Direct Aqueous Mineral Carbonation of Waste Slate Using Ammonium Salt Solutions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Leaching Tests
2.3. Carbonation Tests
2.4. Chemical Analysis and Material Characterization
3. Results and Discussion
3.1. Raw Material
| Materials | Composition (wt. %) | Minerals (XRD) | ||||
|---|---|---|---|---|---|---|
| Ca | Mg | Al | Fe | Si | ||
| Waste slate | 25.2 | 2.2 | 1.4 | 1.3 | 1.4 | Calcite, Chrysotile, and Ca-Mg-Al-Si-oxide minerals |
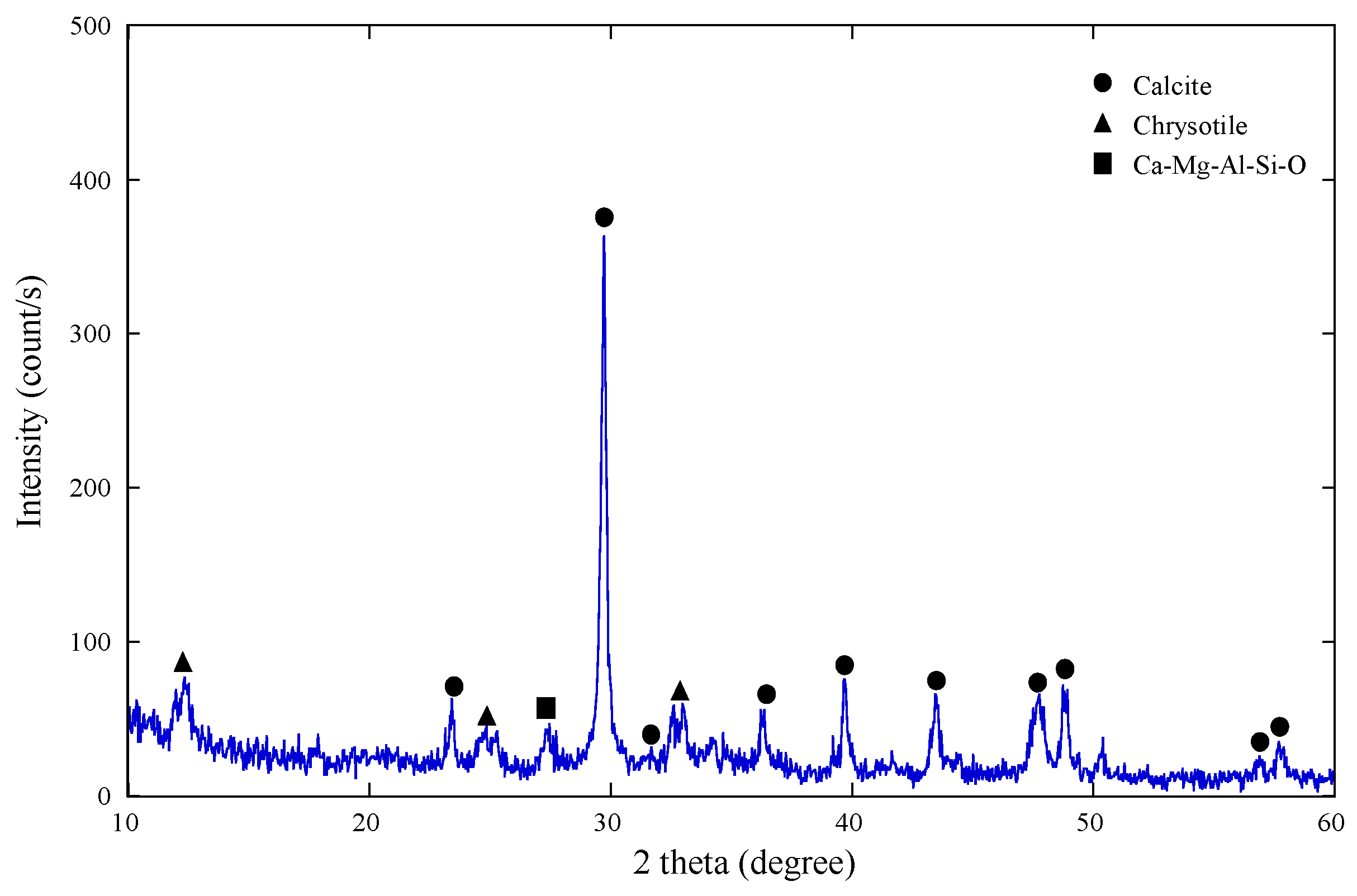

3.2. Leaching Behaviors


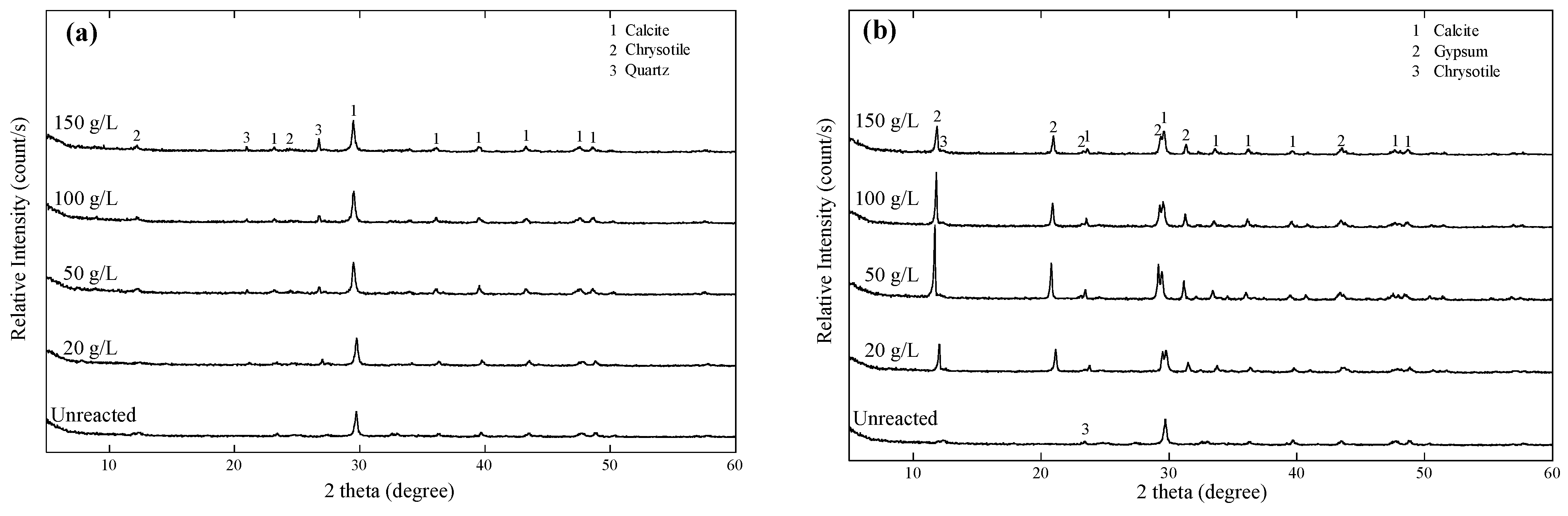
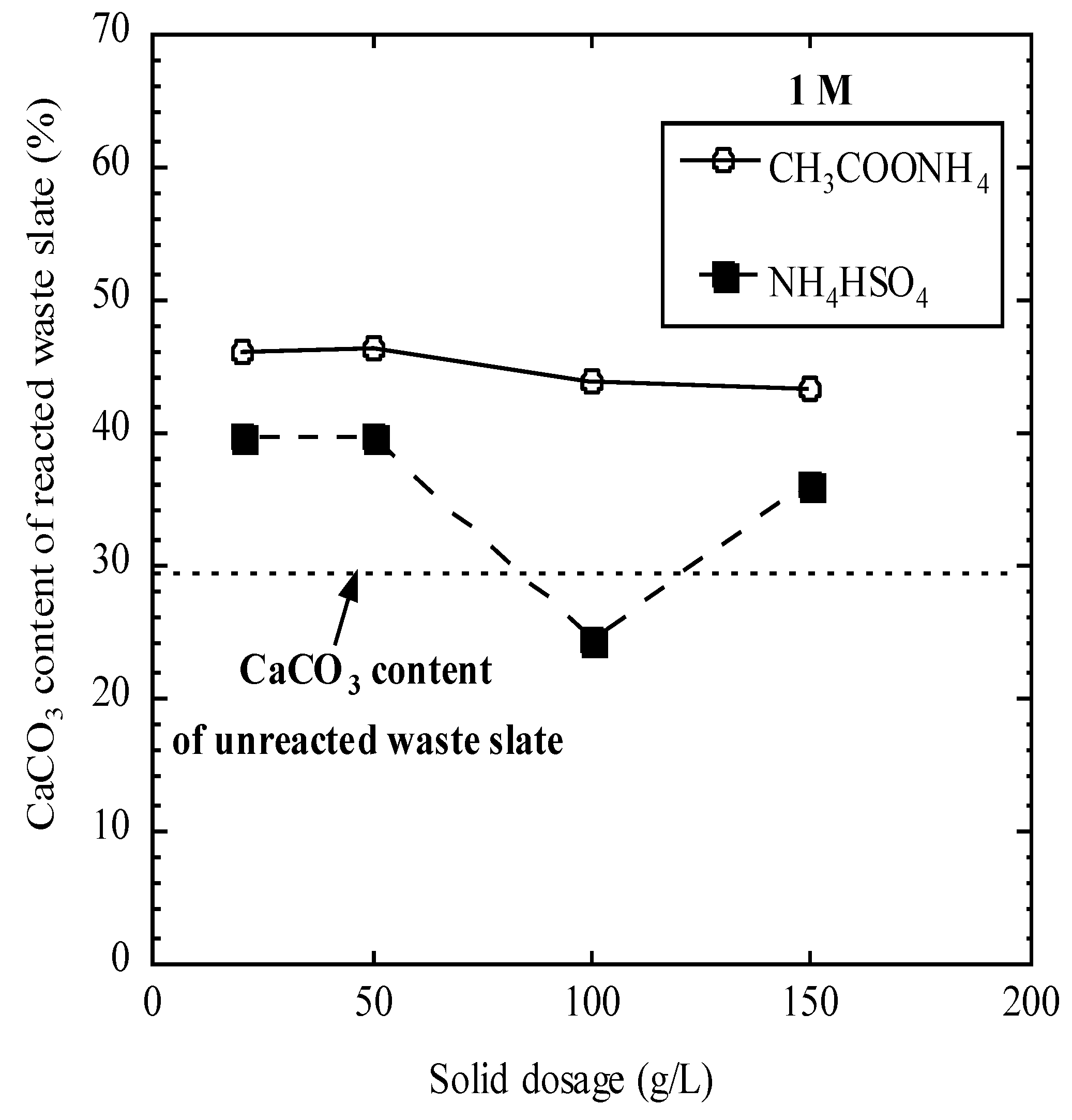
3.3. Carbonation Behaviors
| Solvent | Solid dosage (g/L) | pH | Cation Concentration (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Leaching | Ca | Mg | Fe | Si | ||||||
| Leaching | Carbonation | Leaching | Carbonation | Leaching | Carbonation | Leaching | Carbonation | |||
| 1 M CH3COONH4 | 20 | 9.0 | 1707.2 | 375.2 | 51.3 | 136.8 | 0.1 | 0.9 | 44.3 | 21.6 |
| 50 | 9.4 | 3533.2 | 652.3 | 77.5 | 345.9 | 0.2 | 0.6 | 41.3 | 32.1 | |
| 100 | 9.7 | 6002.2 | 1955.0 | 91.9 | 784.1 | 0.2 | 0.5 | 39.9 | 24.7 | |
| 150 | 9.8 | 7429.2 | 2597.0 | 87.4 | 1200 | 0.7 | 0.4 | 37.0 | 30.2 | |
| 1 M NH4HSO4 | 20 | 9.0 | 778.1 | 709.9 | 100.9 | 154.4 | 1.0 | 0.6 | 40.5 | 16.4 |
| 50 | 9.2 | 822.9 | 797.5 | 210.3 | 342.7 | 0.1 | 0.4 | 50.3 | 24.0 | |
