One-Step Extraction of Antimony in Low Temperature from Stibnite Concentrate Using Iron Oxide as Sulfur-Fixing Agent
Abstract
:1. Introduction
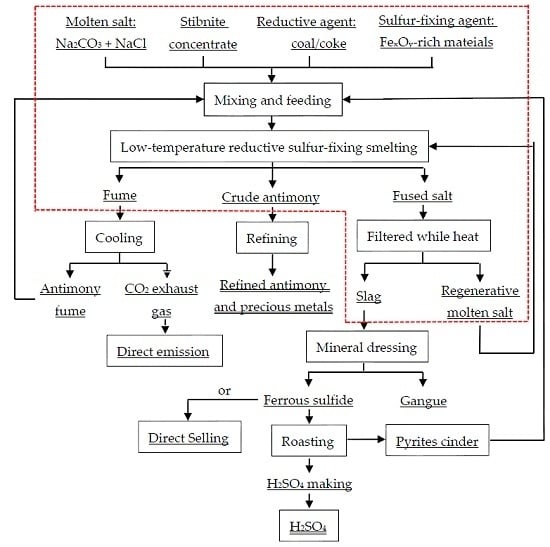
2. Materials and Methods
2.1. Materials
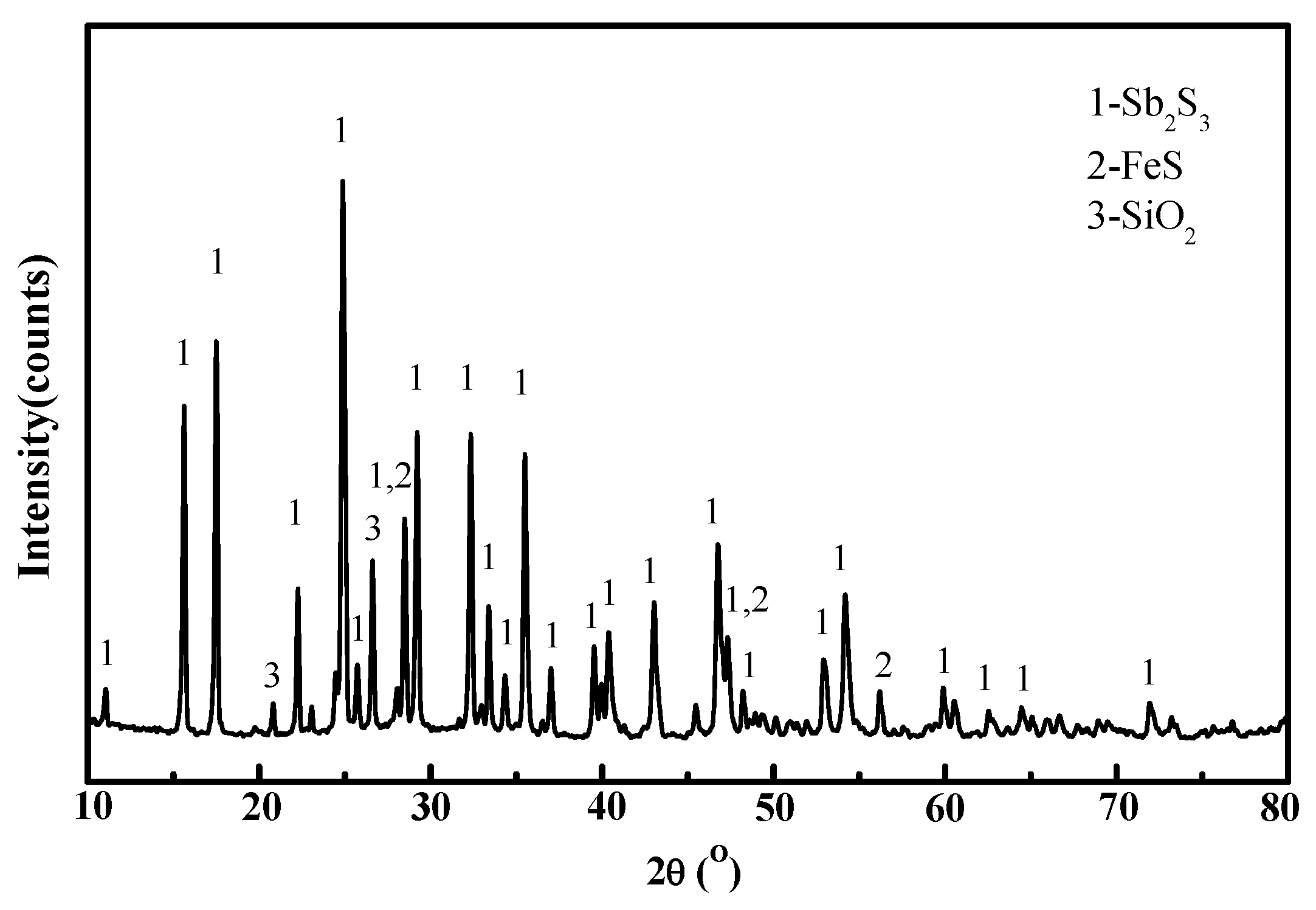
2.2. Methods
2.3. Thermodynamic Considerations
3. Results and Discussion
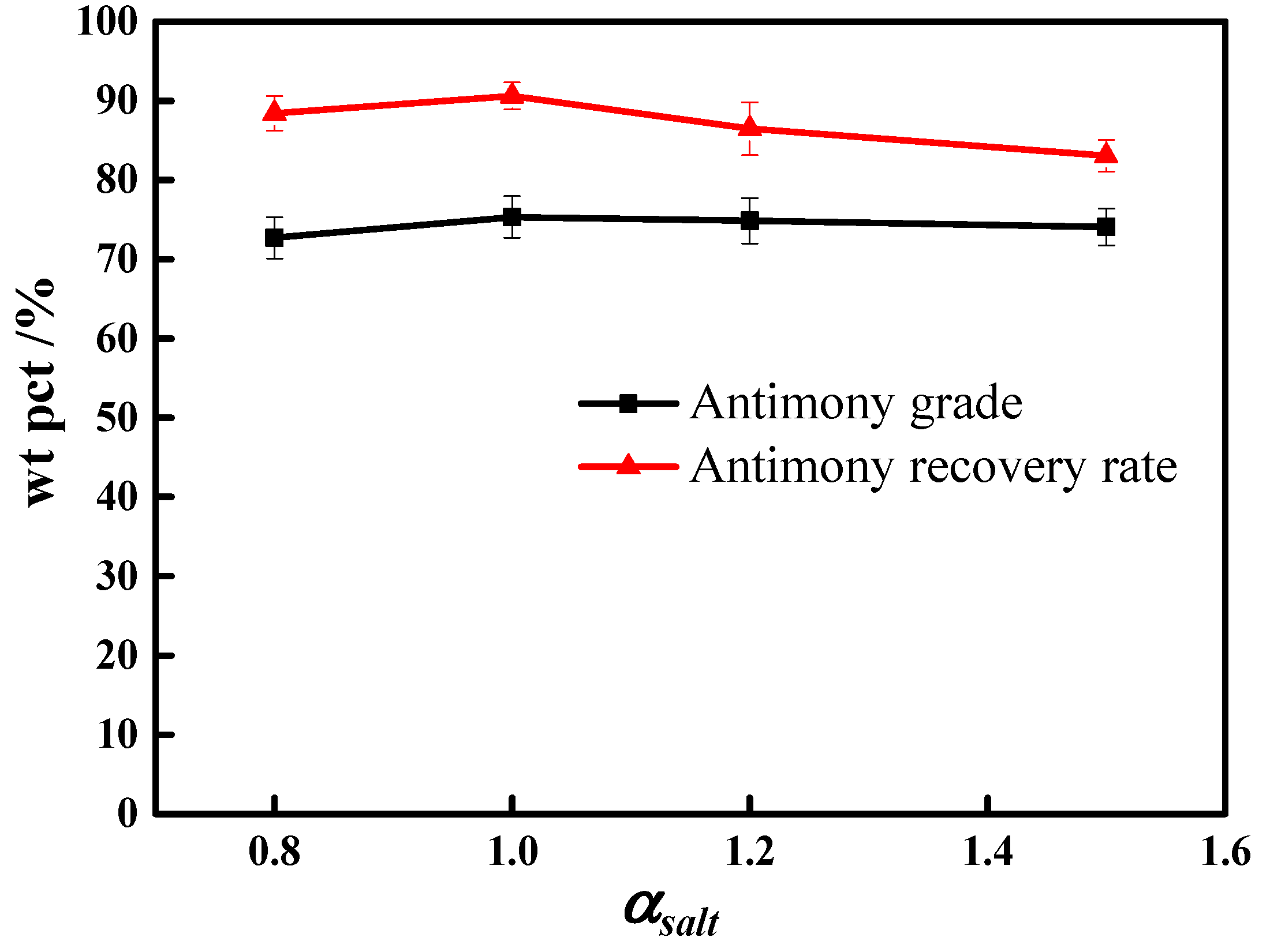
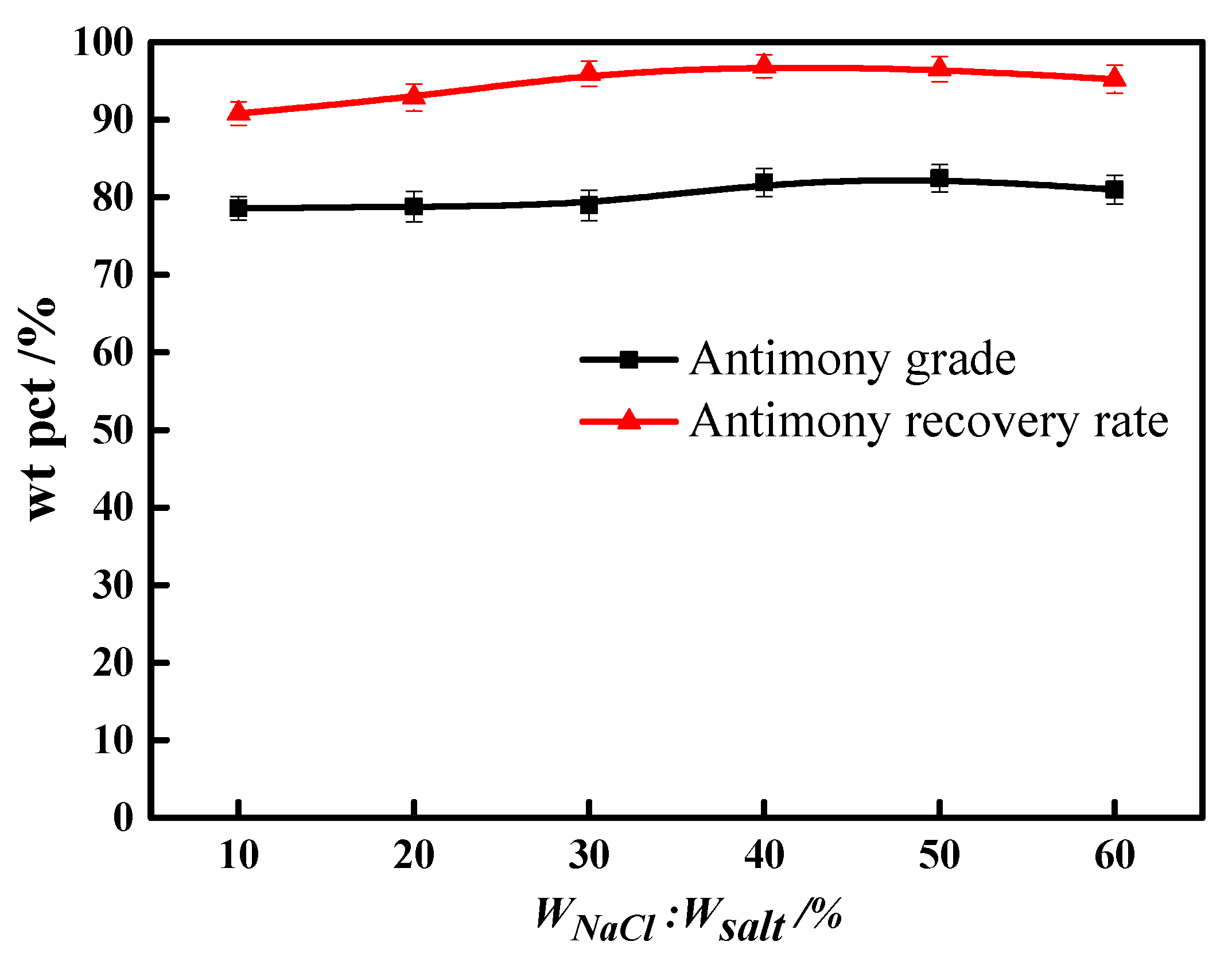
3.1. Molten Salt Dosage
3.2. Molten Salt Composition
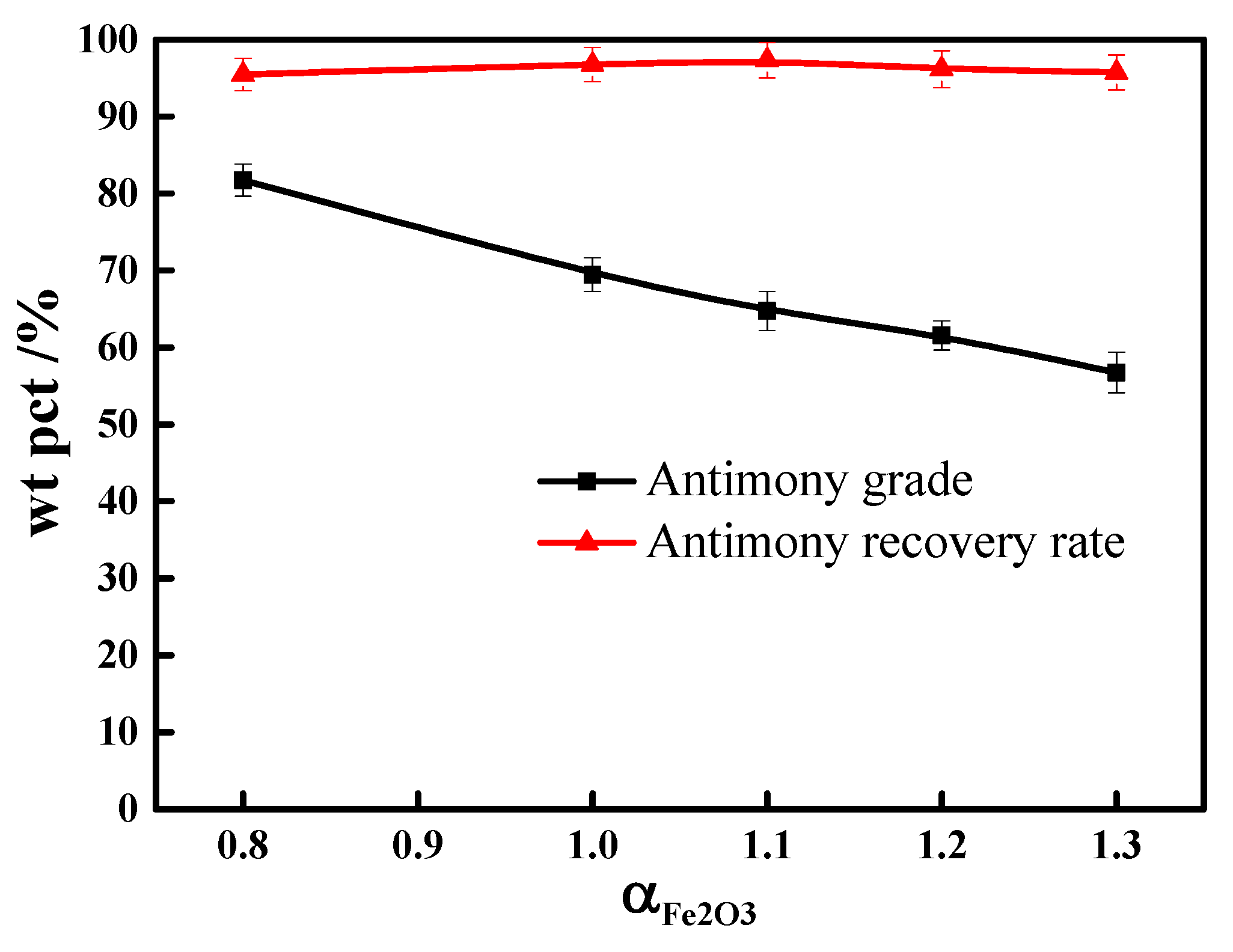
3.3. Ferric Oxide Dosage
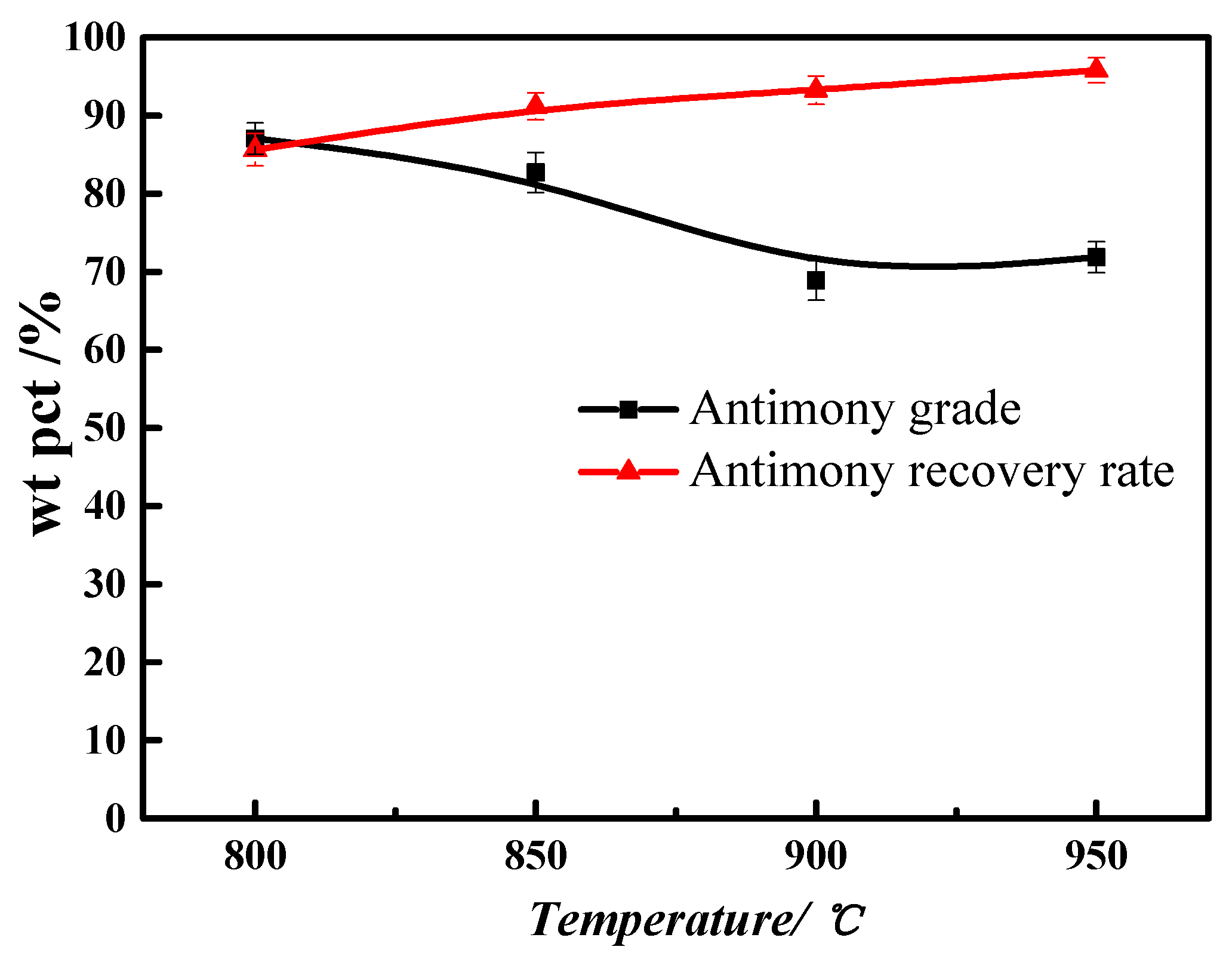
3.4. Smelting Temperature
3.5. Smelting Duration
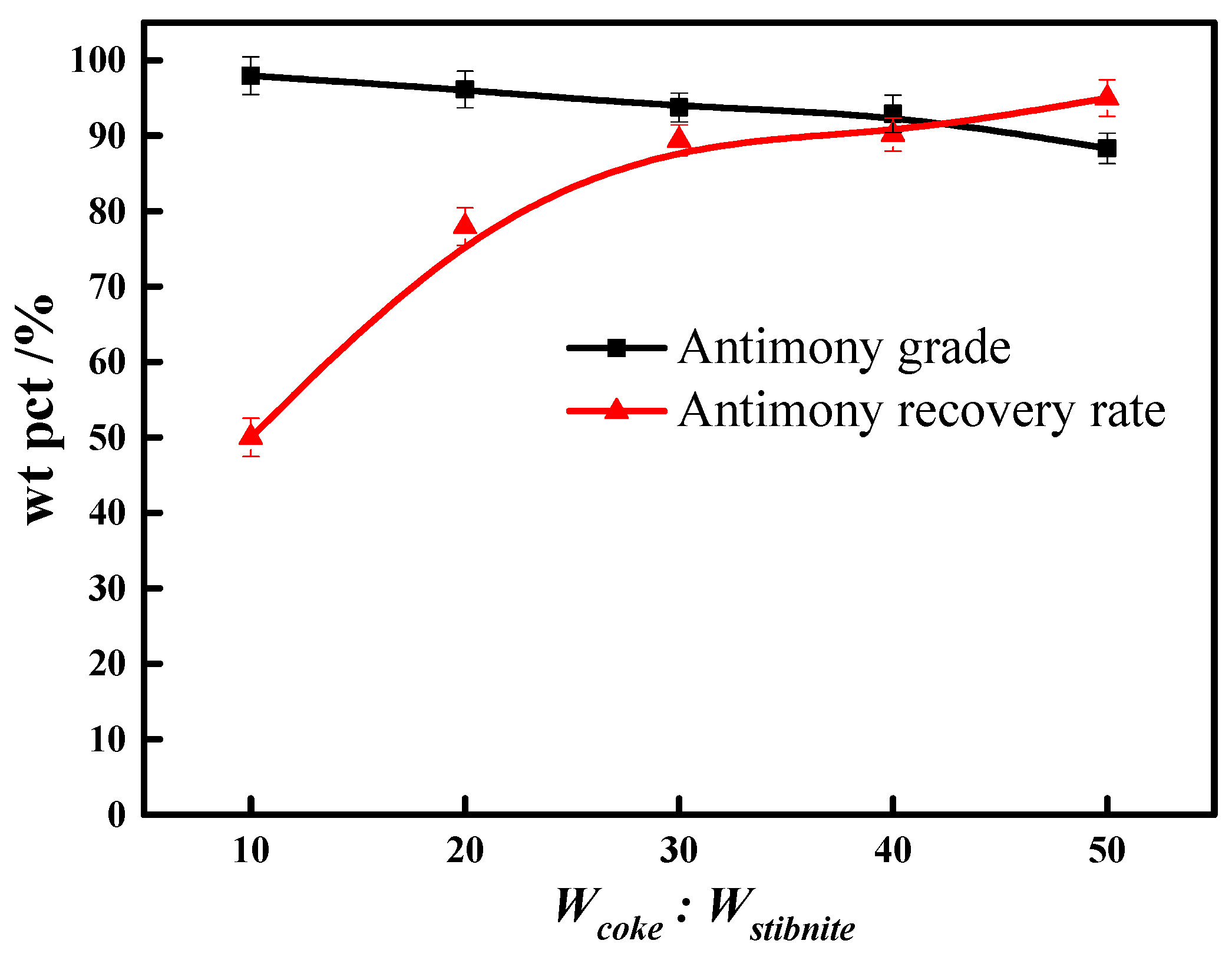
3.6. Reductive Agent Dosage
3.7. Confirmation Experiments
