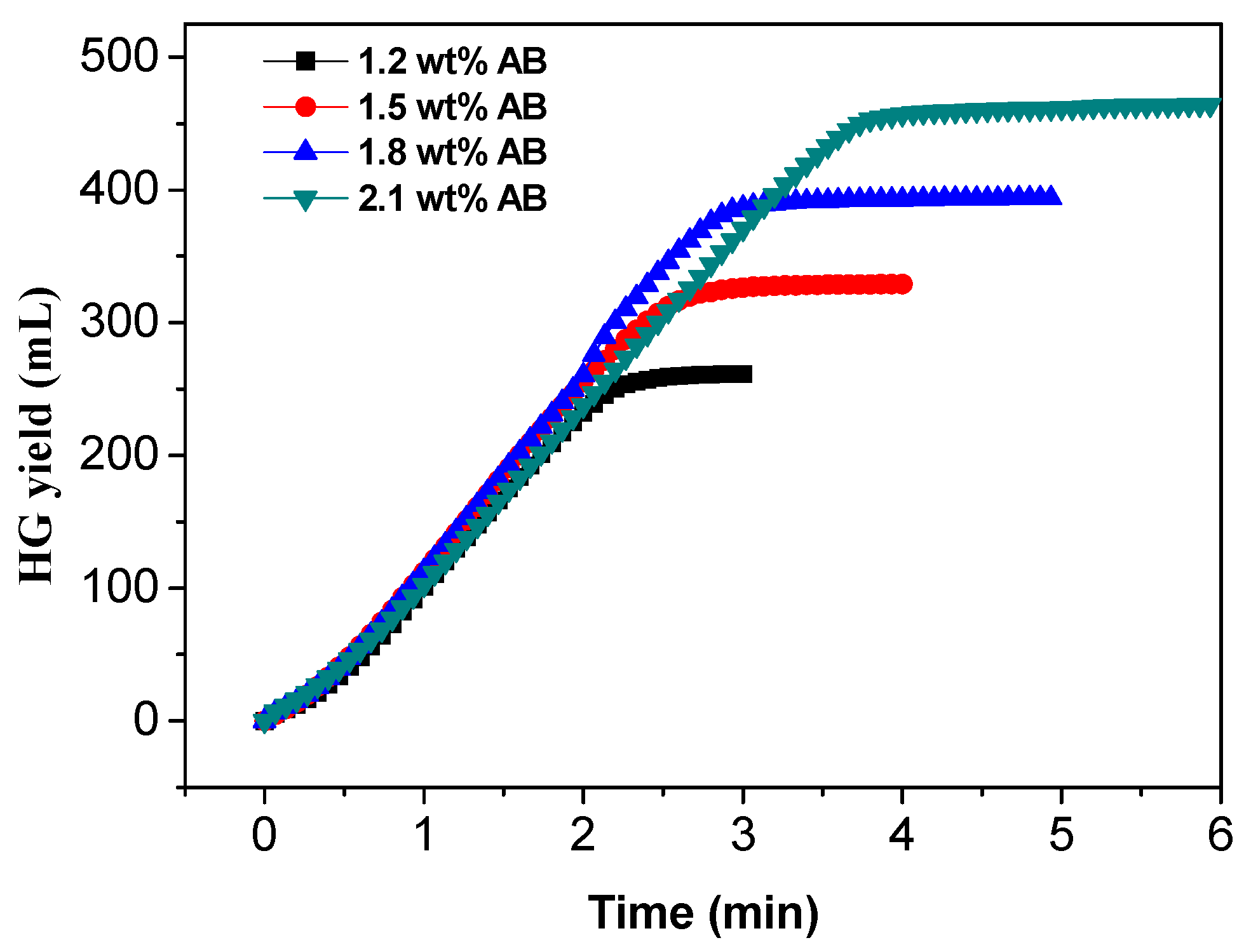
3.6. Effect of AB Concentration on Hydrogen Generation
3.7. Kinetics of Hydrolysis Using Co-Ni-B/PAC
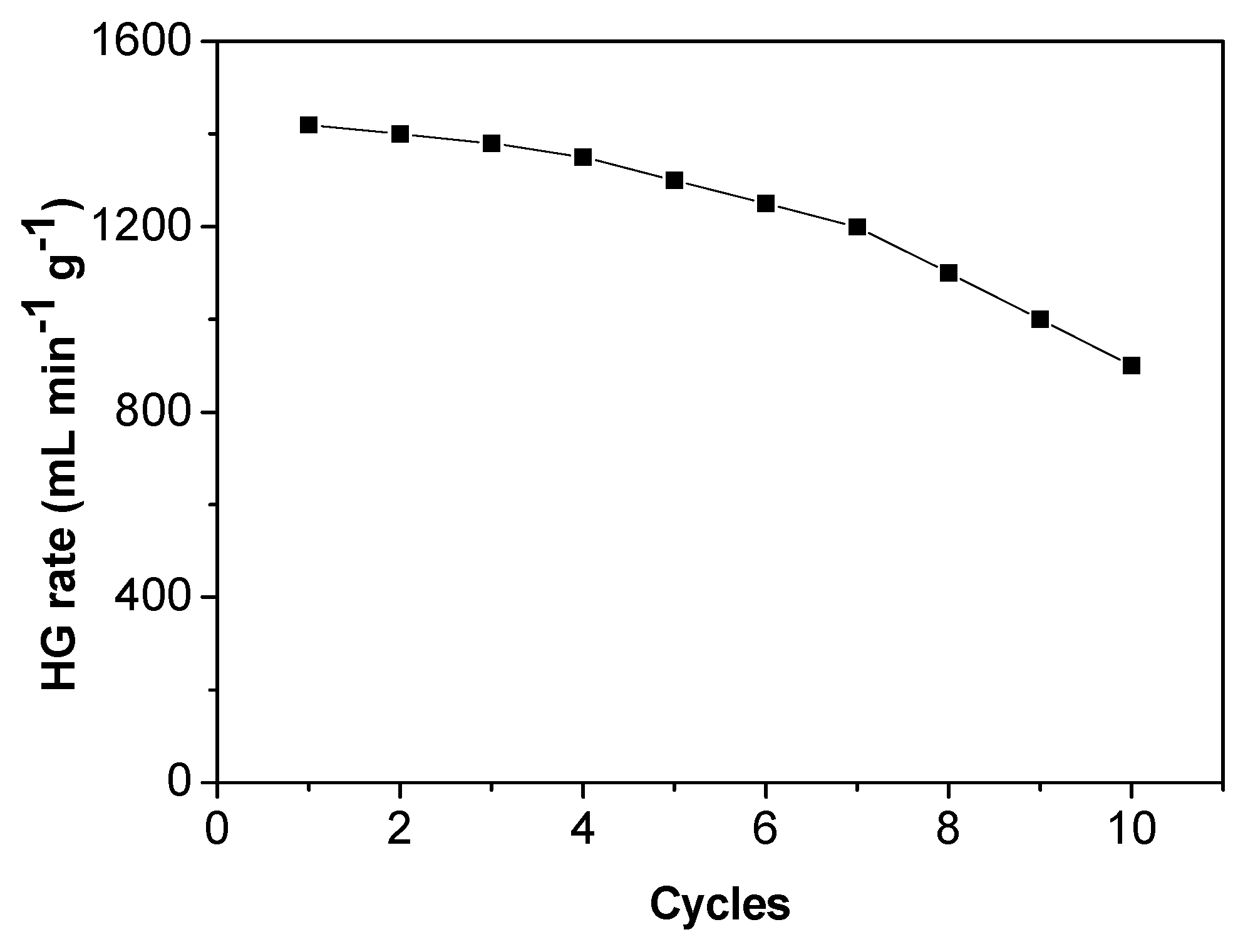
3.8. Stability of Co-Ni-B/PAC
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Z.; Lu, Z.H.; Chen, X. Ultrafine Ni-Pt alloy nanoparticles grown on graphene as highly efficient catalyst for complete hydrogen generation from hydrazine borane. ACS Sustain. Chem. Eng. 2015, 3, 1255–1261. [Google Scholar] [CrossRef]
- Xiang, C.; Jiang, D.; She, Z.; Zou, Y.; Chu, H.; Qiu, S.; Zhang, H.; Xu, F.; Tang, C.; Sun, L. Hydrogen generation by hydrolysis of alkaline sodium borohydride using a cobaltezinceboron/graphene nanocomposite treated with sodium hydroxide. Int. J. Hydrog. Energy 2015, 40, 4111–4118. [Google Scholar] [CrossRef]
- Hu, L.; Zheng, B.; Lai, Z.; Huang, K.W. Room temperature hydrogen generation from hydrolysis of ammonia-borane over an efficient NiAgPd/C catalyst. Int. J. Hydrog. Energy 2014, 39, 20031–20037. [Google Scholar] [CrossRef]
- Qiu, F.; Dai, Y.; Li, L.; Xu, C.; Huang, Y.; Chen, C.; Wang, Y.; Jiao, L.; Yuan, H. Synthesis of Cu@FeCo core-shell nanoparticles for the catalytic hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2014, 39, 436–441. [Google Scholar] [CrossRef]
- Shan, X.; Du, J.; Cheng, F.; Liang, J.; Tao, Z.; Chen, J. Carbon-supported Ni3B nanoparticles as catalysts for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2014, 39, 6987–6994. [Google Scholar] [CrossRef]
- Ma, H.; Na, C. Isokinetic temperature and size-controlled activation of ruthenium-catalyzed ammonia borane hydrolysis. ACS Catal. 2015, 5, 1726–1735. [Google Scholar] [CrossRef]
- Li, Y.; Dai, Y.; Tian, X.K. Controlled synthesis of monodisperse PdxSn100-x nanoparticles and their catalytic activity for hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrog. Energy 2015, 40, 9235–9243. [Google Scholar] [CrossRef]
- Rakap, M. Poly(N-vinyl-2-pyrrolidone)-stabilized palladium-platinum nanoparticles-catalyzed hydrolysis of ammonia borane for hydrogen generation. J. Power Sources 2015, 276, 320–327. [Google Scholar] [CrossRef]
- Chou, C.C.; Chen, B.H. Hydrogen generation from deliquescence of ammonia borane using Ni-Co/r-GO catalyst. J. Power Sources 2015, 293, 343–350. [Google Scholar] [CrossRef]