| 100 | 9.7 | 755.9 | 751.9 | 263.3 | 824.1 | 0.4 | 1.2 | 52.5 | 16.1 | |
| 150 | 9.8 | 655.1 | 852.7 | 214.3 | 1234 | 0.3 | 0.5 | 48.9 | 26.3 | |

3.4. Characteristics of Reacted Asbestos-Containing Waste Slate

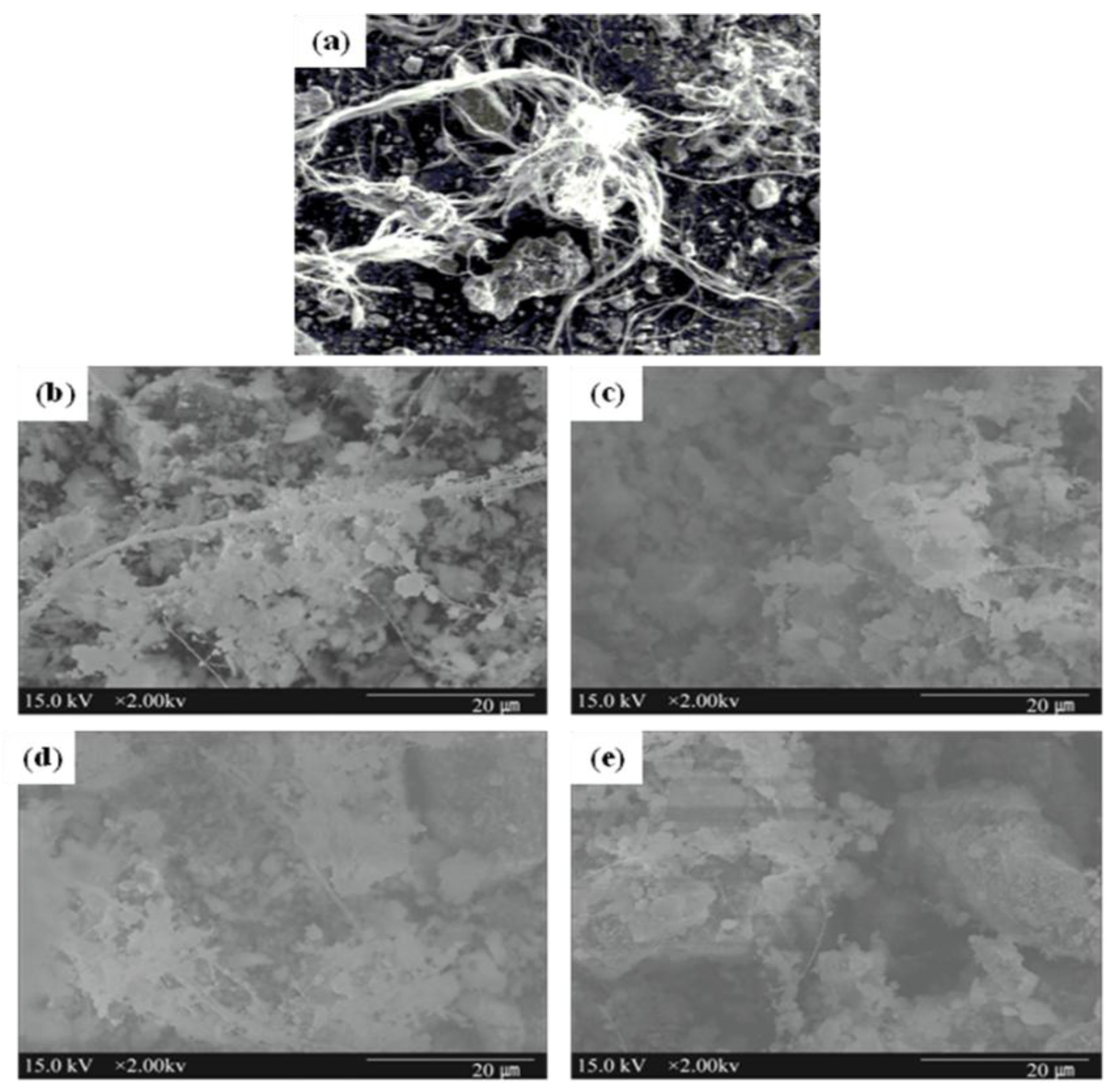
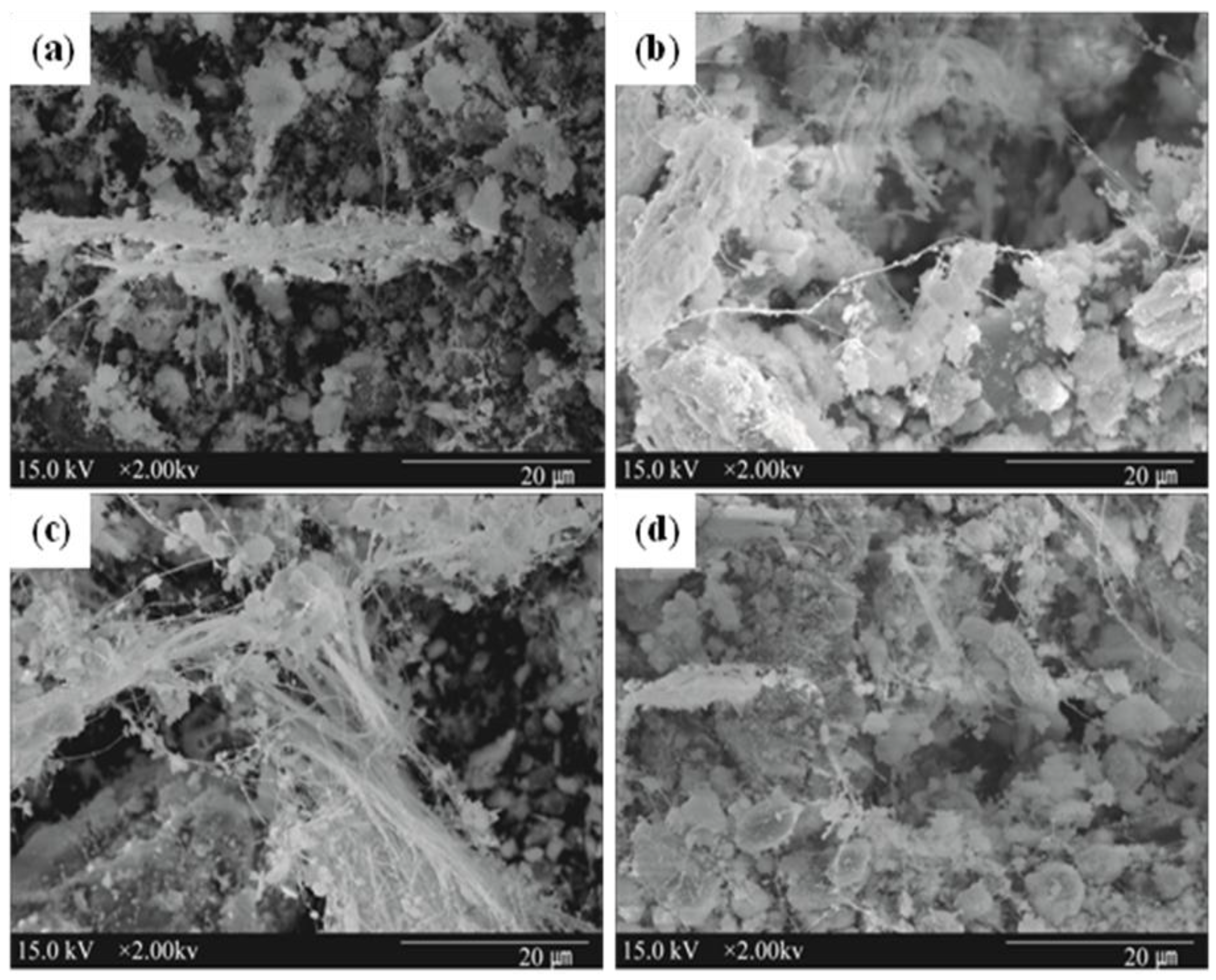
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, B.L.; Sharp, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Sipilä, J.; Teir, S.; Zevenhoven, R. Carbon dioxide sequestration by mineral carbonation: Literature review update 2005–2007; Report VT; Abo Akademi University: Turku, Finland, 2008. [Google Scholar]
- Intergovernmental Panel on Climate Change. Climate change 2007: Mitigation of climate change: Contribution of working group III to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press: Cambridge, NY, USA, 2007. [Google Scholar]
- Jo, H.; Jo, H.Y.; Jang, Y.N. Effect of leaching solutions on carbonation of cementitious materials in aqueous solutions. Environ. Technol. 2012, 33, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Nishimoto, T.; Yamamoto, N.; Yogo, K.; Yamada, K. Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution. Energy 2008, 33, 776–784. [Google Scholar] [CrossRef]
- Wang, X.; Maroto-Valer, M.M. Dissolution of serpentine using recyclable ammonium salts for CO2 mineral carbonation. Fuel 90, 1229–1237. [CrossRef]
