Microstructures and Mechanical Properties of Austempering SUS440 Steel Thin Plates
Abstract
:1. Introduction
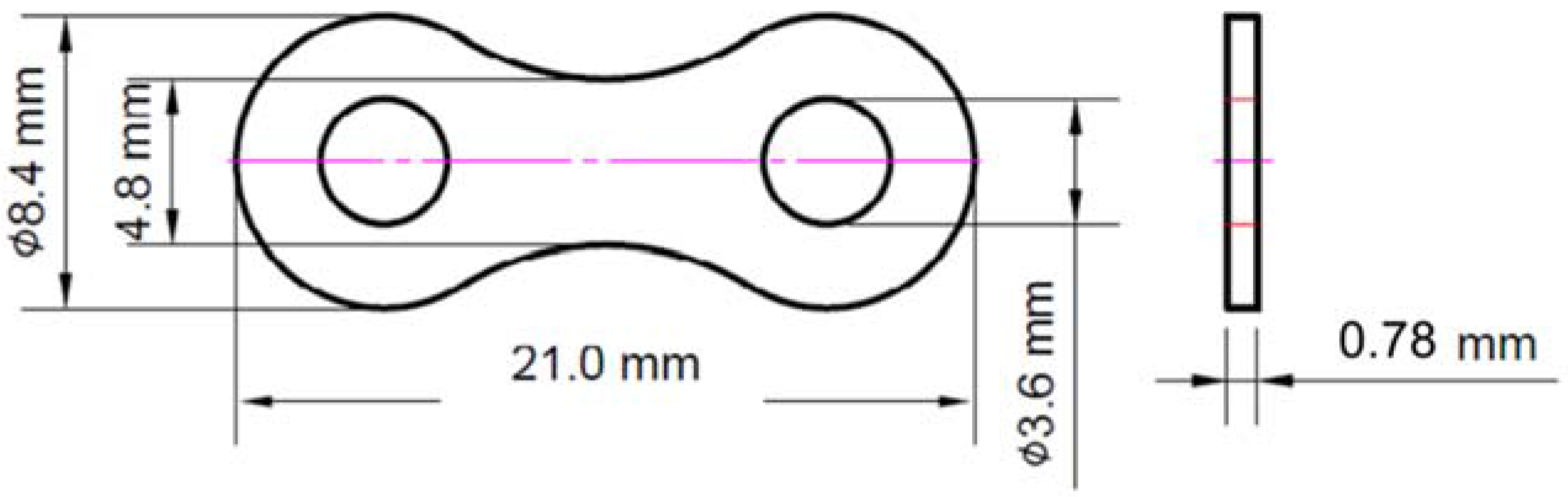
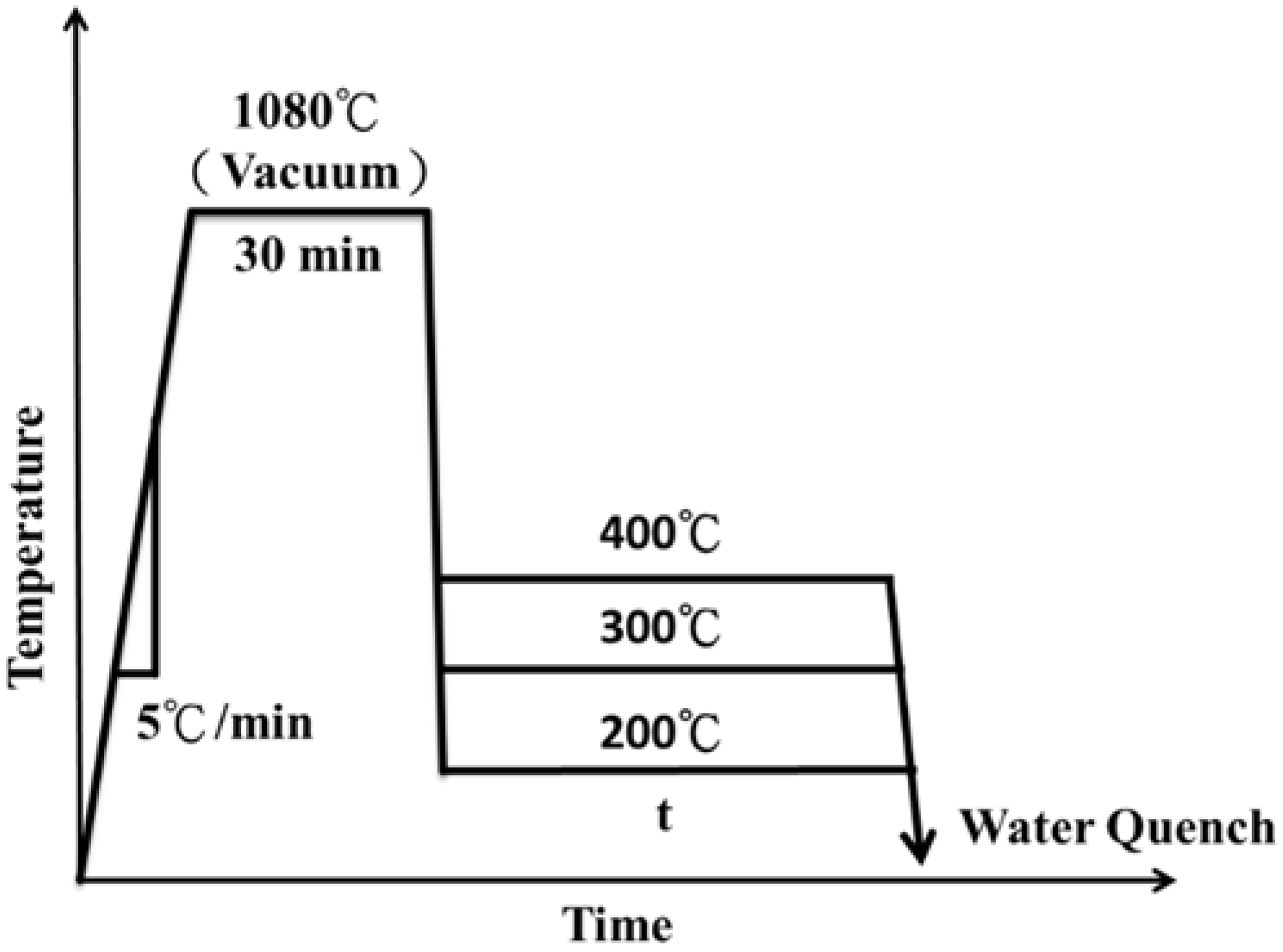
2. Experimental Procedure
| Element | C | Mn | Si | P | S | V | Cr | Mo | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Content | 0.75 | 1.00 | 1.00 | 0.04 | 0.04 | 0.15 | 18.20 | 0.30 | Bal. |
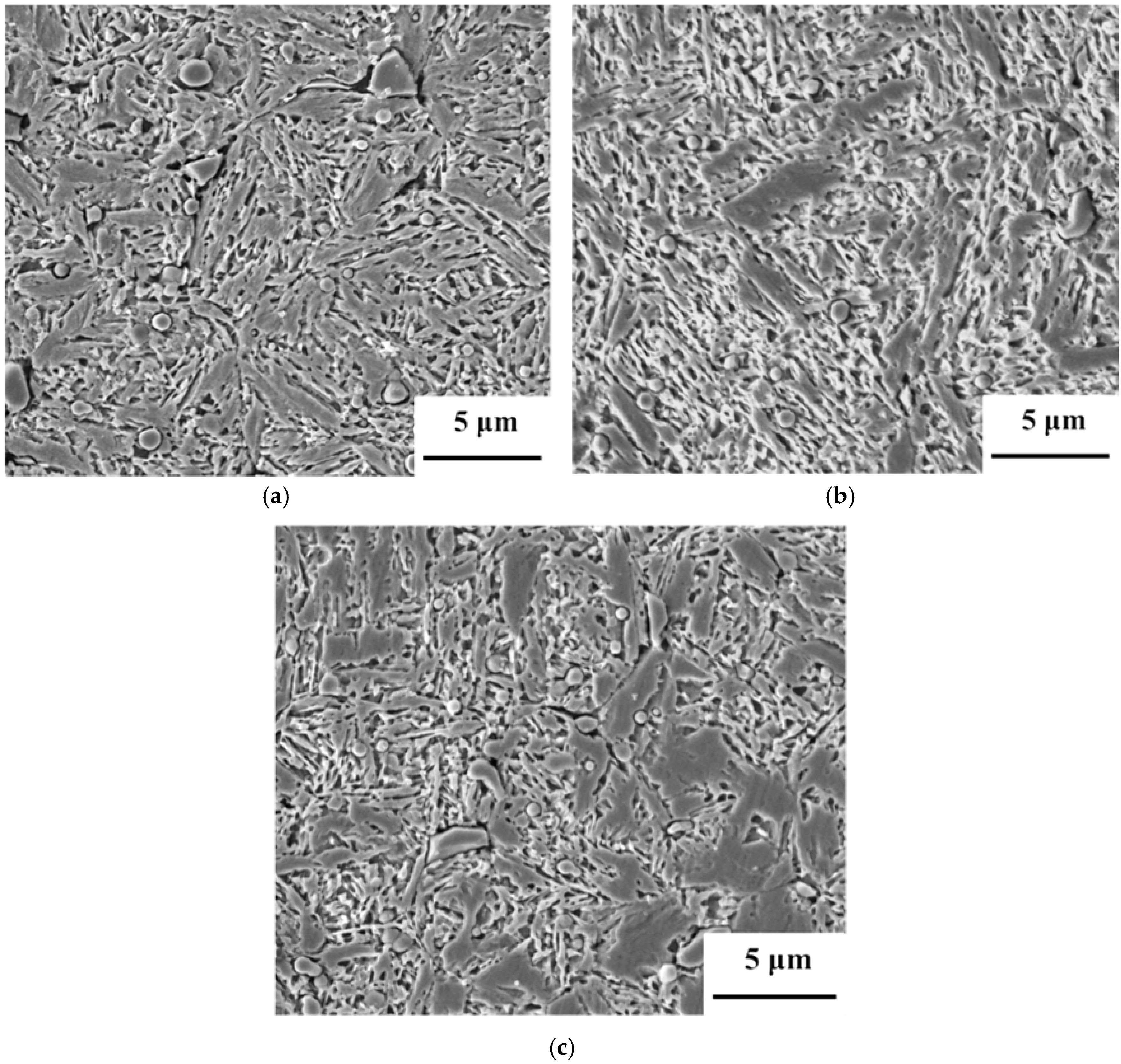
3. Results and Discussion
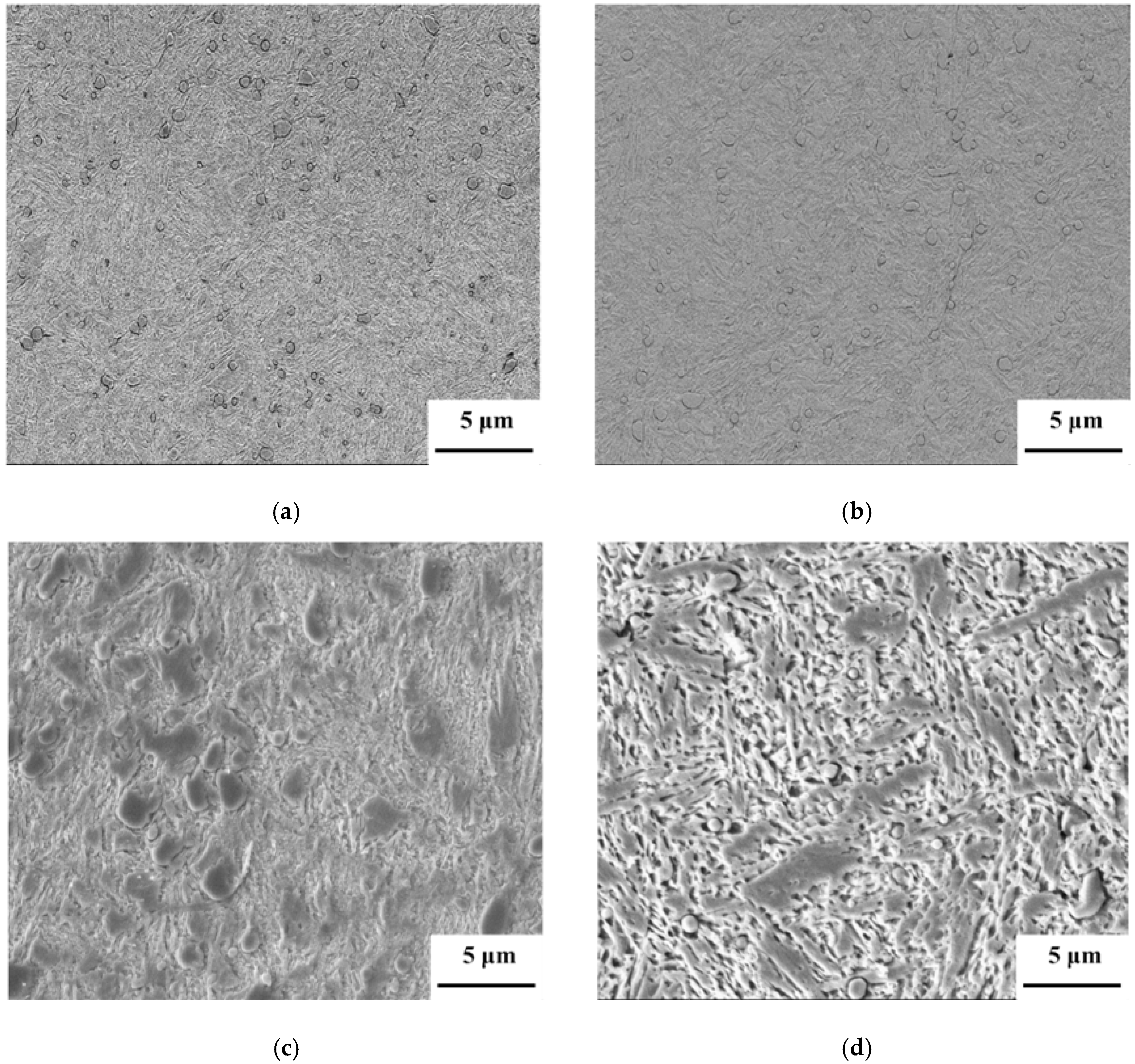
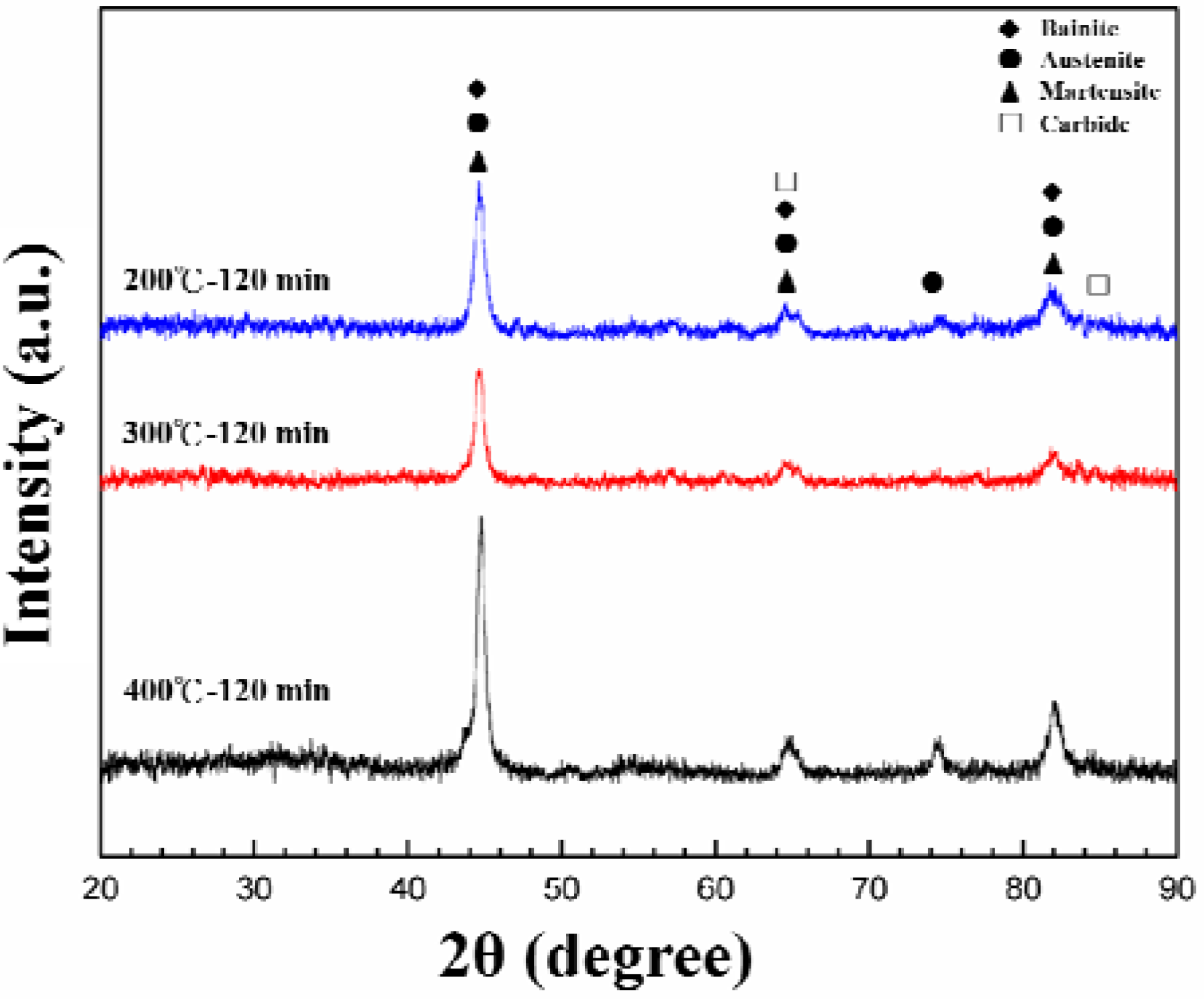
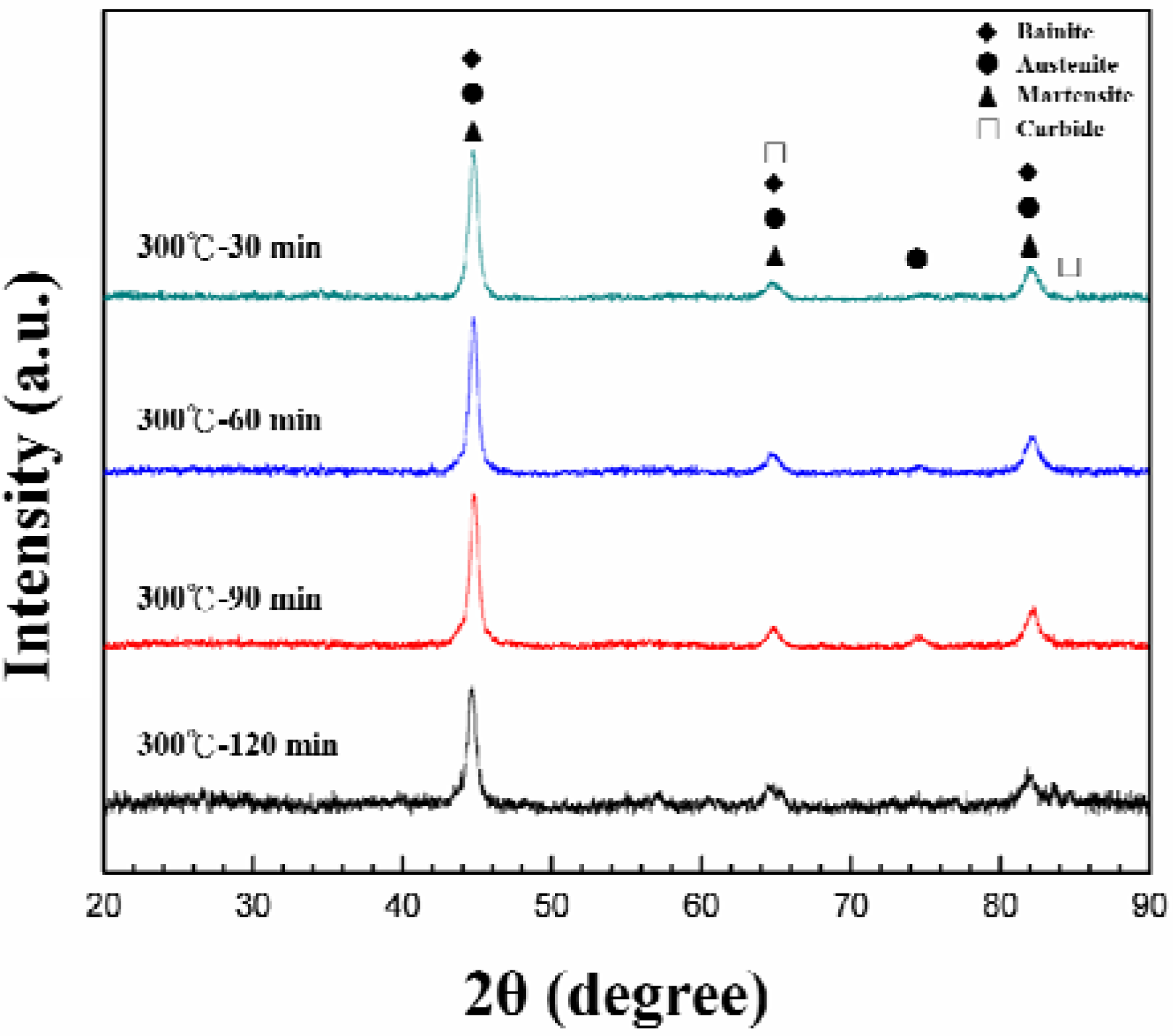

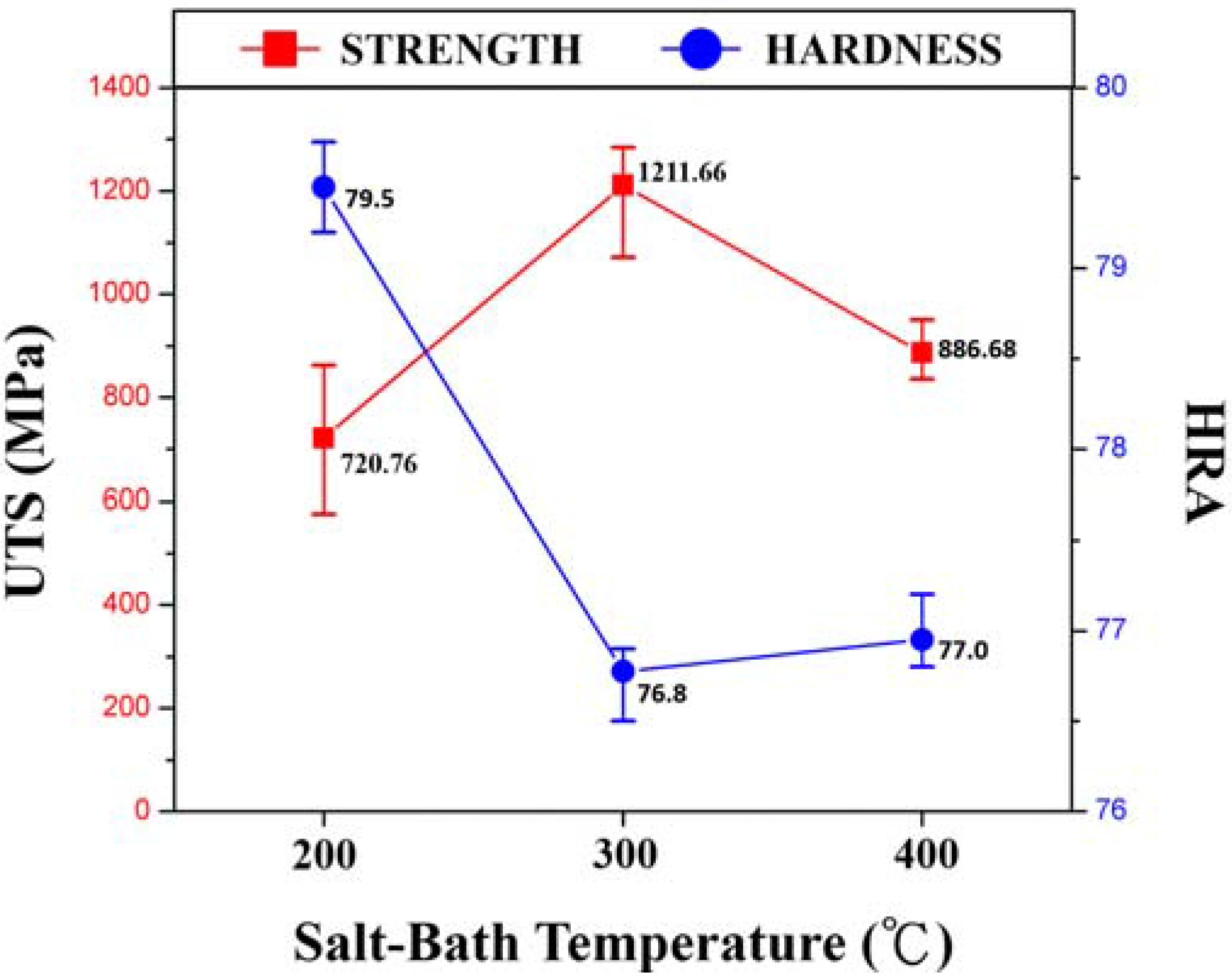
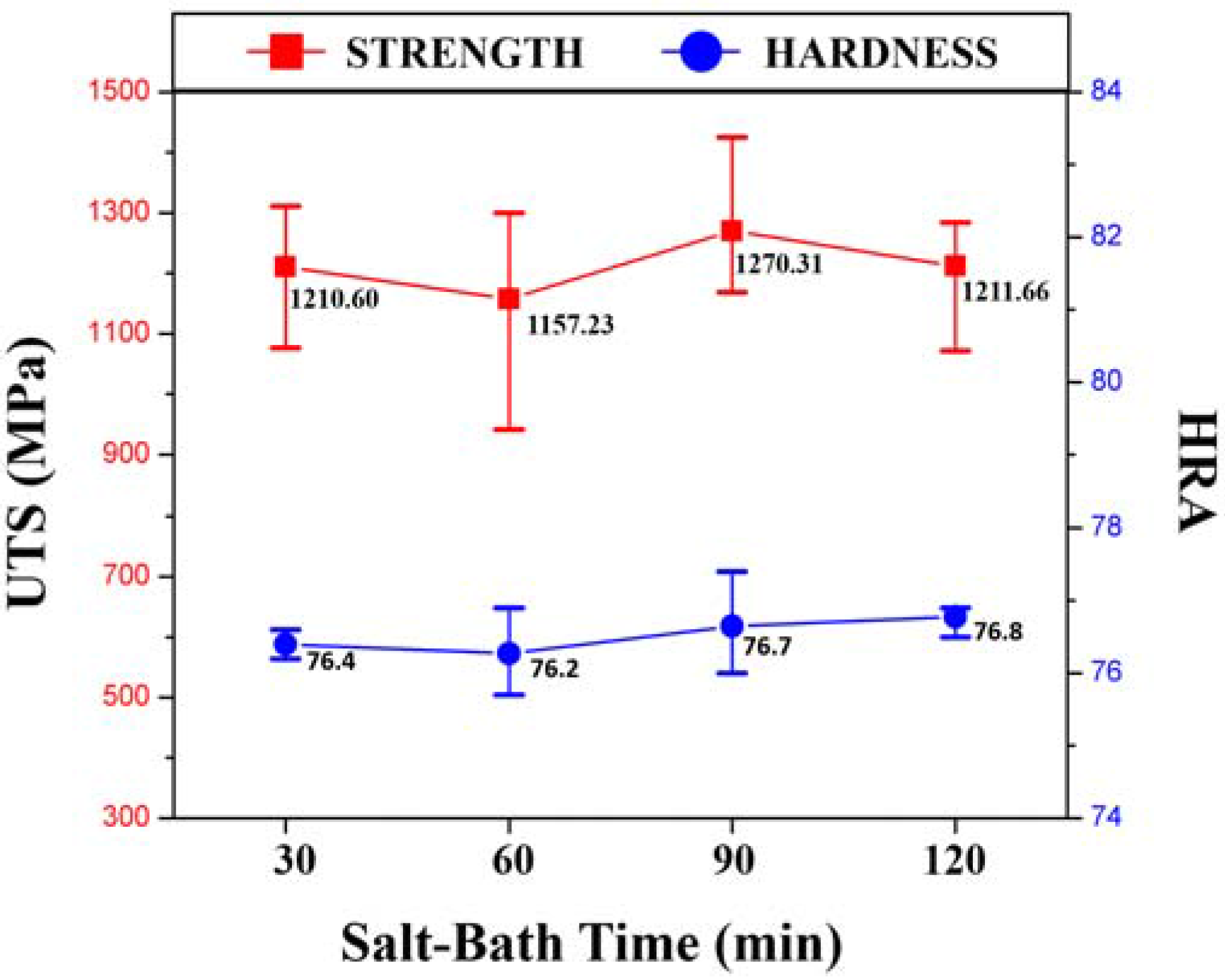
