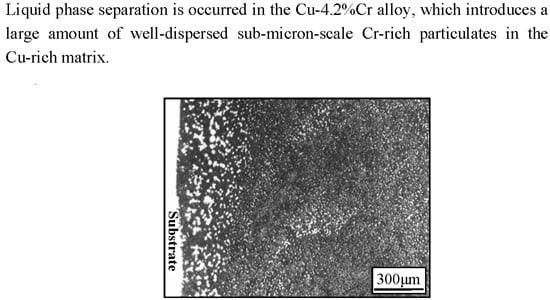
Liquid Phase Separation and the Aging Effect on Mechanical and Electrical Properties of Laser Rapidly Solidified Cu100−xCrx Alloys
Abstract
:1. Introduction
2. Experimental Section
3. Results
3.1. Solidification Behavior and Microstructures
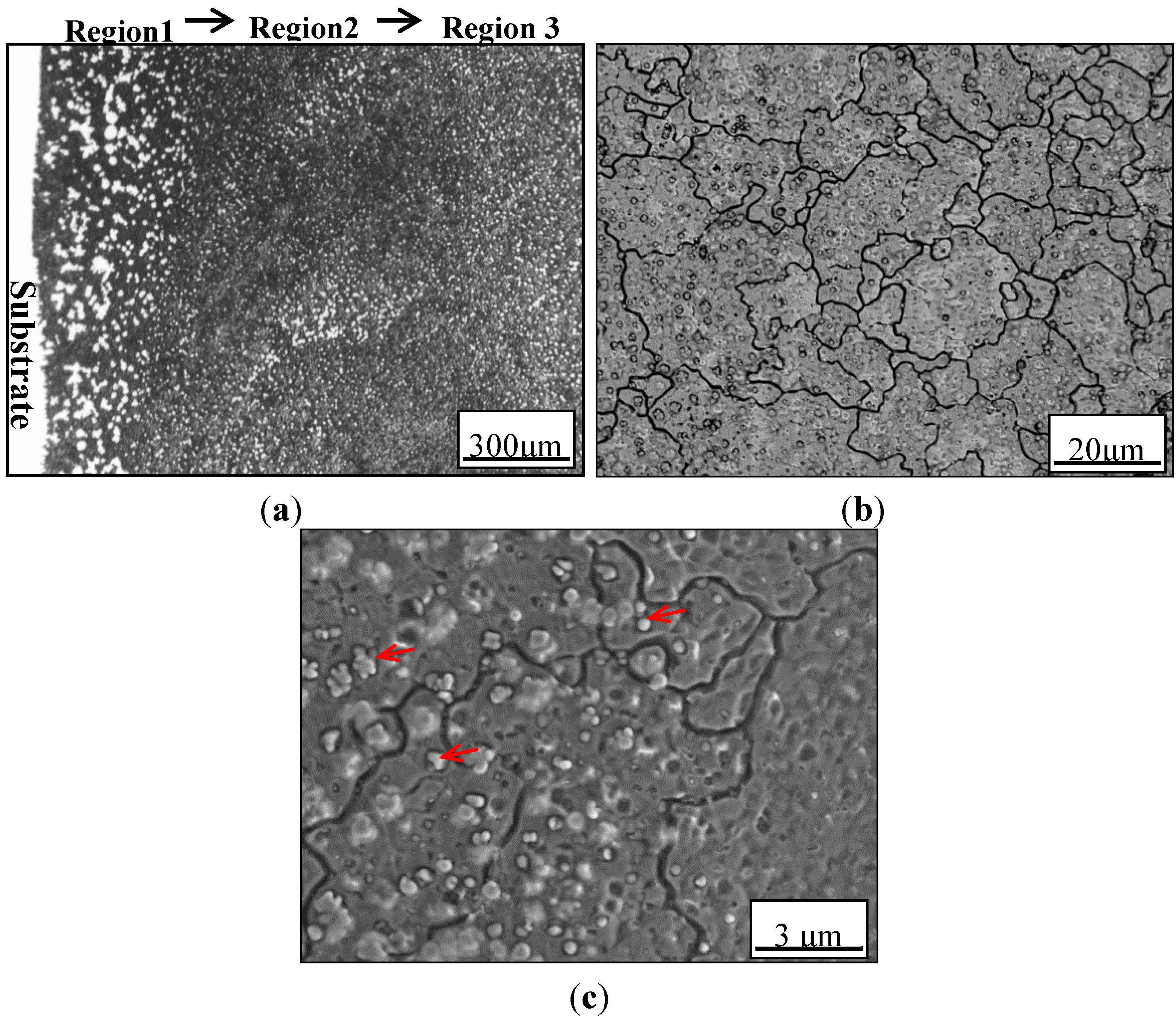
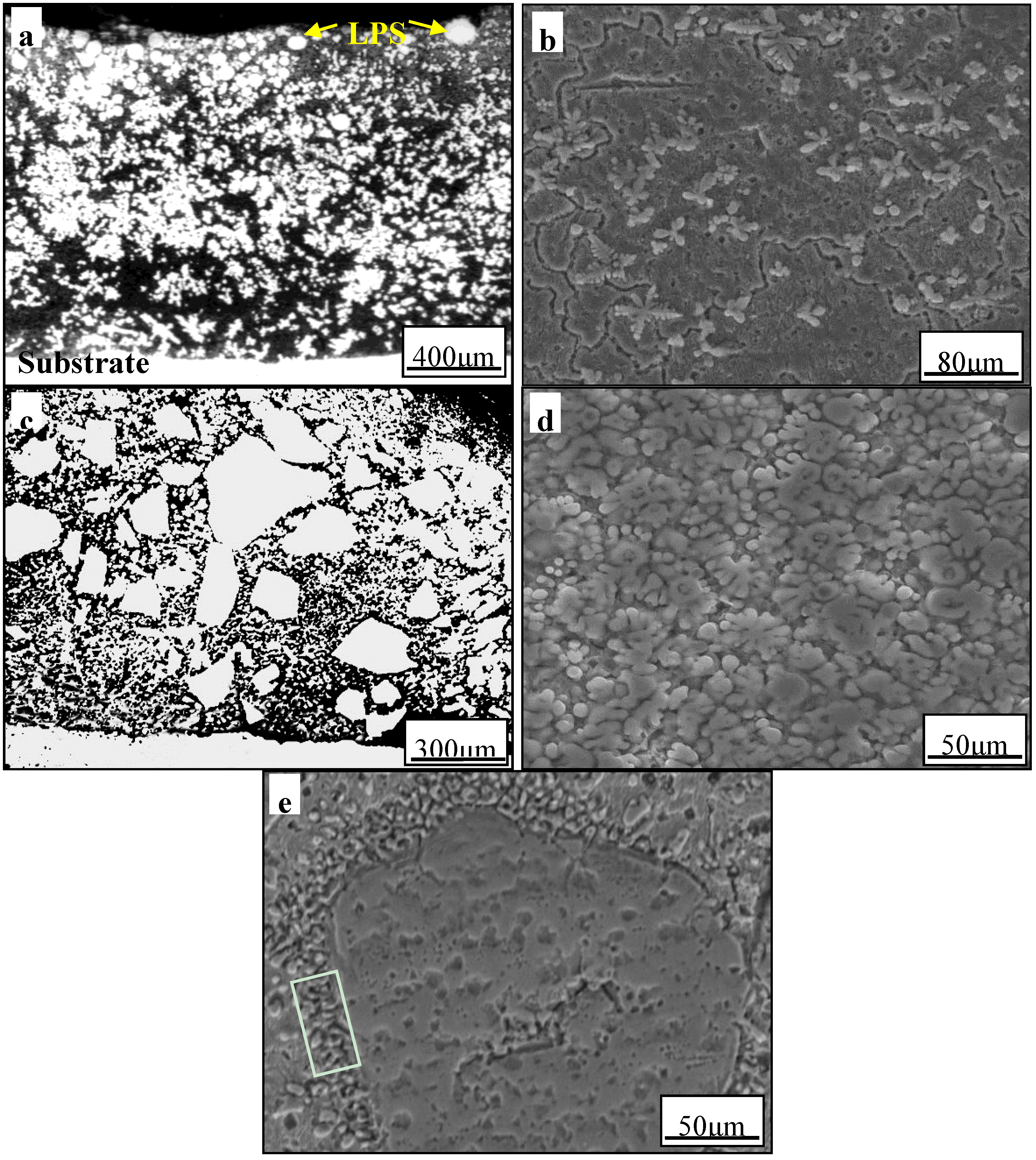
3.2. The Effect of Aging
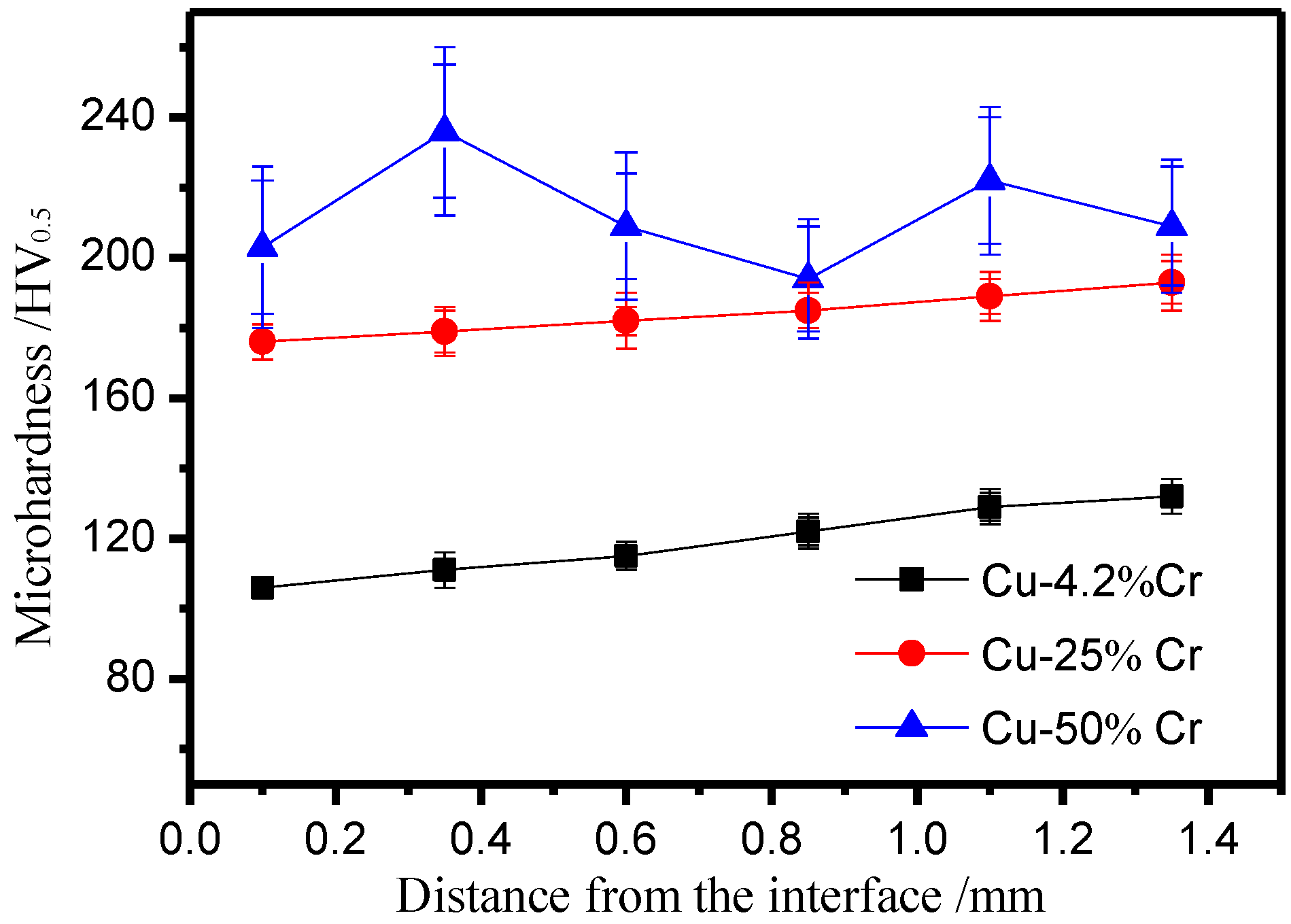
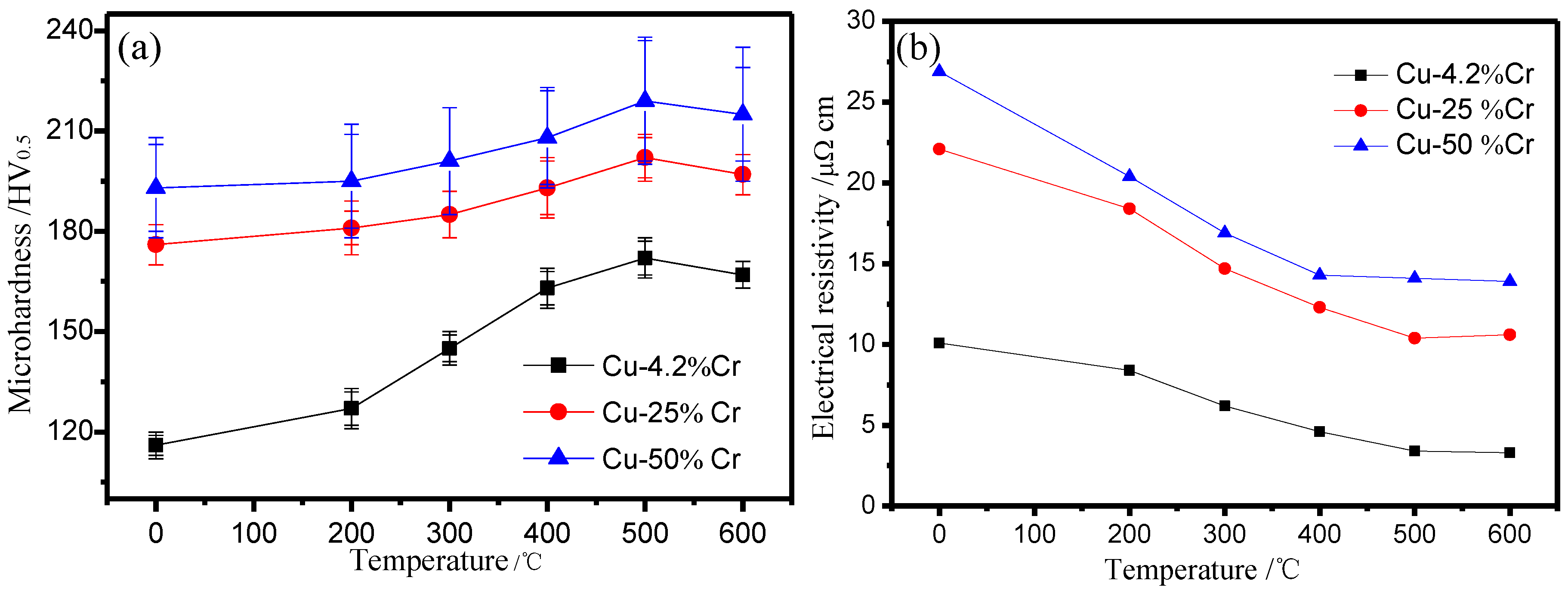
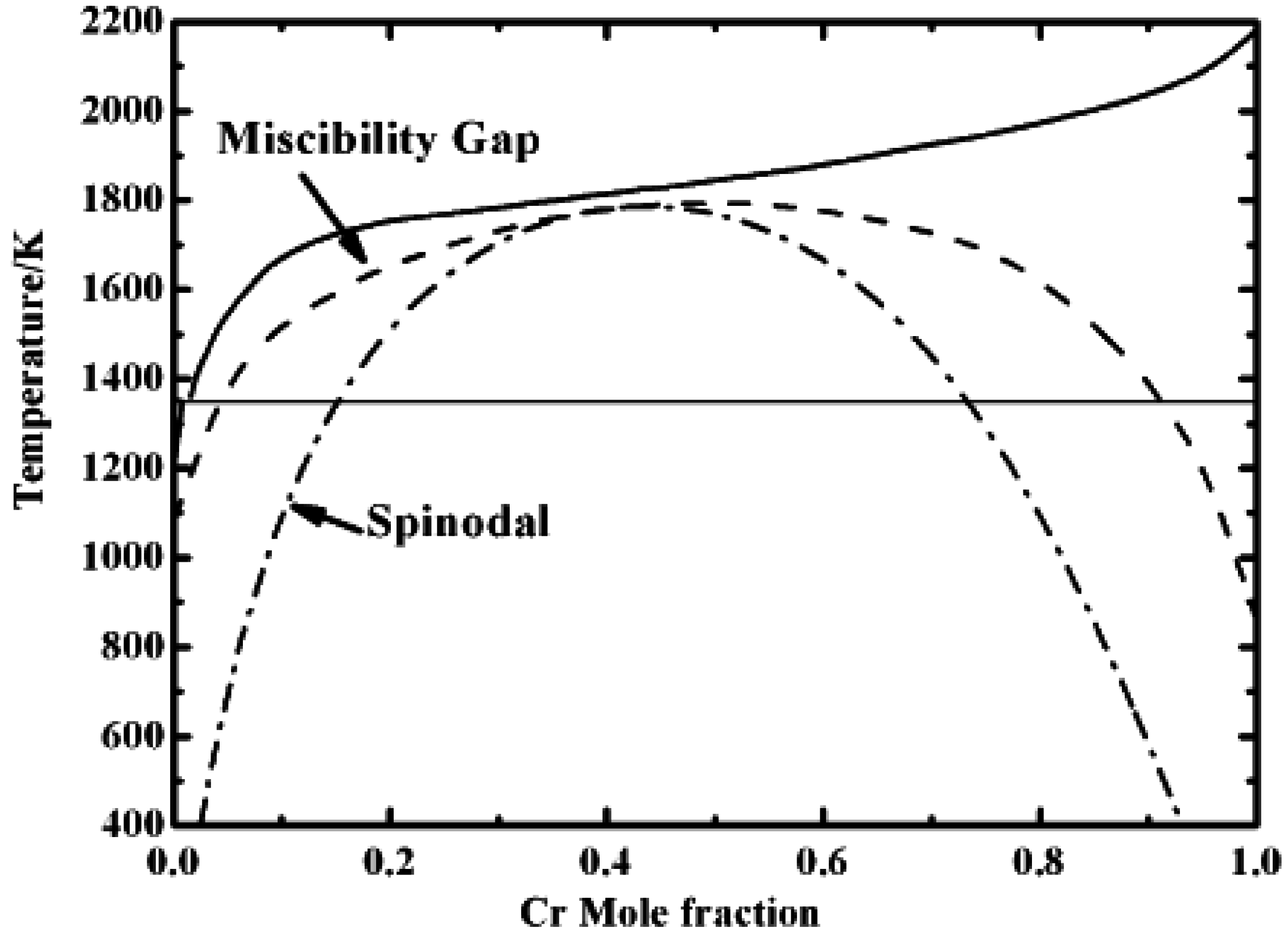
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bachmaie, A.; Rathmayr, G.B.; Bartosik, M.; Apel, D.; Zhang, Z.; Pippan, R. New insights on the formation of supersaturated solid solutions in the Cu-Cr system deformed by high-pressure torsion. Acta Mater. 