- Eloneva, S.; Said, A.; Fogelholm, C.J.; Zevenhoven, R. Preliminary assessment of a method utilizing carbon dioxide and steelmaking slags to produce precipitated calcium carbonate. Appl. Energy 2012, 90, 329–334. [Google Scholar] [CrossRef]
- Jo, H.; Park, S.H.; Jang, Y.N.; Chae, S.C.; Lee, P.K.; Jo, H.Y. Metal leaching and indirect mineral carbonation of waste cement material using ammonium salt solutions. Chem. Eng. J. 2012, 254, 313–323. [Google Scholar] [CrossRef]
- Park, A.H.A.; Fan, L.S. CO2 mineral sequestration: Physically activated dissolution of serpentine and pH swing process. Chem. Eng. Sci. 2004, 59, 5241–5247. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Witkamp, G.J.; Comans, R.N.J. Mineral CO2 sequestration by steel slag carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, Y.; Yamasaki, A.; Iizuka, A.; Fujii, M.; Kumagai, K.; Yanagisawa, Y. Development of a process for producing high-purity calcium carbonate (CaCO3) from waste cement using pressurized CO2. Environ. Prog. 2005, 24, 162–170. [Google Scholar] [CrossRef]
- Baciocchi, R.; Polettini, A.; Pomi, R.; Prigiobbe, V.; Zedwitz, V.N.V.; Steinfeld, A. CO2 sequestration by direct gas-solid carbonation of air pollution control (APC) residues. Energy Fuels 2006, 20, 1933–1940. [Google Scholar] [CrossRef]
- Teir, S.; Kuusik, R.; Fogelholm, C.J.; Zevenhoven, R. Production of magnesium carbonates from serpentinite for long-term storage of CO2. Int. J. Miner. Process. 2007, 85, 1–15. [Google Scholar] [CrossRef]
