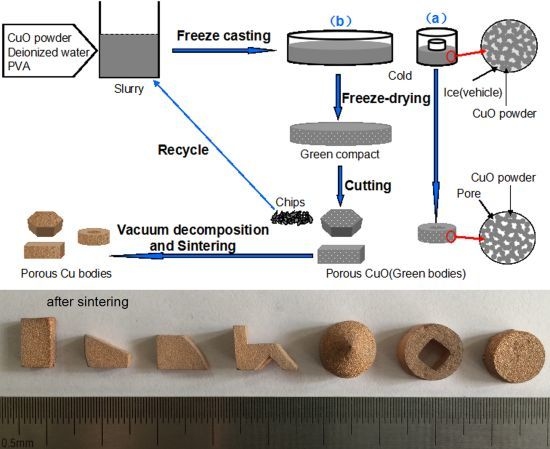
3. Results and Discussion
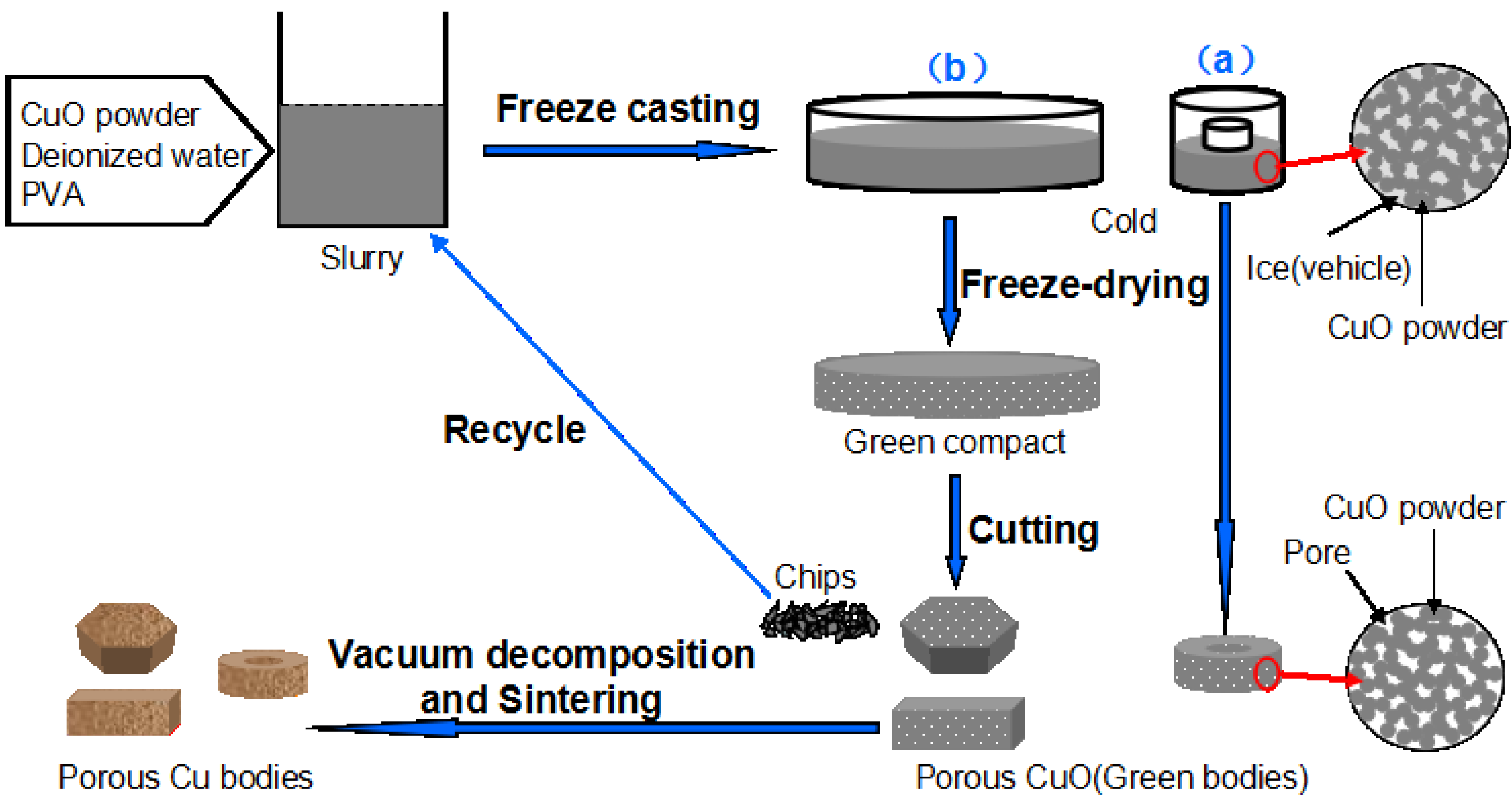
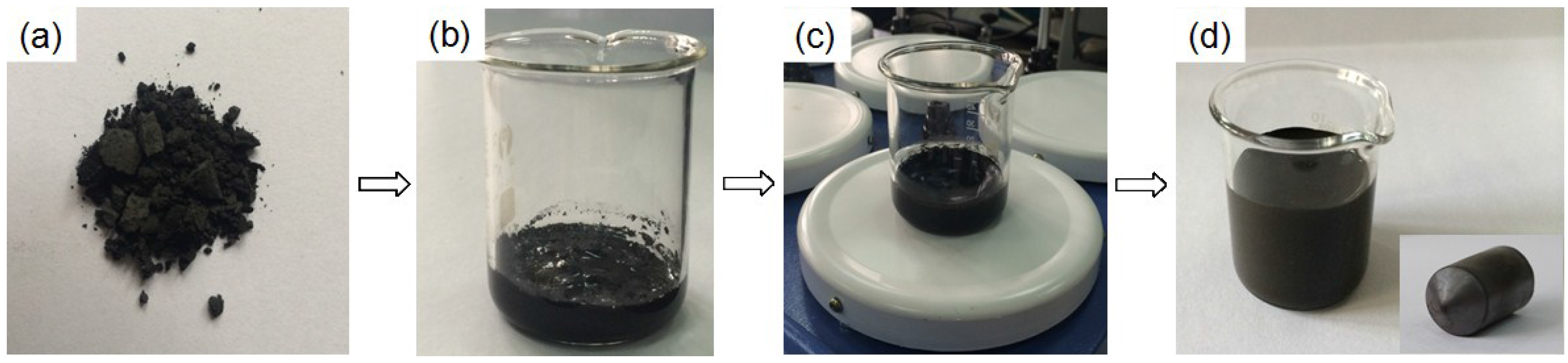
Figure 3 shows the macro photograph and XRD patterns of the porous bodies (30 vol.%) before and after sintering. The graceful complex-shaped porous Cu bodies are fabricated using the described technology, as shown in
Figure 1. The color change shows that CuO is fully decomposed into Cu by sintering (
Figure 3a), and these observations are also in good agreement with XRD results (
Figure 3b). The sintered bodies show no noticeable macroscopic defects, such as cracking or distortion. The complex shape is due to the slurry having good fluidity which can flow into all corners of the molds. In addition, the basis of the freeze-dried CuO green compact is powder which bonds with a PVA binder and is easy to machine into different shapes with a knife.
XRD patterns show only the peaks associated with the CuO phase before sintering, indicating that the additives did not react with CuO powders. Moreover, the CuO powder decomposition at 900 °C for 2 h in vacuum was composed only of Cu phase without any second phases in porous Cu bodies. This indicated that CuO can transform to Cu metal via thermal vacuum decomposition [
9]. The weights of porous bodies were measured before and after vacuum sintering, and the mass loss was calculated at around 23.3%, which is consistent with the results of CuO to Cu (loss of 20.1%) and decomposition of additive (loss of 3%), including a little water. On the basis of these results, the vacuum decomposition of CuO green body was established.
