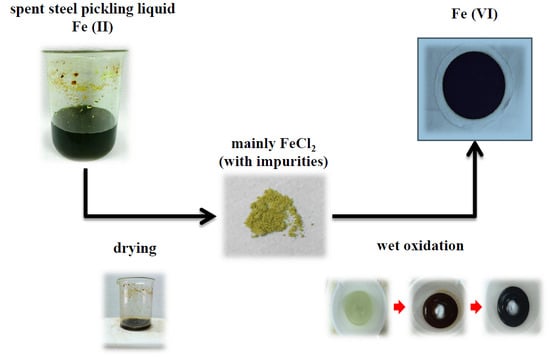
Preparation of Potassium Ferrate from Spent Steel Pickling Liquid
Abstract
:1. Introduction
1.1. Environmental Application of Potassium Ferrate
1.2. Source of Spent Pickling Liquid
1.3. Fe(VI) Preparation with Wet Oxidation Method
1.4. Stability of Fe(VI)
1.5. Quantification of Fe(VI)
2. Materials and Methods
2.1. Materials
| Chemical | Purity | Producer |
|---|---|---|
| Fe(NO3)3·9H2O | 98%–101% (reagent grade) | JT Baker, Center Valley, PA, USA |
| n-C5H12 | 99% (reagent grade) | Merck, Darmstadt, Germany |
| C2H5OH | 96% (reagent grade) | Merck, Germany. |
| (C2H5)2O | 99.5% (reagent grade) | Merck, Germany |
| FeCl2·4H2O | 98% (reagent grade) | Sigma-Aldrich, St. Louis, MO, USA |
| FeCl3·6H2O | 97% (reagent grade) | Sigma-Aldrich, USA |
| KOH | 85% (reagent grade) | Sigma-Aldrich, USA |
| NaOH | 99% (reagent grade) | Sigma-Aldrich, USA |
| NaOCl solution | 6%–14% (reagent grade) | Sigma-Aldrich, USA |
| K2FeO4 | purity ≥ 90% | Sigma-Aldrich, USA |
| MB | 100% | Sigma-Aldrich, USA |
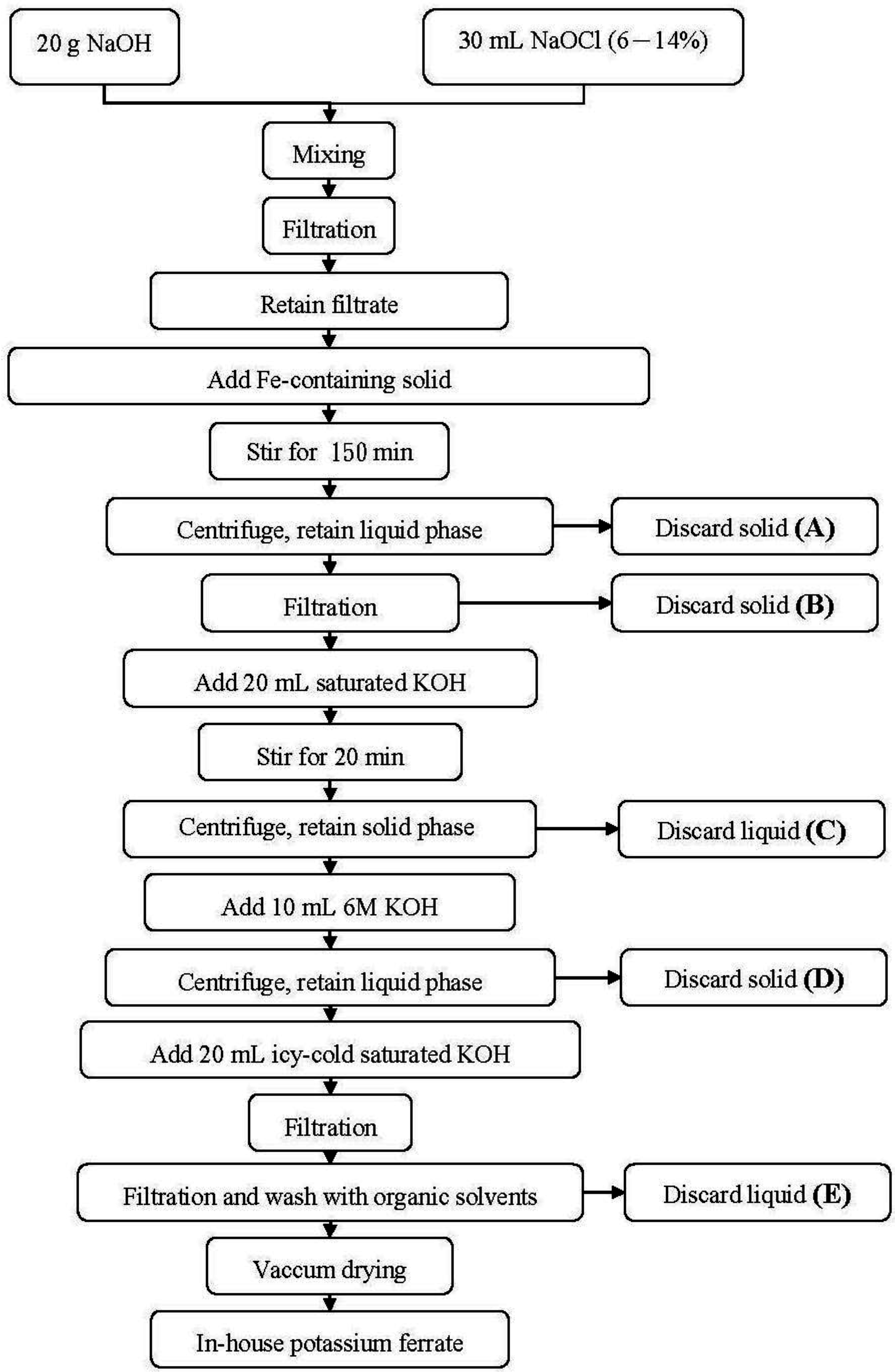