- Zou, Y.; Cheng, J.; Wang, Q.; Xiang, C.; Chu, H.; Qiu, S.; Zhang, H.; Xu, F.; Liu, S.; Tang, C.; et al. Cobalt-boron/nickel-boron nanocomposite with improved catalytic performance for the hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2015, 40, 13423–13430. [Google Scholar] [CrossRef]
- Yao, Q.; Lu, Z.H.; Wang, Y.; Chen, X.; Feng, G. Synergetic catalysis of non-noble bimetallic Cu-Co nanoparticles embedded in SiO2 nanospheres in hydrolytic dehydrogenation of ammonia borane. J. Phys. Chem. C 2015, 119, 14167–14174. [Google Scholar]
- Chandra, M.; Xu, Q. A high-performance hydrogen generation system: Transition metal-catalyzed dissociation and hydrolysis of ammonia-borane. J. Power Sources 2006, 156, 190–194. [Google Scholar] [CrossRef]
- Xu, Q.; Chandra, M. Catalytic activities of non-noble metals for hydrogen generation from aqueous ammonia-borane at room temperature. J. Power Sources 2006, 163, 364–370. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Yen, H.; Seo, Y.; Kaliaguine, S.; Kleitz, F. Role of metal-support interactions, particle size, and metal-metal synergy in CuNi nanocatalysts for H2 generation. ACS Catal. 2015, 5, 5505–5511. [Google Scholar] [CrossRef]
- Fernandes, R.; Patel, N.; Miotello, A.; Jaiswal, R.; Kothari, D.C. Dehydrogenation of ammonia borane with transition metal-doped Co-B alloy catalysts. Int. J. Hydrog. Energy 2012, 37, 2397–2406. [Google Scholar] [CrossRef]
- Lai, S.W.; Lin, H.L.; Lin, Y.P.; Yu, T.L. Hydrolysis of ammonia-borane catalyzed by an iron-nickel alloy on an SBA-15 support. Int. J. Hydrog. Energy 2013, 38, 4636–4647. [Google Scholar] [CrossRef]
- Liang, H.; Chen, G.; Desinan, S.; Rosei, R.; Rosei, F.; Ma, D. In situ facile synthesis of ruthenium nanocluster catalyst supported on carbon black for hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrog. Energy 2012, 37, 17921–17927. [Google Scholar] [CrossRef]
- Rachiero, G.P.; Demirci, U.B.; Miele, P. Bimetallic RuCo and RuCu catalysts supported on gamma-Al2O3. A comparative study of their activity in hydrolysis of ammonia-borane. Int. J. Hydrog. Energy 2011, 36, 7051–7065. [Google Scholar] [CrossRef]