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Lei, T.; Zhou, C.J.; Zhang, H.P. Antimony Metallurgy, 1st ed.; Metallurgical Industry Press: Beijing, China, 2012; p. 143. [Google Scholar]
- Zhao, T.C. Antimony, 1st ed.; Metallurgical Industry Press: Beijing, China, 1987; pp. 96–99. [Google Scholar]
- Wang, J.K.; Lei, T. Treating low grade antimony ore by bath smelting-continuous fuming process (in Chinese). Nonferr. Metall. 2000, 52, 44–48. [Google Scholar]
- Chen, Y.M.; Huang, C.; Tang, M.T.; Yao, W.Y.; Tang, C.B.; Pi, G.H. Production of antimony by directly reducing-matting smelting of stibnite concentrate (in Chinese). Chin. J. Nonferr. Met. 2005, 15, 1311–1316. [Google Scholar]
- Ye, L.G.; Tang, C.B.; Tang, M.T.; Yang, J.G.; Chen, Y.M.; Yang, S.H.; He, J. Separation antimony from stibnite concentrate through a low temperature smelting (in Chinese). J. Cent. South Univ. (Sci. Technol.) 2012, 43, 3338–3843. [Google Scholar]
- Samuel, A.A.; Åke, S. Selective leaching of arsenic and antimony from a tetrahedrite rich complex sulphide concentrate using alkaline sulphide solution. Miner. Eng. 2010, 23, 1227–1236. [Google Scholar]
- Yang, J.Y.; Gao, L.; Yang, J.G. A New Cleaning Hydrometallurgical Technology for Stibnite (in Chinese). Hydrometall. China 2011, 30, 137–146. [Google Scholar]
- Liu, W.F.; Yang, T.Z.; Chen, L.; Bin, S.; Bin, W.D. Development of Antimony Smelting Technology in China. In Proceedings of the 4th International Symposium on High-Temperature Metallurgical Processing, San Antonio, TX, USA, February 2013; pp. 341–351.