- Bonenfant, D.; Kharoune, L.; Sauve, S.; Hausler, R.; Niquette, P.; Mimeault, M.; Kharoune, M. CO2 Sequestration potential of steel slags at ambient pressure and temperature. Ind. Eng. Chem. Res. 2008, 47, 7610–7616. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Gierke, J.S.; Sutter, L.L.; Kawatra, S.K.; Eisele, T.C. Mineral carbonation for carbon sequestration in cement kiln dust from waste piles. J. Hazard. Mater. 2009, 168, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Baciocchi, R.; Costa, G.; Bartolomeo, E.D.; Polettini, A.; Pomi, R. Carbonation of stainless steel slag as a process for CO2 storage and slag valorization. Waste Biomass Valorization 2010, 1, 467–477. [Google Scholar] [CrossRef]
- Kashef-Haghighi, S.; Ghoshal, S. CO2 Sequestration in concrete through accelerated carbonation curing in a flow-through reactor. Ind. Eng. Chem. Res. 2010, 49, 1143–1149. [Google Scholar] [CrossRef]
- Wang, X.; Maroto-Valer, M.M. Integration of CO2 capture and mineral carbonation by using recyclable ammonium salts. ChemSusChem 4, 1291–1300. [CrossRef] [PubMed]
- Baciocchi, R.; Costa, G.; Gianfilippo, M.D.; Polettini, A.; Pomi, R.; Stramazzo, A. Thin-film versus slurry-phase carbonation of steel slag: CO2 uptake and effects on mineralogy. J. Hazard. Mater. 