Figure 3.
Macro photograph (a), and XRD patterns (b) of the porous bodies (30 vol.%) before and after vacuum sintering.
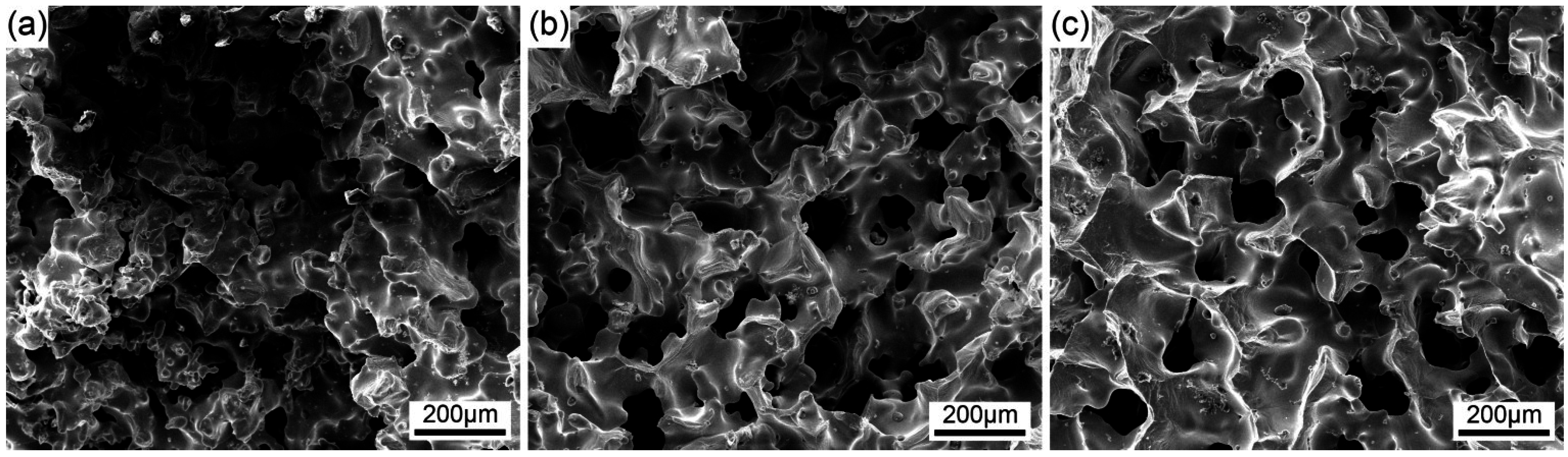
The microstructures of sintered bodies are shown in
Figure 4. The sintered bodies preserved their highly porous structures with interconnected pore tunnels. These pores were generated from sublimation of the ice and the pore morphologies were controllable by the solid content in the slurry. Note that the walls of the tunnels are dense, without any noticeable cracks; the surface of the walls facing tunnels is also smooth. In other words, the fine CuO particles were effectively repelled by growing ice crystals and became concentrated as the slurry solidified, resulting in the formation of highly packed CuO walls; consequently, allowing the preservation of the porous structure without the disintegration of the sample after freeze-drying and heat-treatment. As shown in