2.2. Synthesis Flowchart for K2FeO4
2.3. Crystalline Phase Study with XRD
2.4. Molecular Study of Potassium Ferrates
3. Results and Discussion
3.1. Characteristics of Spent Steel Pickling Liquid
3.1.1. pH and Density of Pickling Liquid
3.1.2. Composition of Spent Steel Pickling Liquid
| Compositional Element | Content (mg L−1) | Spike Recovery d (%) |
|---|---|---|
| Cr a | 3.63 ± 0.23 | (24 ± 1.2) |
| Bi a | 46.48 ± 0.95 | (31 ± 4) |
| Ag a | 6.86 ± 0.07 | (25 ± 3.6) |
| Al a | 6.73 ± 0.04 | (76 ± 2) |
| Tl a | 60.15 ± 0.13 | (52 ± 3.1) |
| Ca b | 1552 ± 0.3 | (97 ± 2.5) |
| Cd a | 3.08 ± 0.02 | (30 ± 2.4) |
| Co a | 5.10 ± 0.036 | (31 ± 2.5) |
| Cu a | 39.15 ± 0.085 | (78 ± 1.4) |
| Fe b | 133,000 ± 2160 | (89 ± 1.2) |
| K a | 7.69 ± 0.00 | (111 ± 3.7) |
| Mg a | 65.28 ± 0.07 | (56 ± 2.6) |
| Mn a | 1387 ± 1 | (73 ± 0.2) |
| Na b | 256.3 ± 1.4 | (87 ± 4) |
| Ni a | 19.72 ± 0.10 | (30 ± 4.1) |
| Pb a | 15.08 ± 0.60 | (23 ± 2.6) |
| Zn b | 188.6 ± 2.1 | (91 ± 2.5) |
| Cl c | 351,500 ± 3100 | (88 ± 3.3) |
3.1.3. Fe Speciation in Solid Raw Material Recycled