2015, 283, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Kirchofer, A.; Becker, A.; Brandt, A.; Wilcox, J. CO2 mitigation potential of mineral carbonation with industrial alkalinity sources in the United States. Environ. Sci. Technol., 2013, 47, 7548–7554. [Google Scholar]
- Jang, Y.N.; Chae, S.C.; Lee, M.K.; Won, H.I.; Ryu, K.W. Method of removing asbestos from asbestos-containing materials by 99% through low temperature heat treatment. US9079055, 14 July 2014. [Google Scholar]
- Gadikota, G.; Natali, C.; Boschi, C.; Park, A.H.A. Morphological changes during enhanced carbonation of asbestos containing material and its comparison to magnesium silicate minerals. J. Hazard. Mater. 2014, 264, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.N.; Jeong, S.; Lim, H. Thermochemical destruction of asbestos-containing roofing slate and the feasibility of using recycled waste sulfuric acid. J. Hazard. Mater. 2014, 265, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Pacella, A.; Fantauzzi, M.; Turci, F.; Cremisini, C.; Montereali, M.R.; Nardi, E.; Atzei, D.; Rossi, A.; Andreozzi, G.B. Surface alteration mechanism and topochemistry of iron in tremolite asbestos: A step toward understanding the potential hazard of amphibole asbestos. Chem. Geol. 2015, 405, 28–38. [Google Scholar] [CrossRef]
