Mechanical Alloying Synthesis of Co9S8 Particles as Materials for Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
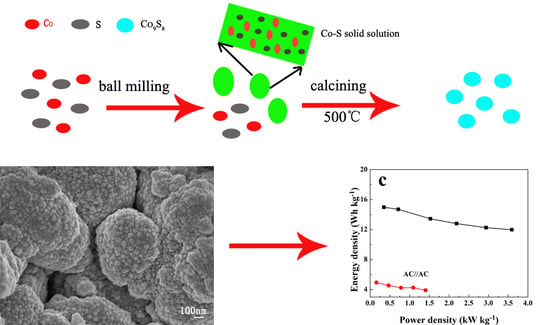
2.1. Materials Synthesis
2.2. Structure Characterization and Electrochemical Measurement
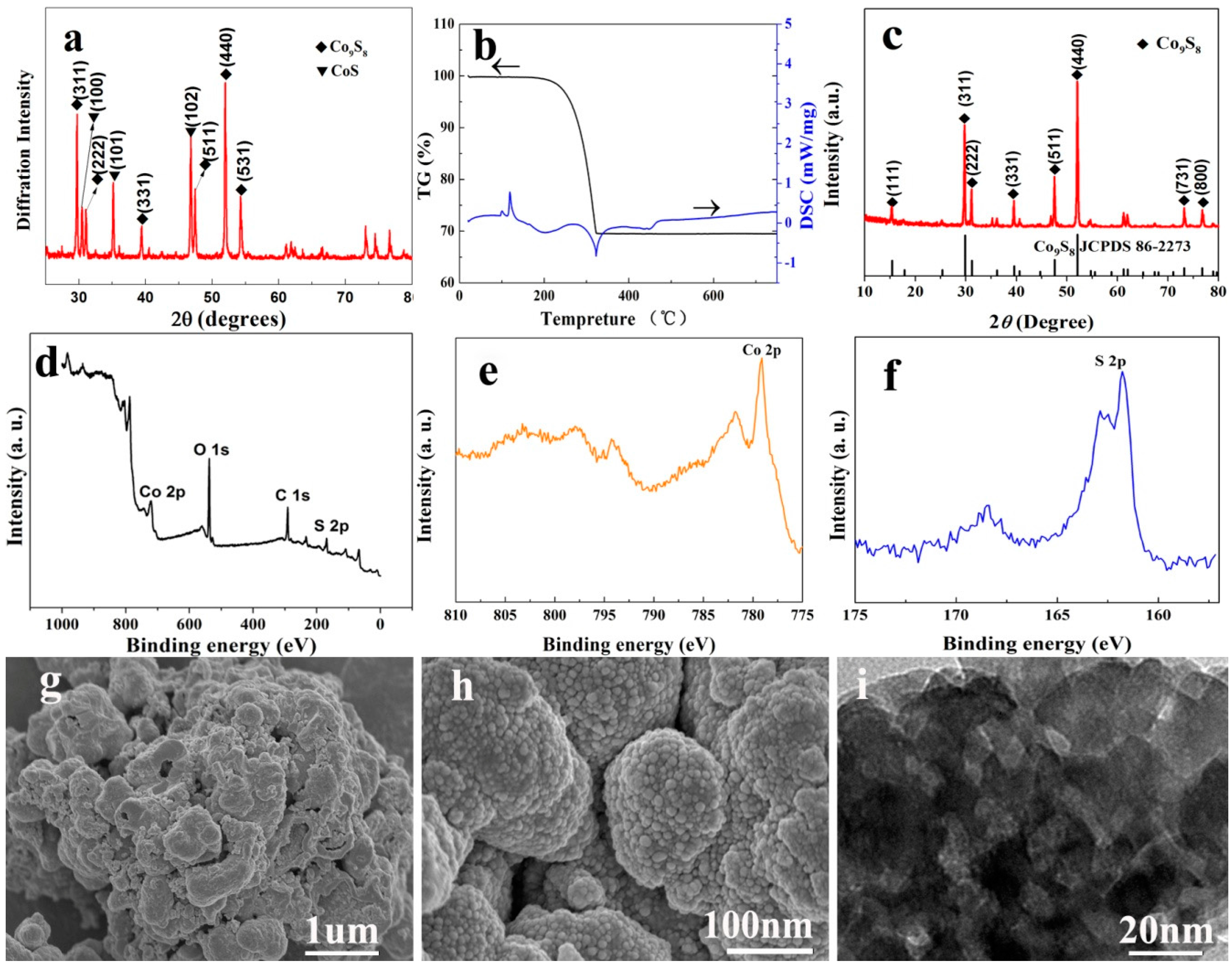
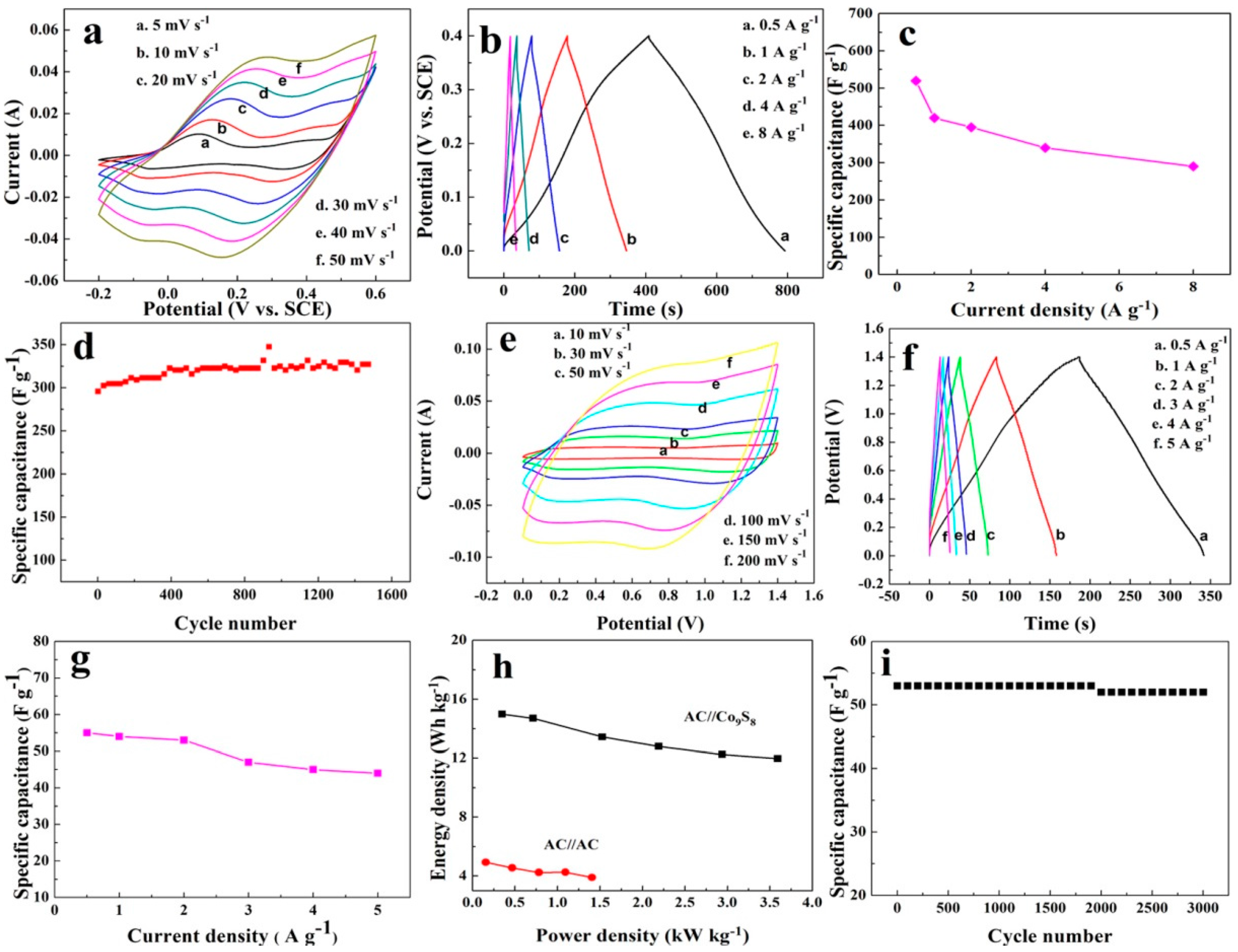
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mondal, A.K.; Su, D.W.; Chen, S.Q.; Zhang, J.Q.; Ung, A.; Wang, G.X. Microwave-assisted synthesis of spherical β-Ni(OH)2 superstructures for electrochemical capacitors with excellent cycling stability. Chem. Phys. Lett. 2014, 610, 115–120. [Google Scholar] [CrossRef]
- He, G.Y.; Li, J.H.; Chen, H.Q.; Shi, J.; Sun, X.Q.; Chen, S.; Wang, X. Hydrothermal preparation of Co3O4@graphene nanocomposite for supercapacitor with enhanced capacitive performance. Mater. Lett. 2012, 82, 61–63. [Google Scholar] [CrossRef]
- Wang, Y.L.; Chang, B.B.; Guan, D.X.; Pei, K.M.; Chen, Z.; Yang, M.S.; Dong, X.P. Preparation of nanospherical porous NiO by a hard template route and its supercapacitor application. Mater. Lett. 2014, 135, 172–175. [Google Scholar] [CrossRef]
- Wang, H.; Köster, T.K.J.; Trease, N.M.; Ségalini, J.; Taberna, P.L.; Simon, P.; Gogotsi, Y.; Grey, C.P. Real-Time NMR Studies of Electrochemical Double-Layer Capacitors. J. Am. Chem. Soc. 2011, 133, 19270–19273. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.B.; Sun, X.X.; Wang, H.J.; Liu, Z.H.; Zhao, X.S. Platelet CMK-5 as an Excellent Mesoporous Carbon to Enhance the Pseudocapacitance of Polyaniline. ACS Appl. Mater. Interfaces 2013, 5, 7501–7508. