On the Step Cooling Treatment for the Assessment of Temper Embrittlement Susceptibility of Heavy Forgings in Superclean Steels
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bruscato, R. Temper embrittlement and creep embrittlement in 2-1/4 Cr-1 Mo shielded metal arc weld deposits. Weld. J. Res. Suppl. 1970, 49, 148s–156s. [Google Scholar]
- Wada, T. Report RP-32-74-03; Climax Molybdenum Company of Michigan: Detroit, MI, USA, 1975. [Google Scholar]
- King, B.L.; Wigmore, G. Temper embrittlement in a 3-pct Cr-Mo turbine disc steel. Metall. Trans. A 1976, 7, 1761–1767. [Google Scholar] [CrossRef]
- Briant, C.L.; Banerji, S.K. Tempered martensite embrittlement in phosphorus doped steels. Metall. Trans. A 1979, 10, 1729–1737. [Google Scholar]
- Kameda, J.; McMahon, C.J., Jr. The effects of Sb, Sn, and P on the strength of grain boundaries in a Ni-Cr Steel. Metall. Trans. A 1981, 12, 31–37. [Google Scholar] [CrossRef]
- A Study of Temper Embrittlement during Stress Relieving of 5Ni-CrMo-V Steels. Available online: http://www.astm.org/DIGITAL_LIBRARY/STP/PAGES/STP46473S.htm (accessed on 8 October 2016).
- Temper Embrittlement of Rotor Steels. Available online: http://www.astm.org/DIGITAL_LIBRARY/STP/PAGES/STP46478S.htm (accessed on 8 October 2016).
- Temper Brittleness—An Interpretive Review. Available online: http://www.astm.org/DIGITAL_LIBRARY/STP/PAGES/STP46479S.htm (accessed on 8 October 2016).
- Mulford, R.A.; McMahon, C.J., Jr.; Pope, D.P.; Feng, H.C. Temper embrittlement of Ni-Cr Steels by phosphorus. Metall. Trans. A 1976, 7, 1183–1195. [Google Scholar] [CrossRef]
- Briant, C.L.; Banerji, S.K. Intergranularfailure in steel: The role of grain-boundary composition. Int. Met. Rev. 1978, 23, 164–199. [Google Scholar] [CrossRef]
- Lea, C.; Seah, M.P. Site competition in surface segregation. Surf. Sci. 1975, 53, 272–285. [Google Scholar] [CrossRef]
- Guttmann, M.; Dumiulin, P.; Wayman, M. The thermodynamics of interactive co-segregation of phosphorus and alloying elements in iron and temper-brittle steels. Metall. Trans. A 1982, 13, 1693–1711. [Google Scholar] [CrossRef]
- Erhart, H.; Grabke, H.J. Equilibrium segregation of phosphorus at grain boundaries of Fe–P, Fe–C–P, Fe–Cr–P, and Fe–Cr–C–P alloys. Met. Sci. 1981, 15, 401–408. [Google Scholar] [CrossRef]
- Yuan, Z.X.; Song, S.H.; Faulkner, R.G.; Xu, T.D. Effect of cerium on temper embrittlement of P-doped Mn structural-steels. Acta Metall. Mater. 1994, 42, 127–132. [Google Scholar]
- Pilkington, R.; Dicken, R.; Peura, P.; Lorimer, G.W.; Allen, G.C.; Holt, M.; Younes, C.M. Trace element embrittlement in a 2.25%Cr-1%Mo steel. Mater. Sci. Eng. A 1996, 212, 191–205. [Google Scholar] [CrossRef]
- Phythian, W.J.; English, C.A. Microstructural evolution in reactor pressure vessel steels. J. Nucl. Mater. 1993, 205, 162–177. [Google Scholar] [CrossRef]
- Wada, T.; Hagel, W.C. Effect of trace elements, molybdenum, and intercritical heat treatment on temper embrittlement of 2-1/4Cr-1 Mo steel. Metall. Mater. Trans. A 1976, 7, 1419–1426. [Google Scholar] [CrossRef]
- Low, J.R., Jr.; Stein, D.F.; Turkalo, A.M.; Laforce, R.P. Alloy and impurity effects on temper brittleness of steel. Trans. Metall. Soc. 1968, 242, 14. [Google Scholar]
- Yu, J.; McMahon, C.J. The effects of composition and carbide precipitation on temper embrittlement of 2.25 Cr-1 Mo steel: Part I. Effects of P and Sn. Metall. Trans. A 1980, 11, 277–289. [Google Scholar] [CrossRef]
- Wignarajah, S.; Masumoto, I.; Hara, T. Evaluation and simulation of the microstructural changes and embrittlement in 21/4Cr-1Mo steel due to long term service. ISIJ Int. 1990, 30, 58–63. [Google Scholar] [CrossRef]
- Seah, M.P. Grain boundary segregation and the T–t dependence of temper brittleness. Acta Mater. 1977, 25, 345–357. [Google Scholar] [CrossRef]
- Suzuki, M.; Fukaya, K.; Oku, T. Effect of applied stress on temper embrittlement of 21/4Cr-1Mo steel. Trans. Iron Steel Inst. Jpn. 1982, 22, 862–868. [Google Scholar] [CrossRef]
- API Publication Standard No. 959, 1982. Available online: https://www.scribd.com/document/292425595/API-Publ-959 (accessed on 8 October 2016).