2014, 69, 301–313. [Google Scholar] [CrossRef]
- Fu, Y.B.; Cui, J. Preparation of Cu-Cr-Zr alloy billets by horizontal electromagnetic continuous stirring. Sci. Technol. 2014, 30, 370–376. [Google Scholar] [CrossRef]
- Lin, G.B.; Wang, Z.D.; Zhang, M.K.; Zhang, H.; Zhao, M. Heat treatment method for making high strength and conductivity Cu–Cr–Zr alloy. Mater. Sci. Technol. 2011, 27, 966–969. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Wang, Y.P.; Gao, J.; Kolbe, M. Microstructure of rapidly solidified Cu-25 wt.% Cr alloys. Mater. Sci. Eng. A 2005, 398, 318–322. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.P.; Zhou, Z.M.; Kolbe, M. Phase separation in undercooled Cu-Cr melts. Mater. Sci. Eng. A 2007, 449–451, 654–657. [Google Scholar] [CrossRef]
- He, W.X.; Yu, Y.; Wang, E.D.; Sun, H.F.; Hu, L.X.; Chen, H. Analysis of phase in Cu-15%Cr-0.24%Zr alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 1342–1348. [Google Scholar]
- Zhao, Q.; Shao, Z.B.; Liu, C.J.; Jiang, M.F.; Li, X.T.; Zevenhoven, R.; Saxen, H. Preparation of Cu-Cr alloy powder by mechanical alloying. J. Alloy. Compd. 2014, 607, 118–124. [Google Scholar] [CrossRef]
- Pan, M.; Konda, G.; Prashanth, S.S.; Jia, Y.D.; Wang, H.W.; Zou, C.M.; Wei, Z.J.; Jürgen, E. Influence of Annealing on Mechanical Properties of Al-20Si Processed by Selective Laser melting. Metals 2014, 4, 28–36. [Google Scholar]