- Yao, Q.; Shi, W.; Feng, G.; Lu, Z.H.; Zhang, X.; Tao, D.; Kong, D.; Chen, X. Ultrafine Ru nanoparticles embedded in SiO2 nanospheres: Highly efficient catalysts for hydrolytic dehydrogenation of ammonia borane. J. Power Sources 2014, 257, 293–299. [Google Scholar] [CrossRef]
- Cheng, J.; Xiang, C.; Zou, Y.; Chu, H.; Qiu, S.; Zhang, H.; Sun, L.; Xu, F. Highly active nanoporous Co-B-TiO2 framework for hydrolysis of NaBH4. Ceram. Int. 2015, 41, 899–905. [Google Scholar] [CrossRef]
- Yao, Q.; Lu, Z.H.; Yang, K.; Che, X.; Zhu, M. Ruthenium nanoparticles confined in SBA-15 as highly efficient catalyst for hydrolytic dehydrogenation of ammonia borane and hydrazine borane. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Hu, B.; Chen, T.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Activated carbons prepared from peanut shell and sunflower seed shell for high CO2 adsorption. Adsorption 2015, 21, 125–133. [Google Scholar] [CrossRef]
- Almasoudi, A.; Mokaya, R. Preparation and hydrogen storage capacity of templated and activated carbons nanocast from commercially available zeolitic imidazolate framework. J. Mater. Chem. 2012, 22, 146–152. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G.; Pašti, I.; Gavrilov, N.; Janošević, A.; Mentus, S. Carbonised polyaniline and polypyrrole: Towards advanced nitrogen-containing carbon materials. Chem. Pap. 2013, 67, 781–813. [Google Scholar] [CrossRef]
- Zou, Y.; Pisciotta, J.; Baskakov, I.V. Nanostructured polypyrrole-coated anode for sun-powered microbial fuel cells. Bioelectrochemistry 2010, 79, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Kale, A.; Miotello, A. Improved dehydrogenation of ammonia borane over Co-P-B coating on Ni: A single catalyst for both hydrolysis and thermolysis. Appl. Catal. B Environ. 2012, 111–112, 178–184. [Google Scholar] [CrossRef]
- Dai, H.B.; Gao, L.L.; Liang, Y.; Kang, X.D.; Wang, P. Promoted hydrogen generation from ammonia borane aqueous solution using cobalt-molybdenum-boron/nickel foam catalyst. J. Power Sources 2010, 195, 307–312. [Google Scholar] [CrossRef]