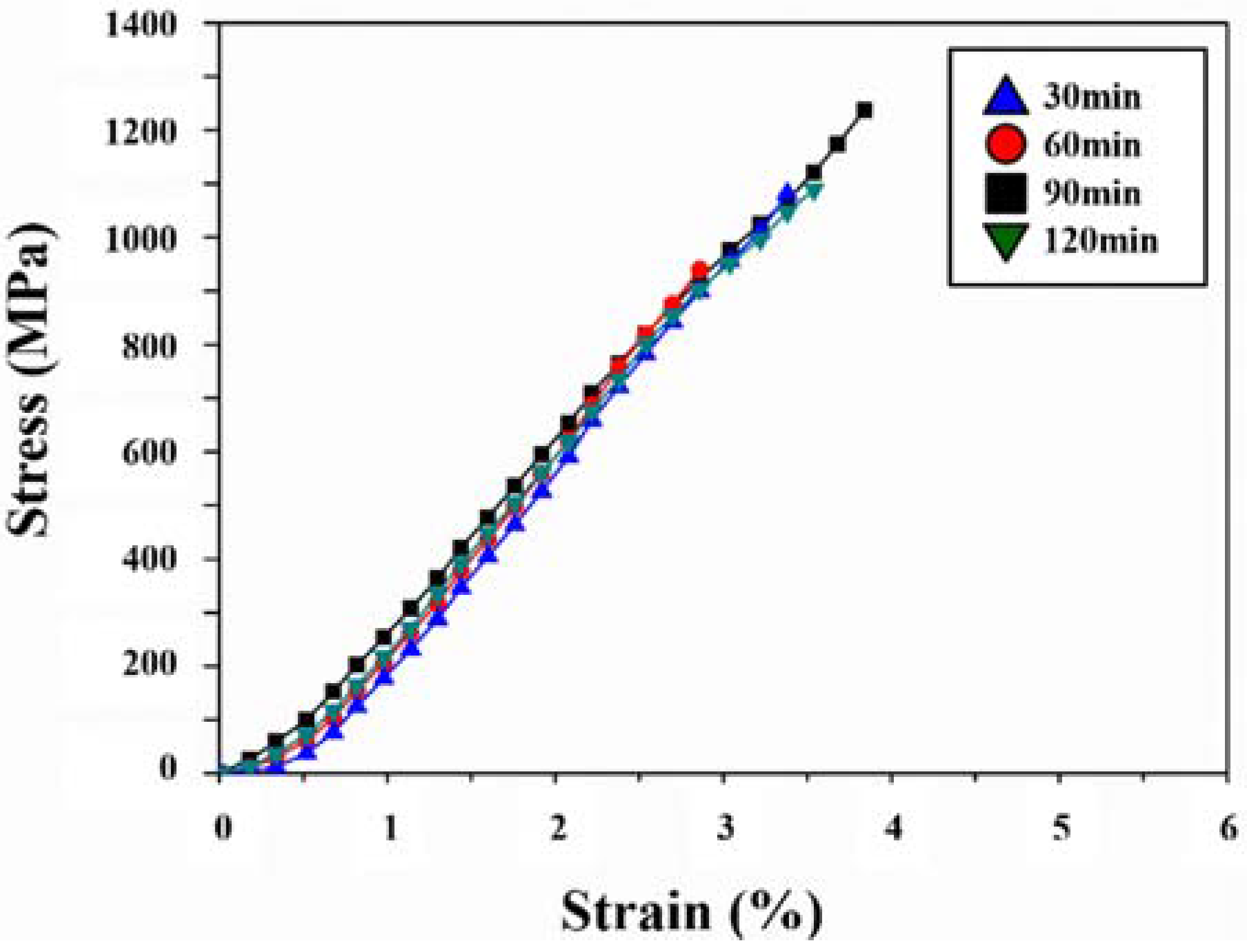
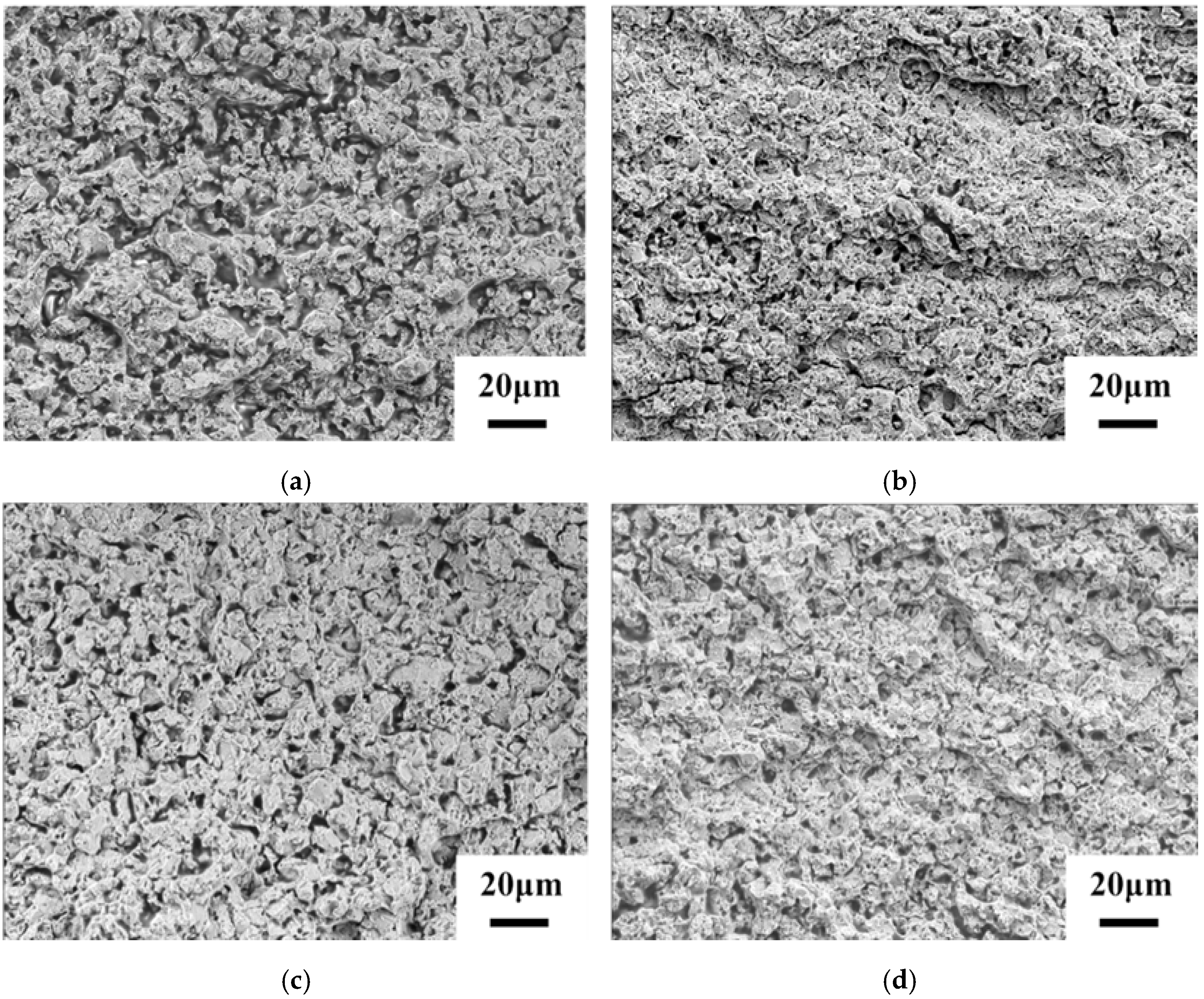
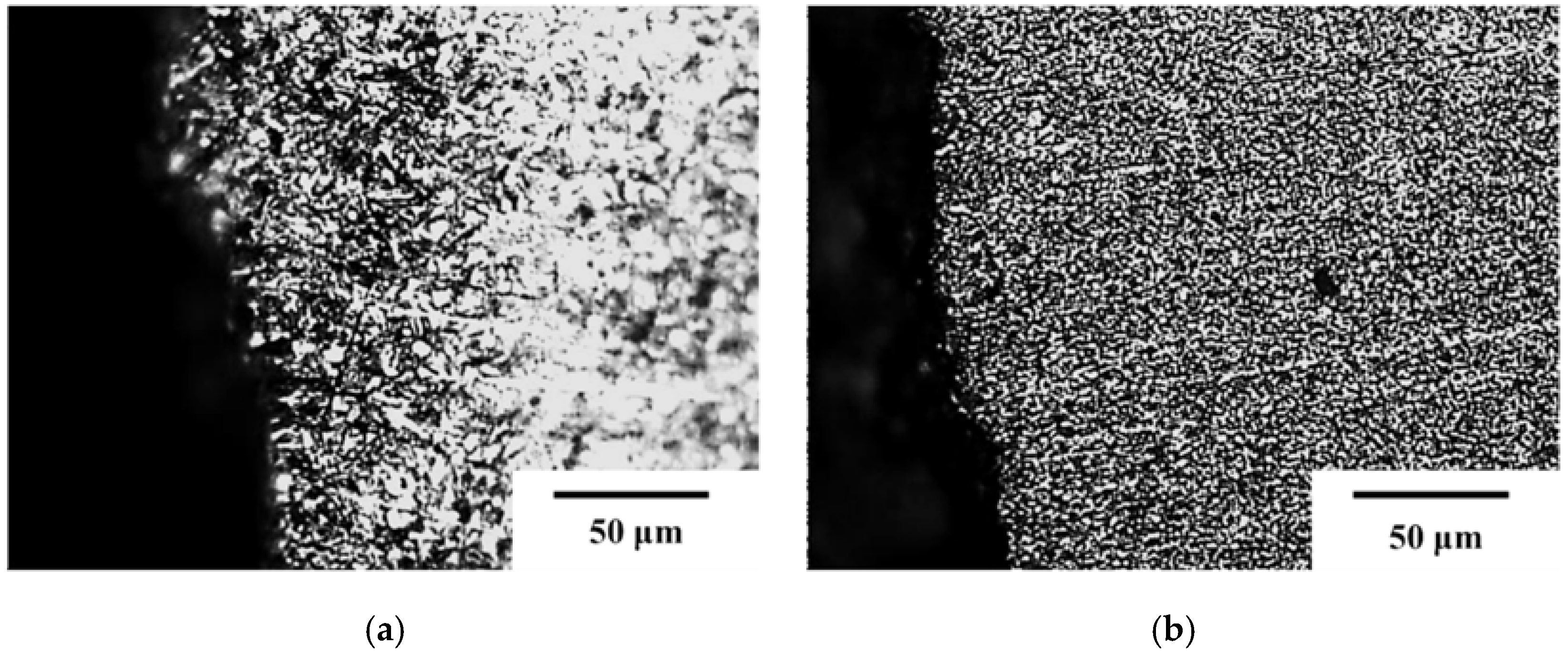
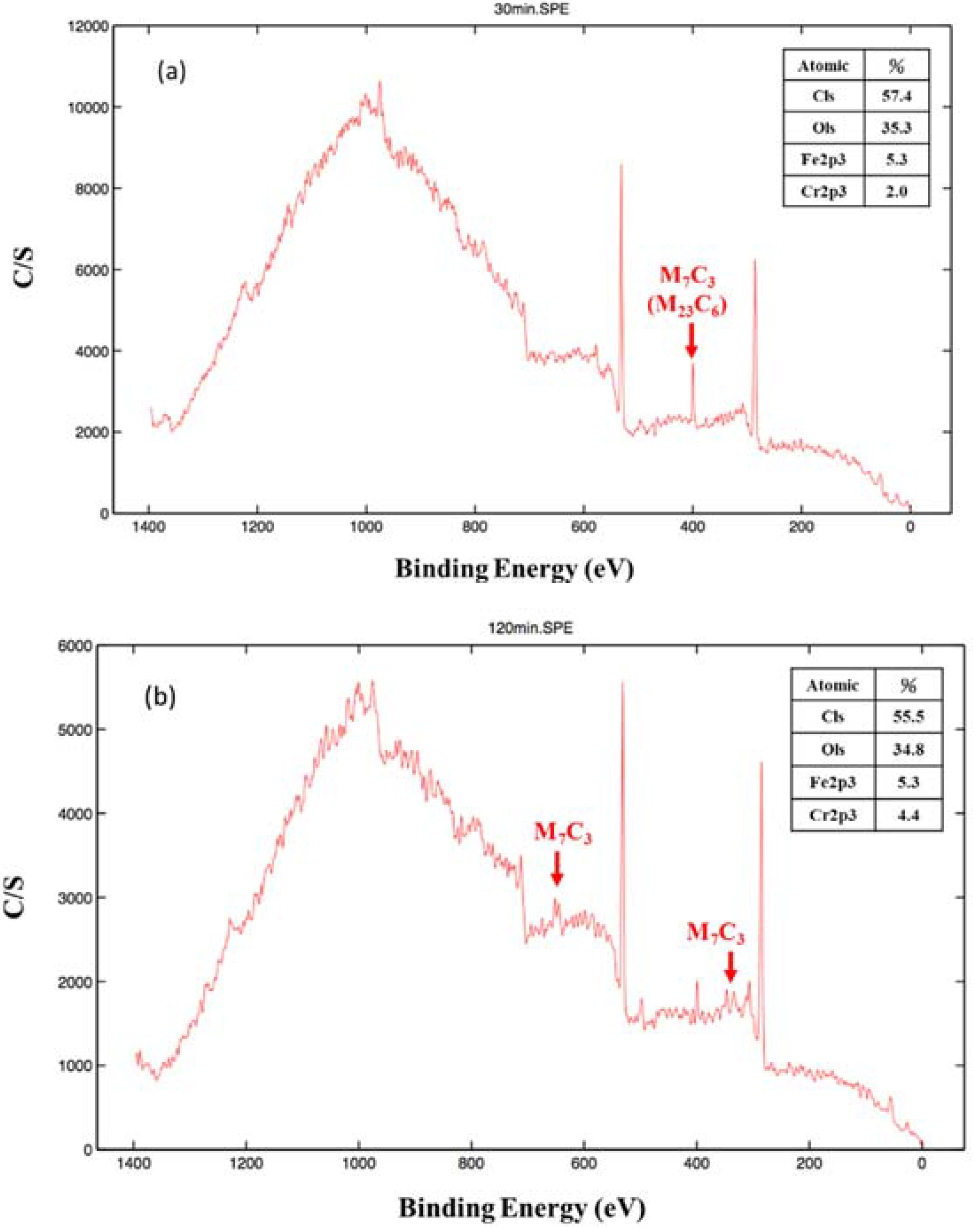

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bee, J.V.; Powell, G.L.F.; Bednarz, B. A substructure within the austenitic matrix of high chromium white irons. Scr. Metall. Mater. 1994, 31, 1735–1736. [Google Scholar] [CrossRef]
- Salleh, S.H.; Omar, M.Z.; Syarif, J. Carbide formation during precipitation hardening of SS440C steel. Eur. J. Sci. Res. 2009, 34, 83–91. [Google Scholar]
- Lin, Y.L.; Lin, C.; Tsai, T. Microstructure and mechanical properties of 0.63C-12.7Cr martensitic