- Zhang, H.; He, Y.Z.; Pan, Y. Enhanced hardness and fracture toughness of the laser-solidified FeCoNiCrCuTiMoAlSiB0.5 high-entropy alloy by martensite strengthening. Scr. Mater. 2013, 69, 342–345. [Google Scholar] [CrossRef]
- Holger, S.; Konda, G.P.; Lukas, L.; Uta, K.; Jürgen, E.; Poprawe, R. Selective Laser Melting of Ti-45Nb Alloy. Metals 2015, 5, 686–694. [Google Scholar]
- Yue, T.M.; Xie, H.; Lin, X.; Yang, H.O.; Meng, G.H. Microstructure of Laser Re-Melted AlCoCrCuFeNi High Entropy Alloy Coatings Produced by Plasma Spraying. Entropy 2013, 15, 2833–2845. [Google Scholar] [CrossRef]
- Muller, R. Arc-melted Cu-Cr alloys as contact materials for vacuum interrupters. SiemensForsch—UEntwick—Ber Bd. 1988, 17, 105–115. [Google Scholar]
- Wei, X.; Wang, J.P.; Yang, Z.M.; Sun, Z.B.; Yu, D.M.; Song, X.P.; Ding, B.J.; Yang, S. Liquid phase separation of Cu–Cr alloys during the vacuum breakdown. J. Alloy. Compd. 2011, 509, 7116–7120. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, S.-H.; Zhang, H.; He, Y.-Z.; Li, M.-X.; Guo, S. Liquid Phase Separation and the Aging Effect on Mechanical and Electrical Properties of Laser Rapidly Solidified Cu100−xCrx Alloys. Metals 2015, 5, 2119-2127. https://doi.org/10.3390/met5042119
Si S-H, Zhang H, He Y-Z, Li M-X, Guo S. Liquid Phase Separation and the Aging Effect on Mechanical and Electrical Properties of Laser Rapidly Solidified Cu100−xCrx Alloys. Metals. 2015; 5(4):2119-2127. https://doi.org/10.3390/met5042119
Chicago/Turabian StyleSi, Song-Hua, Hui Zhang, Yi-Zhu He, Ming-Xi Li, and Sheng Guo. 2015. "Liquid Phase Separation and the Aging Effect on Mechanical and Electrical Properties of Laser Rapidly Solidified Cu100−xCrx Alloys" Metals 5, no. 4: 2119-2127. https://doi.org/10.3390/met5042119
APA StyleSi, S.-H., Zhang, H., He, Y.-Z., Li, M.-X., & Guo, S. (2015). Liquid Phase Separation and the Aging Effect on Mechanical and Electrical Properties of Laser Rapidly Solidified Cu100−xCrx Alloys. Metals, 5(4), 2119-2127. https://doi.org/10.3390/met5042119