- Dai, H.B.; Liang, Y.; Wang, P.; Yao, X.D.; Rufford, T.; Lu, M.; Cheng, H.M. High-performance cobalt-tungsten-boron catalyst supported on Ni foam for hydrogen generation from alkaline sodium borohydride solution. Int. J. Hydrog. Energy 2008, 33, 4405–4412. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Guo, Q.; Ji, S. Catalytic behavior of carbon supported Ni-B, Co-B and Co-Ni-B in hydrogen generation by hydrolysis of KBH4. Fuel Process. Technol. 2011, 92, 1606–1610. [Google Scholar] [CrossRef]
- Dai, H.M.; Su, J.; Hu, K.; Luo, W.; Cheng, G.Z. Pd nanoparticles supported on MIL-101 as high-performance catalysts for catalytic hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2014, 39, 4947–4953. [Google Scholar] [CrossRef]
- Yao, C.F.; Zhuang, L.; Cao, Y.L.; Ai, X.P.; Yang, H.X. Hydrogen release from hydrolysis of borazane on Pt- and Ni-based alloy catalysts. Int. J. Hydrog. Energy 2008, 33, 2462–2467. [Google Scholar] [CrossRef]
- Park, J.W.; Lai, S.W.; Cho, S.O. Catalytic hydrogen generation from hydrolysis of ammonia borane using octahedral Au@Pt nanoparticles. Int. J. Hydrog. Energy 2015, 40, 16316–16322. [Google Scholar] [CrossRef]
- Rakap, M. PVP-stabilized Ru-Rh nanoparticles as highly efficient catalysts for hydrogen generation from hydrolysis of ammonia borane. J. Alloy. Compd. 2015, 649, 1025–1030. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, L.; Han, M.; Tao, Z.; Cheng, F.; Chen, J. CuCo nanoparticles supported on hierarchically porous carbon as catalysts for hydrolysis of ammonia borane. J. Alloy. Compd. 2015, 651, 382–388. [Google Scholar] [CrossRef]
- Yao, Q.; Lu, Z.H.; Zhang, Z.; Chen, X.; Lan, Y. One-pot synthesis of core-shell Cu@SiO2 nanospheres and their catalysis for hydrolytic dehydrogenation of ammonia borane and hydrazine borane. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Lu, Z.H.; Jia, Y.; Chen, X.; Liu, X. In situ facile synthesis of Rh nanoparticles supported on carbon nanotubes as highly active catalysts for H2 generation from NH3BH3 hydrolysis. Int. J. Hydrog. Energy 2015, 40, 2207–2215. [Google Scholar] [CrossRef]
- Yurderi, M.; Bulut, A.; Ertas, İ.E.; Zahmakiran, M.; Kaya, M. Supported copper-copper oxide nanoparticles as active, stable and low-cost catalyst in the methanolysis of ammonia-borane for chemical hydrogen storage. Appl. Catal. B Environ. 2015, 165, 169–175. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Luo, H.; Qiao, M. Liquid phase hydrogenation of furfural to furfuryl alcohol over Mo-doped Co-B amorphous alloy catalysts. App. Catal. A Gen. 2002, 233, 13–20. [Google Scholar] [CrossRef]