stainless steel during various tempering treatments. Mater. Manuf. Proc. 2010, 25, 246–248. [Google Scholar] [CrossRef]
- Salih, A.A.; Omar, M.Z.; Junaidi, S.; Sajuri, Z. Effect of different heat treatment on the SS440C martensitic stainless steel. Aust. J. Basic Appl. Sci. 2011, 5, 867–871. [Google Scholar]
- Bhadeshia, H. Martensite and bainite in steels: Transformation mechanism & mechanical properties. J. Phys. IV 1997, 7, 367–376. [Google Scholar]
- Lee, H.Y.; Yen, H.; Chang, H.; Yang, J. Substructures of martensite in Fe-1C-17Cr stainless steel. Scr. Mater. 2010, 62, 670–673. [Google Scholar] [CrossRef]
- Salleh, S.H. Investigation of microstructures and properties of 440C martensitic stainless steel. Int. J. Mech. Mater. Eng. 2009, 4, 123–126. [Google Scholar]
- Carpenter, S.D.; Carpenter, D. X-ray diffraction study of M7C3 carbide within a high chromium white iron. Mater. Lett. 2003, 57, 4456–4459. [Google Scholar] [CrossRef]
- Carpenter, S.D.; Carpenter, D.; Pearce, J.T.H. XRD and electron microscope study of an as-cast 26.6% chromium white iron microstructure. Mater. Chem. Phys. 2004, 85, 32–40. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Z.; Northwood, D.O.; Liu, Y. A new empirical formula for the calculation of MS temperatures in pure iron and super-low carbon alloy steels. J. Mater. Proc. Technol. 