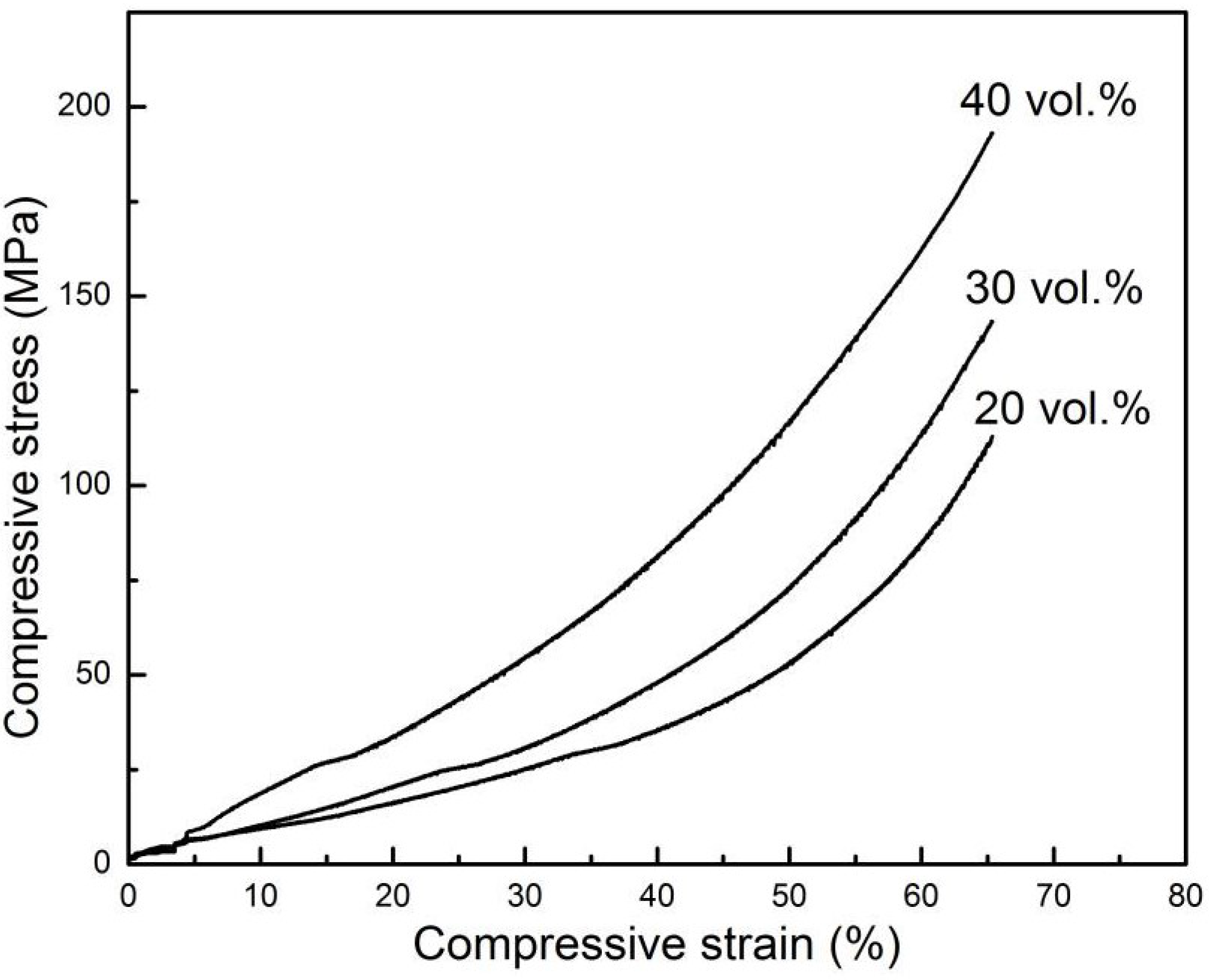
Figure 4, as the initial CuO content increased, the porosity of porous Cu decreased, the pores became smaller and the compressive strength increased (
Figure 5); as is often the case with the freeze-casting method [
8]. In this case, more nucleation sites were available for the aqueous crystals to grow, leading to the pore structure becoming homogenous with solid loading. These pores exist homogenously throughout the sintered body, and the pore tunnels have flat. ellipsoidal. and nearly circular cross-sections.
Figure 4.
SEM of porous Cu bodies produced using different CuO contents: (a) 20 vol.%; (b) 30 vol.%; (c) 40 vol.%.
Figure 5.
The stress-strain curves of porous Cu bodies with various solid content.
Table 1.