- Wen, M.; Zhou, S.; Wu, Q.; Zhang, J.; Wu, Q.; Wang, C.; Sun, Y. Construction of NiCo-Pt nanopolyhedron inlay-structures and their highly efficient catalysis hydrolytic dehydrogenation toward ammonia borane. J. Power Sources 2013, 232, 86–92. [Google Scholar] [CrossRef]
- Figen, A.K.; Coşkuner, B. A novel perspective for hydrogen generation from ammonia borane (NH3BH3) with Co-B catalysts: “Ultrasonic Hydrolysis”. Int. J. Hydrog. Energy 2013, 38, 2824–2835. [Google Scholar] [CrossRef]
- Rakap, M.; Özkar, S. Hydrogen generation from the hydrolysis of ammonia-borane using intrazeolite cobalt(0) nanoclusters catalyst. Int. J. Hydrog. Energy 2010, 35, 3341–3346. [Google Scholar] [CrossRef]
- Liao, J.; Li, H.; Zhang, X. Preparation of Ti supported Co film composed of Co nanofibers as catalyst for the hydrolysis of ammonia borane. Catal. Commun. 2015, 67, 1–5. [Google Scholar] [CrossRef]

| Catalyst Sample | Ea (kJ·mol−1) | Ref. |
|---|---|---|
| Cu@FeCo | 38.75 | [4] |
| Fe-Ni/SBA-15 | 75 | [17] |
| Au@Pt | 44.28 | [33] |
| Ru-Rh@PVP a | 47.4 ± 2.1 | [34] |
| Cu0.2Co0.8/HPC b | 41.7 | [35] |
| Cu@SiO2 | 36 ± 1 | [36] |
| Rh/CNTs c | 32 ± 1 | [37] |
| Cu-Cu2O-CuO/C | 67.9 | [38] |
| Co-Mo-B/Ni | 44 | [39] |
| NiCo-Pt | 45.72 | [40] |
| Co-B | 47.5 | [41] |
| Co-Ni-B/PAC | 30.2 | This work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Gao, Y.; Xiang, C.; Chu, H.; Qiu, S.; Yan, E.; Xu, F.; Tang, C.; Sun, L. Cobalt-Nickel-Boron Supported over Polypyrrole-Derived Activated Carbon for Hydrolysis of Ammonia Borane. Metals 2016, 6, 154. https://doi.org/10.3390/met6070154
Zou Y, Gao Y, Xiang C, Chu H, Qiu S, Yan E, Xu F, Tang C, Sun L. Cobalt-Nickel-Boron Supported over Polypyrrole-Derived Activated Carbon for Hydrolysis of Ammonia Borane. Metals. 2016; 6(7):154. https://doi.org/10.3390/met6070154
Chicago/Turabian StyleZou, Yongjin, Yubo Gao, Cuili Xiang, Hailiang Chu, Shujun Qiu, Erhu Yan, Fen Xu, Chengying Tang, and Lixian Sun. 2016. "Cobalt-Nickel-Boron Supported over Polypyrrole-Derived Activated Carbon for Hydrolysis of Ammonia Borane" Metals 6, no. 7: 154. https://doi.org/10.3390/met6070154