2001, 113, 556–562. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hung, F.; Lui, T.; Chen, L. Microstructures and mechanical properties of austempering Cr-Mo (SCM 435) alloy steel. Mater. Trans. 2013, 54, 56–60. [Google Scholar] [CrossRef]
- Tsuzaki, K.; Maki, T. Some aspects of bainite transformation in Fe-based alloys. J. Phys. IV 1995, 5, 61–70. [Google Scholar] [CrossRef]
- Funatani, K. Low-temperature salt bath nitriding of steels. Met. Sci. Heat Treat. 2004, 46, 277–281. [Google Scholar] [CrossRef]
- Lee, B.-J. On the stability of Cr carbides. Calphad 1992, 16, 121–149. [Google Scholar] [CrossRef]
- Kwok, C.T.; Lo, K.; Cheng, F.; Man, H. Effect of processing conditions on the corrosion performance of laser surface-melted AISI 440C martensitic stainless steel. Surf. Coat. Technol. 2003, 166, 221–230. [Google Scholar] [CrossRef]
- Carpenter, S.D.; Carpenter, D.; Pearce, J.T.H. XRD and electron microscope study of a heat treated 26.6% chromium white iron microstructure. Mater. Chem. Phys. 2007, 101, 49–55. [Google Scholar] [CrossRef]
- Avishan, B.; Yazdani, S.; Nedjad, S.H. Toughness variations in nanostructured bainitic steels. Mater. Sci. Eng. A 2012, 548, 106–111. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, J.; Wang, H.; Wu, X. Effect of tempering on microstructure