Porosity and volume shrinkage of the porous Cu bodies as a function of the solid content in the slurry.
| Solid content (vol%) | Porosity (%) | Volume shrinkage (%) |
|---|
| 20 | 49.7 | 60.2 |
| 25 | 44.3 | 55.1 |
| 30 | 38.3 | 51.4 |
| 35 | 34.9 | 46.2 |
| 40 | 30.8 | 42.2 |
Table 1 shows the porosity and volume shrinkage of the sintered body. The porosity is controllable by the solid content in the slurry, and decreases from 49.7% to 30.8% with the increase in solids content from 20 to 40 vol.%, in agreement with the microstructure (
Figure 4). On the other hand, shrinkage during sintering mainly occurs because of densification of the metal framework, and the degree of shrinkage is also affected by solid content.
Table 1 shows that the volume shrinkage decreases with the increase in solids content, and reaches a minimum 42.2% when the solids content is 40 vol.%. The solids content determines the porosity and volume shrinkage levels of the material.
Figure 6 shows the surfaces of porous Cu (20 vol.%) in different machining conditions. The machining of green freeze-dried porous body was found to be more promising compared with that of sintered porous metal. The machining of sintered porous Cu bodies leads to partial closure of surface pores due to plastic deformation of Cu and to rapid wear of the tool. Also, the residues of lubricants or grinding media mark the porous structure. Owing to the disadvantages of machining of sintered porous metal, the machining of green freeze-dried porous body becomes especially economical for fabricating porous parts with special geometry, and the machined chips can be reused for preparing slurry. This process may be applicable to many other metal oxide slurries for the preparation of porous metal, and the procedure is low-cost and simple.
Figure 6.
The surfaces of porous Cu in different machining conditions: (a) machined in the sintered state; (b) machined in the green body state.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (2012QNA02) and the National Nature Science Foundation of China (51202289).
Author Contributions
Huashen Ran: Sample preparation, and writing the manuscript. Zhangsheng Liu and Jinan Niu: Data analysis. Xiaohong Wang: Development of the process. Peizhong Feng and Haifei Zhang: Supervision of the first authors and writing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Banhart, J. Manufacture, characterization and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Cui, C.X.; Liu, S.J.; Zhao, L.C. Open-celled porous Cu prepared by replication of NaCl space-holders. Mater. Sci. Eng. 2010, 527, 1275–1278. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Sahoo, K.L. Processing, stabilization and applications of metallic foams. Art of science. Mater. Sci. Pol. 2007, 25, 733–753. [Google Scholar]
- Hong, C.Q.; Zhang, X.H.; Han, J.C.; Du, J.C.; Han, W.B. Ultra-high-porosity zirconia ceramics fabricated by novel room-temperature freeze-casting. Scr. Mater. 2009, 60, 563–566. [Google Scholar] [CrossRef]
- Deville, S.; Saiz, E.; Tomsia, A.P. Ice-templated porous alumina structures. Acta Mater. 2007, 55, 1965–1974. [Google Scholar] [CrossRef]
- Bouville, F.; Maire, E.; Meille, S.; Moortèle, B.V.D.; Stevenson, A.J.; Deville, S. Strong, tough and stiff bioinspired ceramics from brittle constituents. Nat. Mater. 2014, 13, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, H.F. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Oh, S.T.; Lee, W.; Chang, S.; Suk, M.J. Microstructure of porous Cu fabricated by freeze-drying process of CuO/camphene slurry. Trans. Nonferrous Met. Soc. China 2012, 22, 688–691. [Google Scholar] [CrossRef]
- Wu, C.; Qiao, G.J.; Wang, H.J.; Jin, Z.H. Preparation of high porosity copper foam by polyurethane sponge impregnation method. Rare Met. Mater. Eng. 2009, 38, 722–725. [Google Scholar]
- Ramos, A.I.C.; Dunand, D.C. Preparation and Characterization of Directionally Freeze-cast Copper Foams. Metals 2012, 2, 265–273. [Google Scholar] [CrossRef]
- Moritz, T.; Richte, H.J. Ice-mould freeze casting of porous ceramic components. J. Eur. Ceram. Soc. 2007, 27, 4595–4601. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).