| Type of Iron Salt | Oxidizing Agent | Salt Weight (g) | Yield (g) | Purity (wt. %) | Yield (Mol. %) | Literature |
|---|---|---|---|---|---|---|
| Fe(NO3)3·9H2O | NaOCl | 25 | 10.43 | 63.40 | 53.9 | [23] |
| FeCl3·6H2O | NaOCl | 25 | 9.64 | 74.71 | 39.3 | [23] |
| Fe(NO3)3·9H2O | NaOCl | - b | - b | 98.5 | - b | [26] |
| Fe(NO3)3·9H2O | NaOCl | - b | - b | 90 | - b | [27] |
| Recycled solid a | NaOCl | 2 | 0.254 | 88 | 11.7 | This study |
3.2. Characteristics of In-House and Commercialized Potassium Ferrates
3.2.1. Chemical Compositions of Potassium Ferrates
| Sigma-Aldrich K2FeO4 Standard | In-House K2FeO4 (This Study) | |||
|---|---|---|---|---|
| Composition | wt. Percentage (%) | Recovery (%) | wt. Percentage (%) | Recovery (%) |
| Cr a | 0.11 ± 0 | (51 ± 2.5) | 0.01 ± 0.0002 | (60 ± 2.8) |
| Bi a | 0.11 ± 0.0344 | (61 ± 1.6) | 0.06 ± 0.000196 | (51 ± 1.8) |
| Al a | 0.01 ± 0.0006 | (75 ± 2.6) | 0.02 ± 0.00098 | (71 ± 2.8) |
| Ba a | 0.00 ± 0 | (82 ± 2.2) | 0.01 ± 0 | (88 ± 2.1) |
| Ca a | 0.00 ± 0.0004 | (73 ± 1.4) | 0.02 ± 0.0002 | (70 ± 2.6) |