- Buscemi, C.D.; Jack, B.I.; Erwin, N.E. Temper embrittlement in 2-1/4 Cr-1 Mo steels after 75,000-hour isothermal aging. J. Eng. Mater. Technol. 1991, 113, 329–335. [Google Scholar] [CrossRef]
- Watanabe, J.; Shindo, Y.; Murakami, Y.; Adachi, T.; Ajiki, S. Temper embrittlement of 21/4 Cr-1Mo pressure vessel steel. In Proceedings of the ASME 29th Petroleum Mechanical Engineering Conference, Dallas, TX, USA, 15–18 September 1974.
- McMahon, C.J., Jr. Temper Embrittlement of Steels: Remaining Issues. Mater. Sci. Forum 1989, 46, 61–76. [Google Scholar] [CrossRef]
- Hickey, J.J.; Bulloch, J.H. The role of reverse temper embrittlement on some low and high temperature crack extension processes in low carbon, low alloy steels: A review. Int. J. Press. Vess. Pip. 1992, 49, 339–386. [Google Scholar] [CrossRef]
- Mclean, D. Grain Boundaries in Metals; Oxford University Press: London, UK, 1957; p. 118. [Google Scholar]
- Sevc, P.; Anovex, J.J.; Lucas, M.; Grabke, H.J. Kinetics of phosphorus segregation in 2.7Cr-0.7Mo-0.3V steels with different phosphorus contents. Steel Res. 1995, 66, 537–542. [Google Scholar]
- Zhang, Z.; Xu, T.; Lin, Q.; Yu, Z. A new interpretation of temper embrittlement dynamics by non-equilibrium segregation of phosphor in steels. J. Mater. Sci. 2001, 36, 2055–2059. [Google Scholar]
- Li, Q.; Li, L.; Liu, E.; Liu, D.; Cui, X. Temper embrittlement dynamics induced by non-equilibrium segregation of phosphorus in steel 12Cr1MoV. Scr. Mater. 2005, 53, 309–313. [Google Scholar] [CrossRef]
- Tanaka, Y.; Azuma, T.; Yaegashi, N. Isothermal aging test results up to 100,000 h of NiCrMoV steels for low pressure steam turbine. In Materials Ageing and Component Life Extension, Proceedings of the International Symposium on Materials Ageing and Component Life Extension, Milan, Italy, 10–13 October 1995.
- Bourrat, J.; Shaff, H. Pitting and stress corrosion cracking of conventional and high purity LP turbine rotor steel. In Proceedings of the Clean Steel, Superclean Steel, London, UK, 6–7 March 1995; p. 157.