- Leonelli, C.; Veronesi, P.; Boccaccini, D.N.; Rivasi, M.R.; Barbieri, L.; Andreola, F.; Lancellotti, I.; Rabitti, D.; Pellacani, G.C. Microwave thermal inertisation of asbestos containing waste and its recycling in traditional ceramics. J. Hazard. Mater. 2006, 135, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Sumi, T.; Ito, S.; Dillert, R.; Kashimura, K.; Yoshikawa, N.; Sato, M.; Shinohara, N. Microwave-driven asbestos treatment and its scale-up for use after natural disasters. Environ. Sci. Technol. 2014, 48, 6882–6890. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Kashimura, K.; Hashiguchi, M.; Sato, M.; Horikoshi, S.; Mitani, T.; Shinohara, N. Detoxification mechanism of asbestos materials by microwave treatment. J. Hazard. Mater. 2015, 284, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Averroes, A.; Sekiguchi, H.; Sakamoto, K. Treatment of airborne asbestos and asbestos-like microfiber particles using atmospheric microwave air plasma. J. Hazard. Mater. 2011, 195, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F.; Tartaglia, A. Thermal decomposition of asbestos and recycling in traditional ceramics. J. Eur. Ceram. Soc. 2000, 20, 1409–1418. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic chemistry: Chemical equilibria and rates in natural water, 3rd ed.Wiley-Interscience publications: New York, NY, USA, 1996. [Google Scholar]
- Pade, C.; Guimaraes, M. The CO2 uptake of concrete in a 100 year perspective. Cem. Concr. Res. 2007, 37, 1348–1356. [Google Scholar] [CrossRef]