and mechanical properties of a non-quenched bainitic steel. Mater. Sci. Eng. A 2010, 527, 3433–3437. [Google Scholar] [CrossRef]
- Yang, J.R.; Yu, T.H.; Wang, C.H. Martensitic transformations in AISI 440C stainless steel. Mater. Sci. Eng. A 2006, 438, 276–280. [Google Scholar] [CrossRef]
- Sajjadi, S.A.; Zebarjad, S.M. Isothermal transformation of austenite to bainite in high carbon steels. J. Mater. Proc. Technol. 2007, 189, 107–113. [Google Scholar] [CrossRef]
- Isfahany, A.N.; Saghafian, H.; Borhani, G. The effect of heat treatment on mechanical properties and corrosion behavior of AISI420 martensitic stainless steel. J. Alloys Compd. 2011, 509, 3931–3936. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Hung, F.-Y.; Lui, T.-S.; Chen, L.-H. Microstructures and Mechanical Properties of Austempering SUS440 Steel Thin Plates. Metals 2016, 6, 35. https://doi.org/10.3390/met6020035
Chen C-Y, Hung F-Y, Lui T-S, Chen L-H. Microstructures and Mechanical Properties of Austempering SUS440 Steel Thin Plates. Metals. 2016; 6(2):35. https://doi.org/10.3390/met6020035
Chicago/Turabian StyleChen, Cheng-Yi, Fei-Yi Hung, Truan-Sheng Lui, and Li-Hui Chen. 2016. "Microstructures and Mechanical Properties of Austempering SUS440 Steel Thin Plates" Metals 6, no. 2: 35. https://doi.org/10.3390/met6020035