- Liu, F.Y.; Le, X.C.; McKnight-Whitford, A.; Xia, Y.L.; Wu, F.C.; Elswick, E.; Johnson, C.C.; Zhu, C. Antimony speciation and contamination of waters in the Xikuangshan antimony mining and smelting area (in Chinese). Chin. Environ. Geochem. Health 2010, 32, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.C.; Lockwood, P.V.; Ashley, P.M.; Tighe, M. The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: A critical review. Environ. Pollut. 2010, 158, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.T.; Tang, C.B.; Chen, Y.M.; Yang, J.G.; Yang, S.H.; He, J.; Ou, Z. A promising low carbon clean metallurgical method—Low-temperature molten salt metallurgy of heavy metal. China Nonferr. Metall. 2010, 4, 49–53. [Google Scholar]
- Yang, J.G.; Tang, C.B.; Chen, Y.M.; Tang, M.T. Separation of antimony from a stibnite concentrate through a low-temperature smelting process to eliminate SO2 emission. Metal. Mater. Trans. B 2011, 42, 30–36. [Google Scholar] [CrossRef]
- Ye, L.G.; Tang, C.B.; Chen, Y.M.; Yang, S.H.; Tang, M.T. The thermal physical properties and stability of the eutectic composition in a Na2CO3-NaCl binary system. Thermochim. Acta 2014, 596, 14–20. [Google Scholar] [CrossRef]
- Ye, L.G.; Tang, C.B.; Chen, Y.M.; Yang, S.H.; Yang, J.G.; Zhang, W.H. One-step extraction of antimony from low-grade stibnite in Sodium Carbonate-Sodium Chloride binary molten salt. J. Clean. Prod. 2015, 93, 134–139. [Google Scholar] [CrossRef]
- Smirnov, M.P. Direct smelting of lead at low temperature. Nonferr. Metall. 1990, 5, 34–36. [Google Scholar]
- Sun, H.; Sun, T.C.; Gao, E.X.; Liu, Z.H.; Xu, Y. Desulfurization mechanism of calcium salts in direct reduction roasting of pyrite cinder. J. Univ. Sci. Technol. BJ 2013, 35, 977–985. [Google Scholar]
- Alp, I.; Deveci, H.; Yazici, E.Y.; Türk, T. Potential use of pyrite cinders as raw material in cement production: Results of industrial scale trial operations. J. Hazard. Mater. 2009, 166, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Q.; Chen, D.; Pan, J.; Süngün, Y.H. One step technology to separate copper, zinc, lead from iron in metallurgical slag and pyrite cinder (I): Laboratory scale test. Miner. Process. Extr. Metall. 2012, 121, 79–85. [Google Scholar] [CrossRef]
- HSC Chemistry, version 6.1; Chemical Reaction and Equilibrium Software with extensive thermochemical database; Outokumpu: Pori, Finland, 2007.