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Rakhi, R.B.; Alshareef, H.N. Morphology-Dependent Enhancement of the Pseudocapacitance of Template-Guided Tunable Polyaniline Nanostructures. J. Phys. Chem. C 2013, 117, 15009–15019. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, S.; Fan, M.Q.; Li, C.; Chen, D.; Tian, G.L.; Shu, K.Y. Bimetallic nickel cobalt selenides: A new kind of electroactive material for high-power energy storage. J. Mater. Chem. A 2015, 3, 23653–23659. [Google Scholar] [CrossRef]
- Li, S.G.; Teng, F.; Chen, M.D.; Li, N.; Hua, X.; Wang, K.; Li, M. Interesting electrochemical properties of novel three-dimensional Ag3PO4 tetrapods as a new super capacitor electrode material. Chem. Phys. Lett. 2014, 601, 59–62. [Google Scholar] [CrossRef]
- Kurra, N.; Wang, R.Q.; Alshareef, H.N. All conducting polymer electrodes for asymmetric solid-state supercapacitors. J. Mater. Chem. A 2015, 3, 7368–7374. [Google Scholar] [CrossRef]
- Rauda, I.E.; Augustyn, V.; Dunn, B.; Tolber, S.H. Enhancing Pseudocapacitive Charge Storage in Polymer Templated Mesoporous Materials. Accounts Chem. Res. 2013, 46, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Rakhi, R.B.; Alhebshi, N.A.; Anjum, D.H.; Alshareef, H.N. Nanostructured cobalt sulfide-on-fiber with tunable morphology as electrodes for asymmetric hybrid supercapacitors. J. Mater. Chem. A 2014, 2, 16190–16198. [Google Scholar] [CrossRef]