| C | Mn | Si | P | S | Cu | Cr | Ni | Mo | Sn | Al | V | Nb | Ti | B | As | Sb | Co |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.26 | 0.04 | 0.02 | 0.003 | 0.001 | 0.03 | 1.66 | 3.62 | 0.40 | 0.004 | 0.004 | 0.087 | 0.003 | 0.002 | 0.0002 | 0.0004 | 0.0004 | 0.006 |
| Steel Condition | Temperature (°C) | Time (h) |
|---|---|---|
| A | 524 | 120 |
| B | 593 | 2 |
| C | 538 | 48 |
| D | not aged material | |
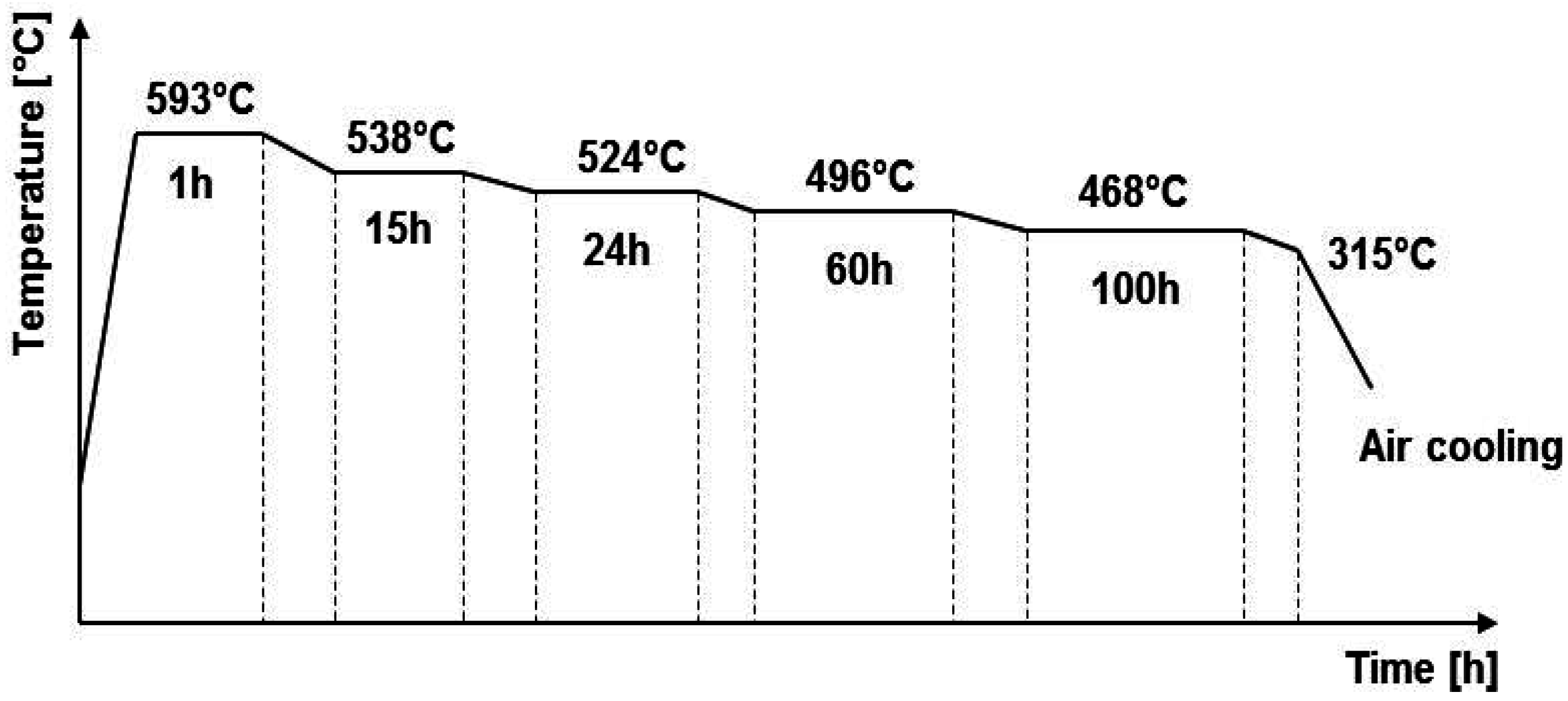
| E | step cooling (Figure 1) | |
| F | 593 | 8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberti, R.; Faccoli, M. On the Step Cooling Treatment for the Assessment of Temper Embrittlement Susceptibility of Heavy Forgings in Superclean Steels. Metals 2016, 6, 239. https://doi.org/10.3390/met6100239
Roberti R, Faccoli M. On the Step Cooling Treatment for the Assessment of Temper Embrittlement Susceptibility of Heavy Forgings in Superclean Steels. Metals. 2016; 6(10):239. https://doi.org/10.3390/met6100239
Chicago/Turabian StyleRoberti, Roberto, and Michela Faccoli. 2016. "On the Step Cooling Treatment for the Assessment of Temper Embrittlement Susceptibility of Heavy Forgings in Superclean Steels" Metals 6, no. 10: 239. https://doi.org/10.3390/met6100239