- Rickard, D. Kinetics of pyrite formation by the H2S oxidation of iron (II) monosulfide in aqueous solutions between 25 and 125 °C: The rate equation. Geochim. Cosmochim. Acta 1997, 61, 115–134. [Google Scholar] [CrossRef]


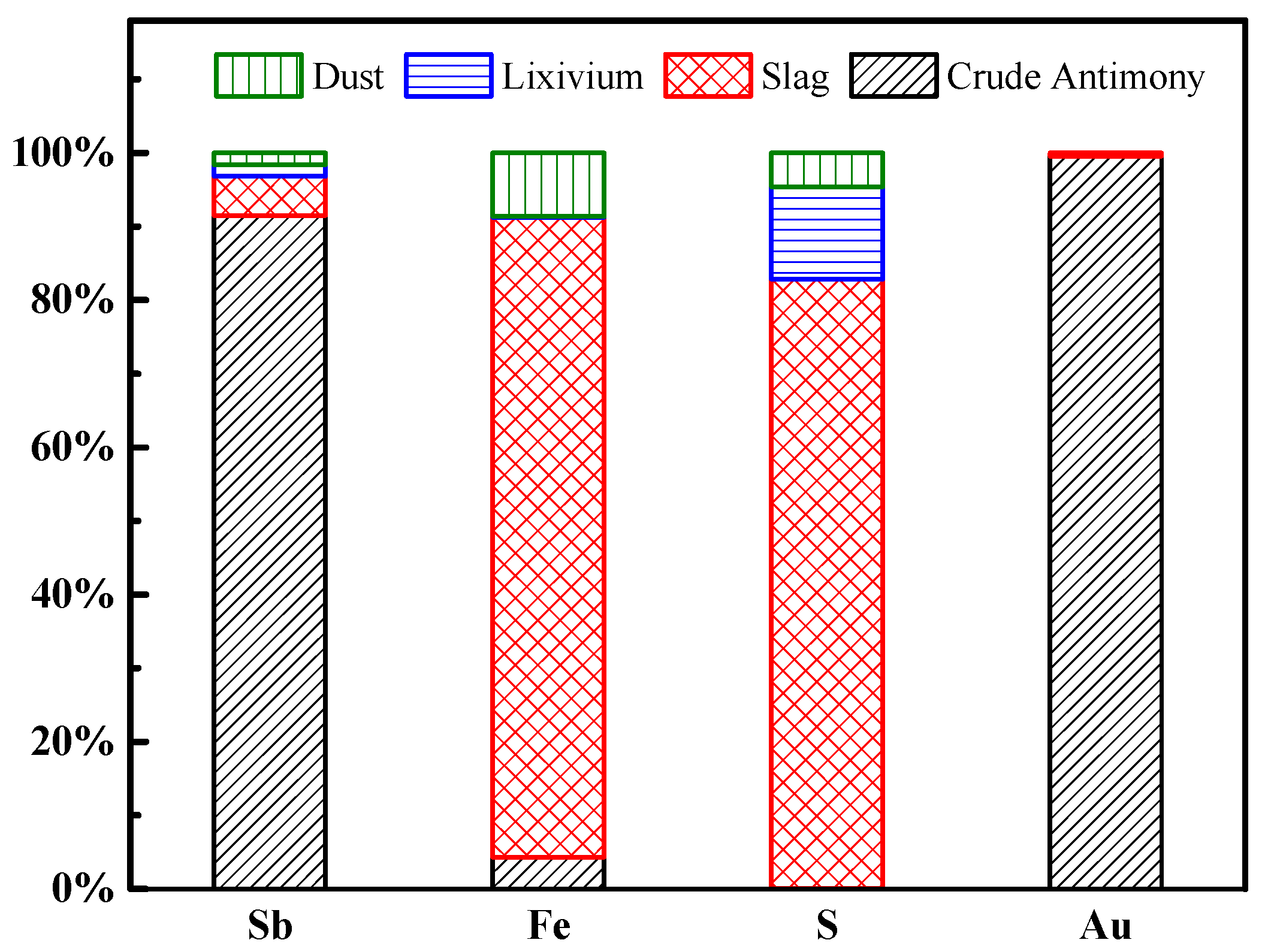
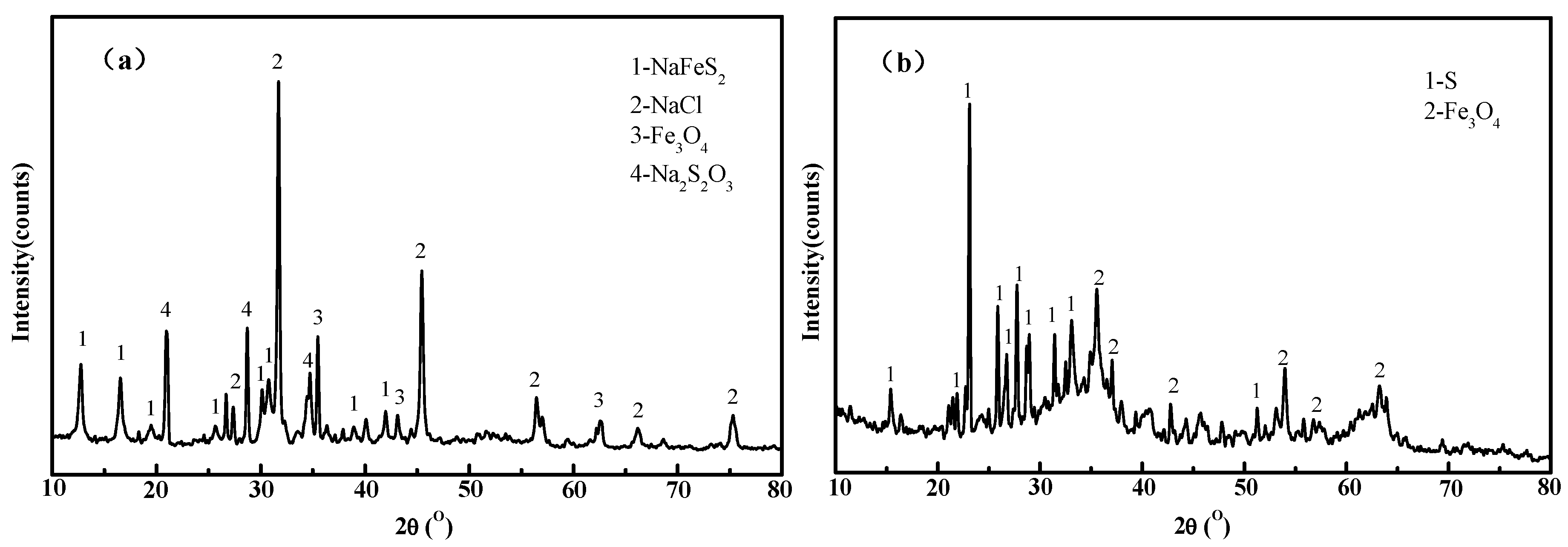
| Materials/% | Sb | S | Fe | Pb | Cu | As | Bi | Au * | SiO2 | CaO |
|---|---|---|---|---|---|---|---|---|---|---|
| Stibnite concentrate | 48.08 | 25.13 | 5.14 | 0.28 | 0.04 | 0.5 | 0.01 | 101.05 | 12.14 | 0.90 |
| Reductant | Industrial analysis | Chemical composition of the ash | LOI | |||||||
| FCd | Vd | Ad | Fetotal | MgO | SiO2 | CaO | Al2O3 | |||
| 81.27 | 3.3 | 15.43 | 25.23 | 0.53 | 41.23 | 6.60 | 25.24 | 82.79 | ||
| Reaction | (kJ/mol) [19] | Equation |
|---|---|---|
| Sb2S3 + 1.5Fe2O3 + 4.5C = 2Sb + 3FeS + 4.5CO (g) | (1) | |
| Sb2S3 + 1.5Fe2O3 + 4.5CO (g) = 2Sb + 3FeS + 4.5CO2 (g) | (2) | |
| Sb2S3 + 3Na2CO3 + 6C = 2Sb + 3Na2S + 9CO (g) | (3) | |
| Sb2S3 + 3Na2CO3 + 3CO (g) = 2Sb + 3Na2S + 6CO2 (g) | (4) | |
| Fe2O3 + 2Na2S + 1.5CO2 (g) + 0.5C = 2FeS + 2Na2CO3 | (5) |
| No. | Chemical Compositions | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude Antimony/% | Slag/% | Lixivium/mg∙L−1 | ||||||||||||