| Fe a | 24.99 ± 0.899 | (84 ± 2.7) | 23.75 ± 0.214 | (85 ± 2.1) |
| K a | 37.77 ± 0.746 | (96 ± 2.5) | 38.69 ± 0.697 | (94 ± 2.8) |
| Li a | 0.20 ± 0.0008 | (69 ± 2.3) | 0.59 ± 0.00982 | (58 ± 1.8) |
| Mn a | 0.07 ± 0.002 | (80 ± 2.6) | 0.10 ± 0.00039 | (79 ± 3.1) |
| Na a | 0.01 ± 0.0002 | (79 ± 1.5) | 0.01 ± 0.00039 | (77 ± 1.8) |
| Ni a | 0.15 ± 0.0656 | (69 ± 3.1) | 0.04 ± 0.009627 | (71 ± 3.0) |

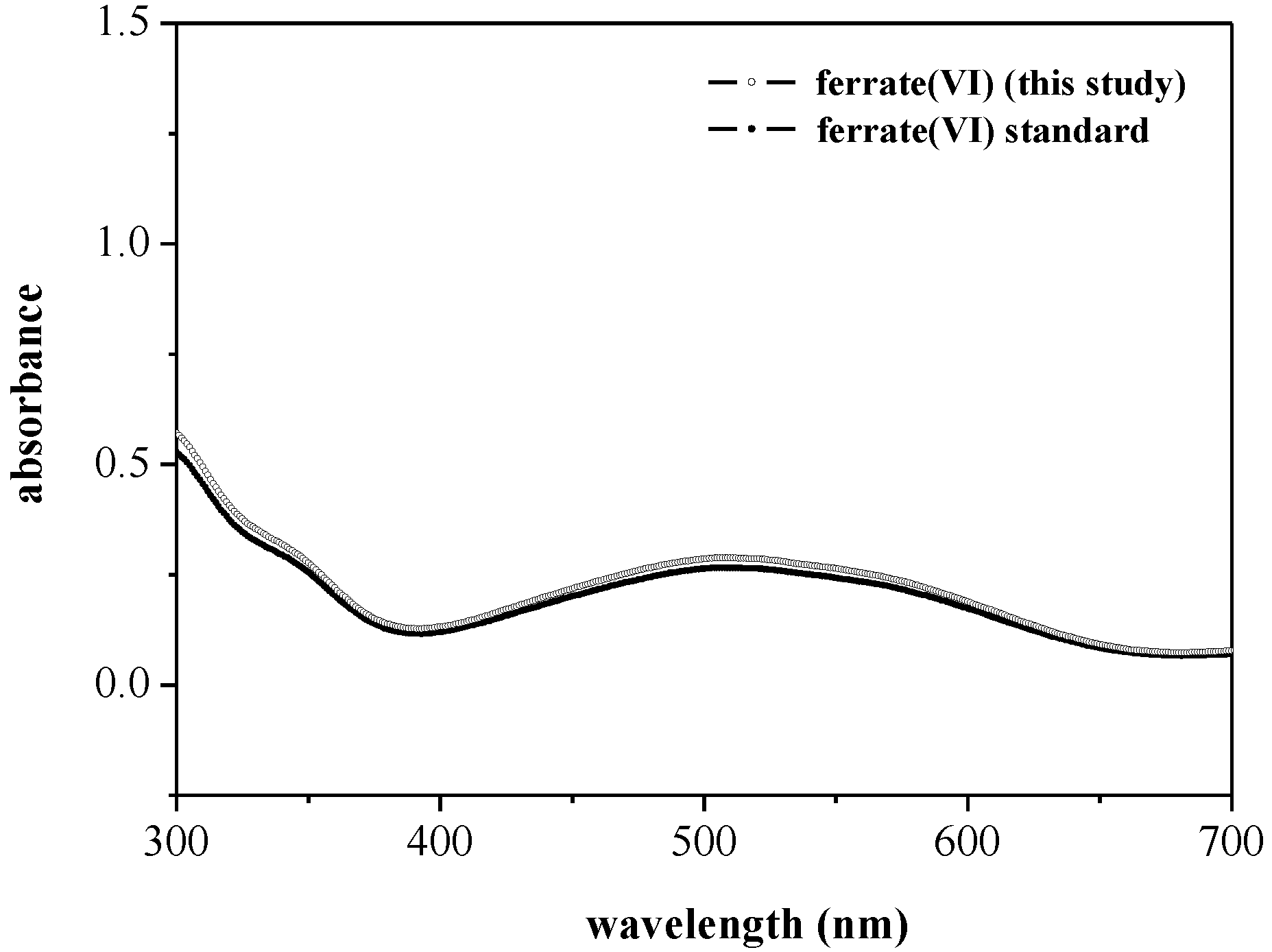
3.2.2. UV-Vis Absorption Spectra
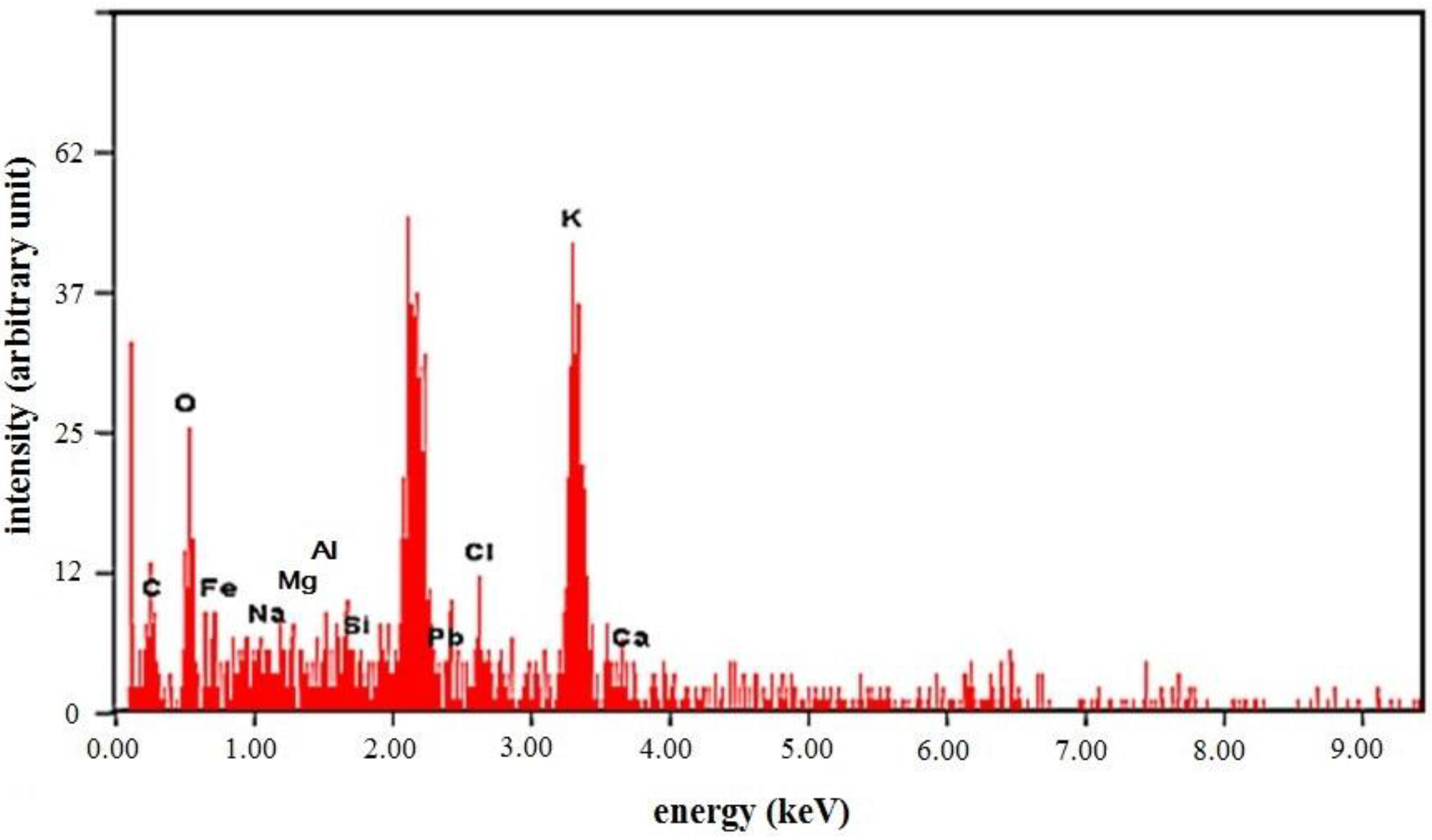
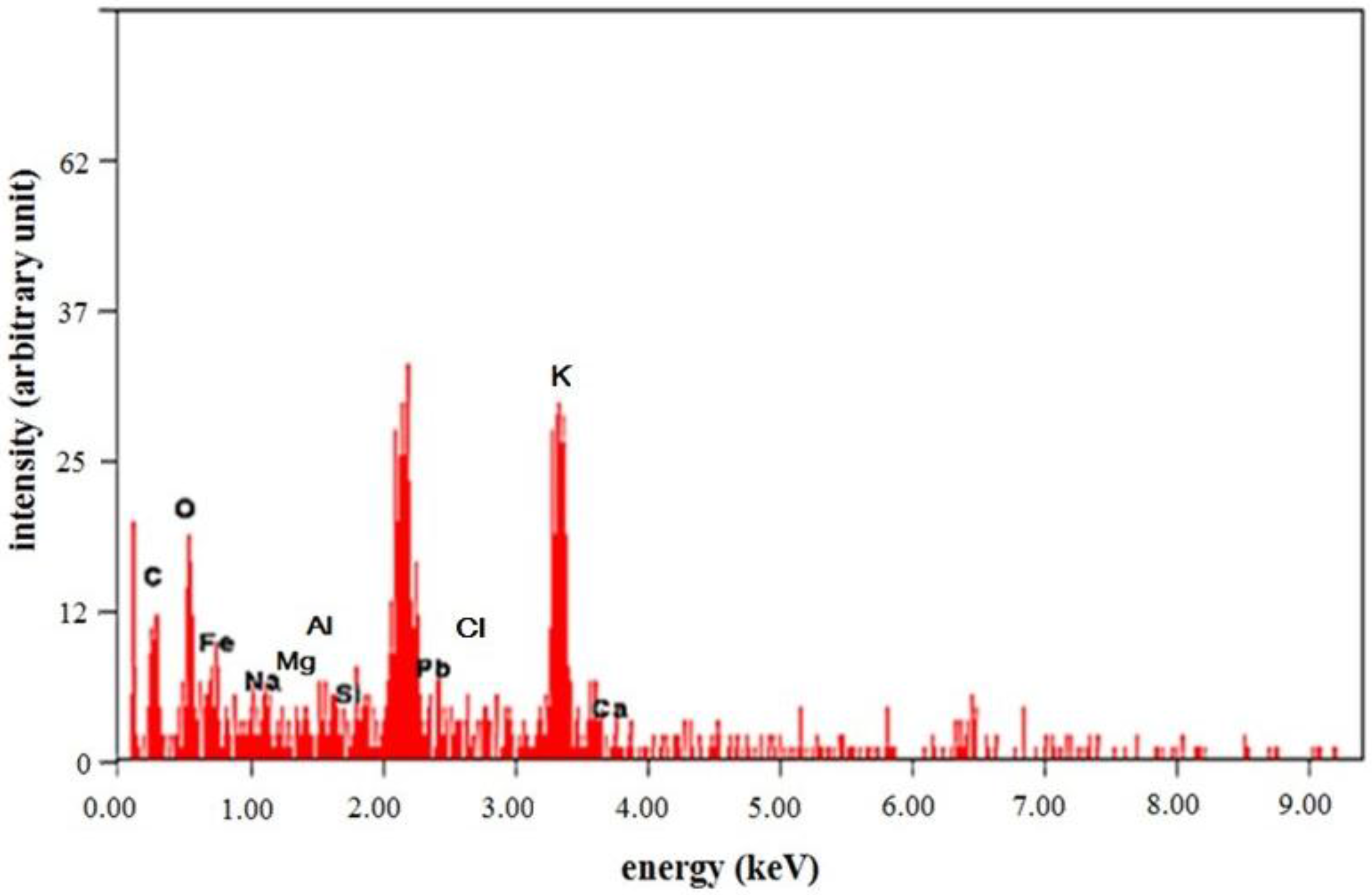
3.2.3. SEM-EDX Results from Potassium Ferrates
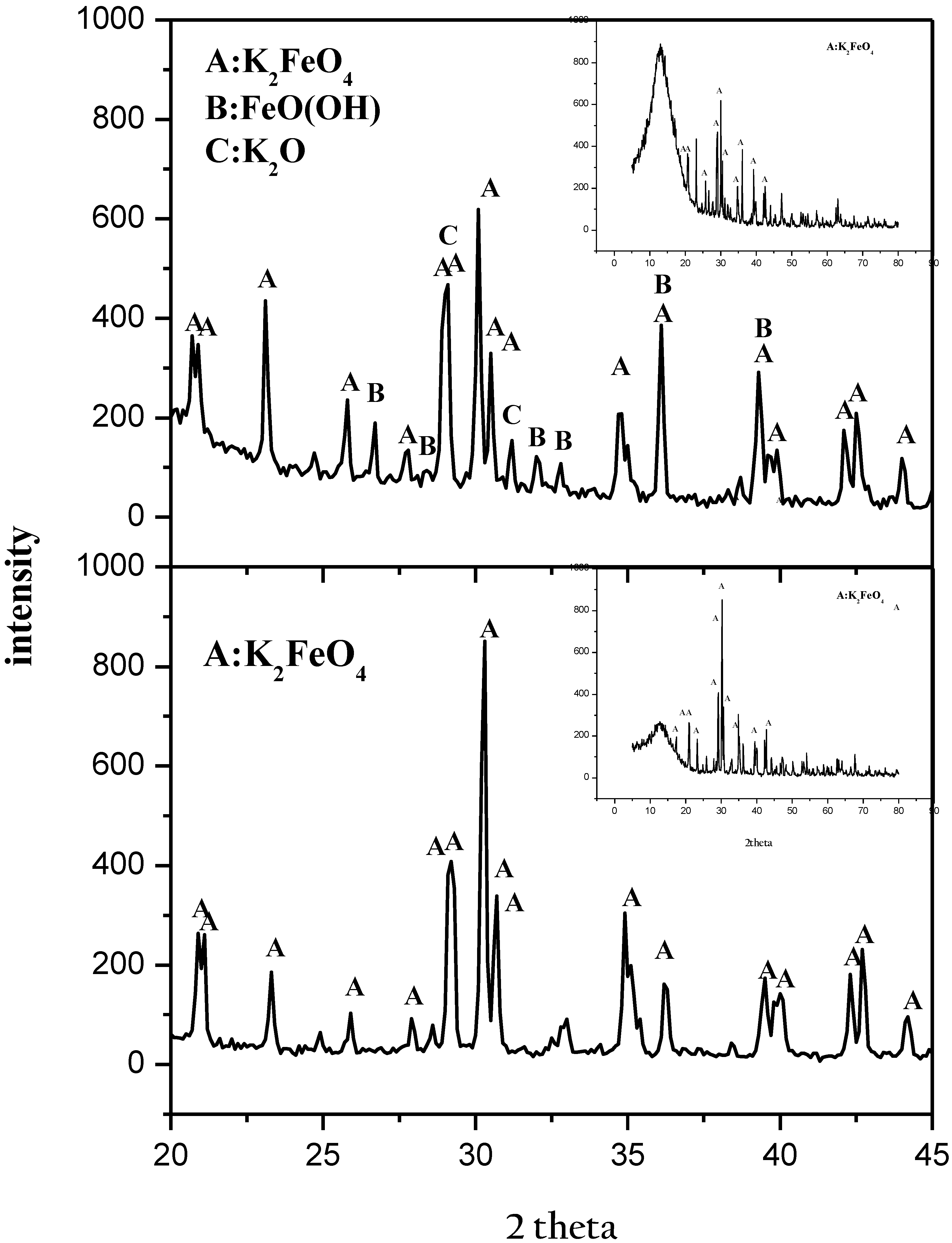
3.2.4. Crystalline Phases of Potassium Ferrates
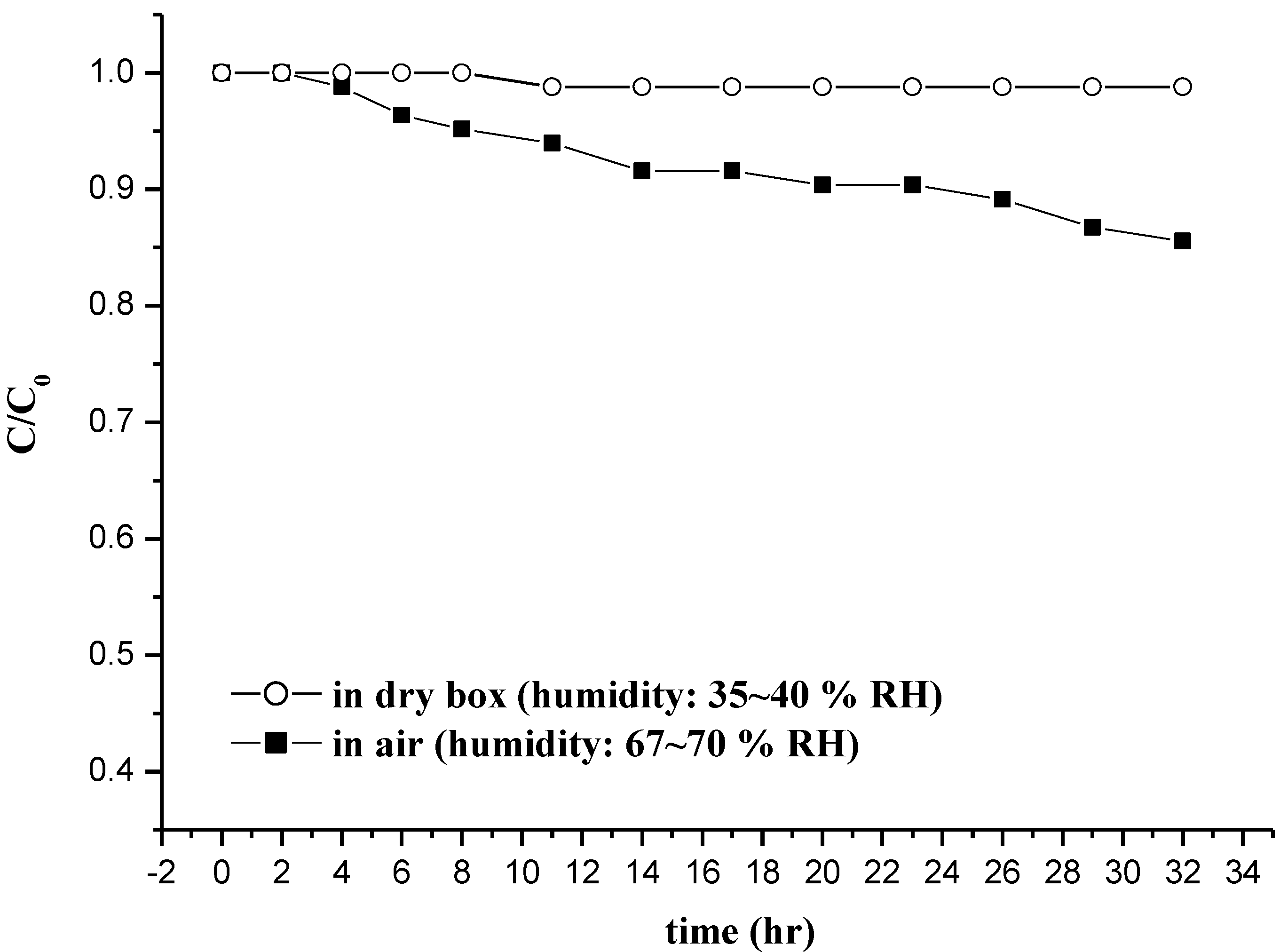
3.2.5. Test of Fe(VI) Stabilization during Storage
3.3. Stepwise Loss of Iron in Fe(VI) Synthesis Flowchart
| Synthesis Step b | Fe Loss a (%) |
|---|---|
| Discarded solid (A) | 54.8 |
| Discarded solid (B) | 26.5 |
| Discarded liquid (C) | 1.49 |
| Discarded solid (D) | 8.25 |
| Discarded liquid (E) | 0.55 |
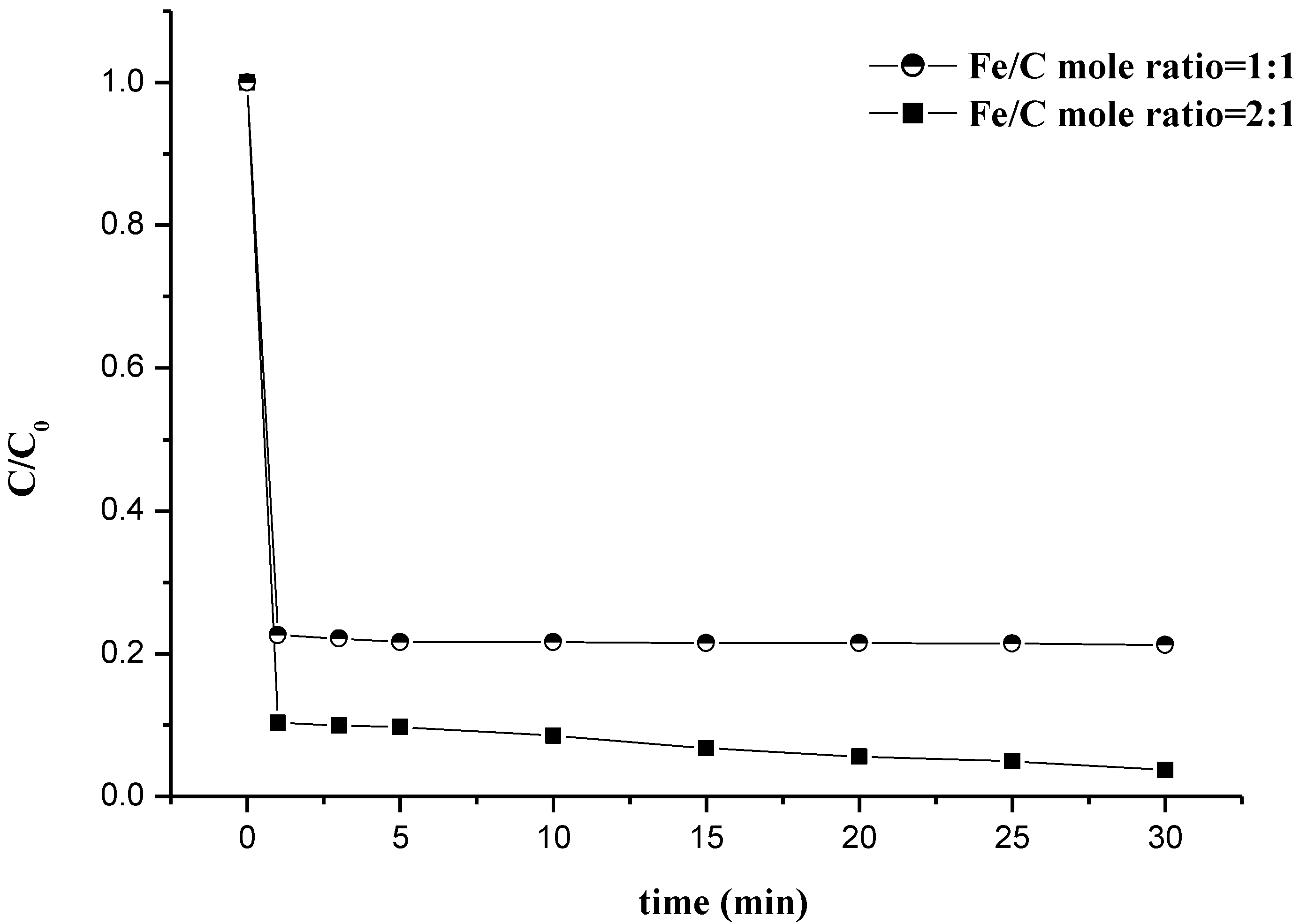