- Back, M.; Kuehn, M.; Stanjek, H.; Peiffer, S. Reactivity of alkaline lignite fly ashes towards CO2 in water. Environ. Sci. Technol. 2008, 42, 4520–4526. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Ryu, K.W.; Jang, Y.N.; Kim, W.; Bang, J.H. Effect of oxalic acid on heat pretreatment for serpentine carbonation. Mater. Trans. 2011, 52, 235–238. [Google Scholar] [CrossRef]
- Ryu, K.W.; Jang, Y.N.; Lee, M.G. Enhancement of chrysotile carbonation in alkali solution. Mater. Trans. 2012, 53, 1349–1352. [Google Scholar] [CrossRef]
- Aust, A.E.; Cook, P.M.; Dodson, R.F. Morphological and chemical mechanisms of elongated mineral particle toxicities. J. Toxic. Environ. Health Part 2011, 14, 40–75. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Jo, H.Y.; Rha, S.; Lee, P.-K. Direct Aqueous Mineral Carbonation of Waste Slate Using Ammonium Salt Solutions. Metals 2015, 5, 2413-2427. https://doi.org/10.3390/met5042413
Jo H, Jo HY, Rha S, Lee P-K. Direct Aqueous Mineral Carbonation of Waste Slate Using Ammonium Salt Solutions. Metals. 2015; 5(4):2413-2427. https://doi.org/10.3390/met5042413
Chicago/Turabian StyleJo, Hwanju, Ho Young Jo, Sunwon Rha, and Pyeong-Koo Lee. 2015. "Direct Aqueous Mineral Carbonation of Waste Slate Using Ammonium Salt Solutions" Metals 5, no. 4: 2413-2427. https://doi.org/10.3390/met5042413