| Sb | Fe | Pb | Au | Sb | Fe | Pb | ST | Na | Sb | Fe | Pb | ST | Na | |
| 1# | 96.7 | 2.29 | 0.55 | 0.03 | 5.46 | 33.67 | 0.09 | 18.5 | 9.26 | 726 | 8.44 | 0.72 | 4642 | >14000 |
| 2# | 95.8 | 2.65 | 0.54 | 0.02 | 5.52 | 34.18 | 0.06 | 19.4 | 8.21 | 715 | 8.80 | 0.67 | 4824 | >14000 |
| 3# | 95.4 | 3.62 | 0.52 | 0.02 | 4.26 | 35.83 | 0.08 | 18.95 | 8.44 | 731 | 8.17 | 0.89 | 5012 | >14000 |
| AVG | 96 | 2.85 | 0.54 | 0.02 | 5.08 | 34.56 | 0.08 | 18.95 | 8.64 | 724 | 8.47 | 0.76 | 4826 | >14000 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, Y.; Xue, H.; Tang, C.; Yang, S.; Tang, M. One-Step Extraction of Antimony in Low Temperature from Stibnite Concentrate Using Iron Oxide as Sulfur-Fixing Agent. Metals 2016, 6, 153. https://doi.org/10.3390/met6070153
Li Y, Chen Y, Xue H, Tang C, Yang S, Tang M. One-Step Extraction of Antimony in Low Temperature from Stibnite Concentrate Using Iron Oxide as Sulfur-Fixing Agent. Metals. 2016; 6(7):153. https://doi.org/10.3390/met6070153
Chicago/Turabian StyleLi, Yun, Yongming Chen, Haotian Xue, Chaobo Tang, Shenghai Yang, and Motang Tang. 2016. "One-Step Extraction of Antimony in Low Temperature from Stibnite Concentrate Using Iron Oxide as Sulfur-Fixing Agent" Metals 6, no. 7: 153. https://doi.org/10.3390/met6070153