3.4. MB De-Colorization with In-House Potassium Ferrate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Audette, R.J.; Quail, J.W.; Smith, P.J. Ferrate(VI) ion, a novel oxidizing agent. Tetrahedron Lett. 1971, 3, 279–282. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, Y.; Cho, M.; Kim, J.Y. Chemistry of ferrate (Fe(IV)) in aqueous solution and its application as a green chemistry. J. Ind. Eng. Chem. 2004, 10, 161–171. [Google Scholar]
- Jiang, J.Q.; Lloyd, B. Progress in the development and use of ferrate(VI) salt as an oxidant and coagulant for water and wastewater treatment. Water Res. 2002, 36, 1397–1408. [Google Scholar] [CrossRef]
- Jiang, J.Q. The role of ferrate(VI) in the remediation of emerging micro pollutants: A review. Desalin. Water Treat. 2015, 55, 828–835. [Google Scholar] [CrossRef]
- Sharma, V.K.; Zboril, R.; Varma, R.S. Ferrates: Greener Oxidants with Multimodal Action in Water Treatment Technologies. Acc. Chem. Res. 2015, 48, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Panditi, V.R.; Gardinali, P.R.; Varma, R.S.; Kim, H.; Sharma, V.K. Ferrate promoted oxidative cleavage of sulfonamides: Kinetics and product formation under acidic conditions. Chem. Eng. J. 2015, 279, 307–316. [Google Scholar] [CrossRef]
- Yates, B.J.; Zboril, R.; Sharma, V.K. Engineering aspects of ferrate in water and wastewater treatment—A review. J. Environ. Sci. Heal. A 2014, 49, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Gombos, E. Ferrate treatment for inactivation of bacterial community in municipal secondary effluent. Bioresour. Technol. 2012, 107, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Prucek, R.; Tucek, J.; Kolarí̌k, J.; Huškováa, I.; Filip, J.; Varma, R.S.; Sharma, V.K.; Zboril, R. Ferrate(VI)-Prompted removal of metals in aqueous media: Mechanistic delineation of enhanced efficiency via metal entrenchment in magnetic oxides. Environ. Sci. Technl. 2015, 49, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Gombos, E.; Barkacs, K.; Felfoldi, T.; Vertes, C.; Mako, M.; Palko, G.; Záray, G. Removal of organic matters in wastewater treatment by ferrate(VI) technology. Microchem. J. 2013, 107, 115–120. [Google Scholar] [CrossRef]