- Tang, J.H.; Shen, J.F.; Li, N.; Ye, M.X. A free template strategy for the synthesis of CoS2-reduced graphene oxide nanocomposite with enhanced electrode performance for supercapacitors. Ceram. Int. 2014, 40, 15411–15419. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerasubramani, G.K.; Kim, S.J. Hydrothermal synthesis, characterization and electrochemical properties of cobalt sulfide nanoparticles. Mat. Sci. Semicond. Proc. 2015, 40, 781–786. [Google Scholar] [CrossRef]
- Wan, H.Z.; Ji, X.; Jiang, J.J.; Yu, J.W.; Miao, L.; Zhang, L.; Bie, S.W.; Chen, H.C.; Ruan, Y.J. Hydrothermal synthesis of cobalt sulfide nanotubes: The size control and its application in supercapacitors. J. Power Sources 2013, 243, 396–402. [Google Scholar] [CrossRef]
- Yin, L.; Wang, L.Q.; Liu, X.H.; Gai, Y.S.; Su, L.H.; Qu, B.H.; Gong, L.Y. Ultra-Fast Microwave Synthesis of 3D Flower-Like Co9S8 Hierarchical Architectures for High-Performance Supercapacitor Applications. Eur. J. Inorg. Chem. 2015, 14, 2457–2462. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Hu, Y.; Li, J.; Liu, M.; Kong, L.; Hu, Y.; Kang, L. Mechanical Alloying Synthesis of Co9S8 Particles as Materials for Supercapacitors. Metals 2016, 6, 142. https://doi.org/10.3390/met6060142
Li B, Hu Y, Li J, Liu M, Kong L, Hu Y, Kang L. Mechanical Alloying Synthesis of Co9S8 Particles as Materials for Supercapacitors. Metals. 2016; 6(6):142. https://doi.org/10.3390/met6060142
Chicago/Turabian StyleLi, Bo, Yuxia Hu, Jiajia Li, Maocheng Liu, Lingbin Kong, Yumei Hu, and Long Kang. 2016. "Mechanical Alloying Synthesis of Co9S8 Particles as Materials for Supercapacitors" Metals 6, no. 6: 142. https://doi.org/10.3390/met6060142