- Sharma, V.K. Oxidation of inorganic contaminants by ferrates (VI, V, and IV) kinetics and mechanisms: A review. J. Environ. Manag. 2011, 92, 1051–1073. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Q.; Lloyd, B. Preliminary study of ciprofloxacin (cip) removal by potassium ferrate(VI). Sep. Purif. Technol. 2013, 88, 95–98. [Google Scholar] [CrossRef]
- Li, C.; Lib, X.Z.; Grahamc, N.; Gaoa, N.Y. The aqueous degradation of bisphenol A and steroid estrogens by ferrate. Water Res. 2008, 42, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, W.; Zhang, X.; Chen, F.; Wei, T. Formation of disinfection by-products after pre-oxidation with chlorine dioxide or ferrate. Water Res. 2013, 47, 5856–5864. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdulya, A.; Sharma, V.K. Oxidation of benzothiophene, dibenzothiophene, and methyl-dibenzothiophene by ferrate(VI). J. Hazard. Mater. 2015, 279, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Administration, R.O.C. Available online: http://www.epa.gov.tw/ (accessed on 21 September 2015).
- Agrawal, A.; Sahu, K.K. An overview of the recovery of acid from spent acidic solutions from steel and electroplating industries. J. Hazard. Mater. 2009, 171, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Rögener, F.; Buchloh, D.; Reichardt, T.; Schmidt, J.; Knaup, F. Total regeneration of mixed pickling acids from stainless steel production. Stahl und Eisen. 2009, 10, 69–73. [Google Scholar]
- Perfiliev, Y.D.; Benko, E.M.; Pankratov, D.A.; Sharma, V.K.; Dedushenko, S.K. Formation of iron(VI) in ozonalysis of iron(III) in alkaline solution. Inorg. Chim. Acta 2007, 360, 2789–2791. [Google Scholar] [CrossRef]
- Thompson, J.E.; Ockerman, L.T.; Schreyer, J.M. Preparation and purification of potassium ferrate(VI). J. Am. Chem. Soc. 1951, 73, 1379–1381. [Google Scholar] [CrossRef]
- Delaude, L.; Laszlo, P. A Novel Oxidizing Reagent Based on Potassium Ferrate(VI). J. Org. Chem. 1996, 61, 6360–6370. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K. Potassium ferrate(VI): An environmentally friendly oxidant. Adv. Environ. Res. 2002, 6, 143–156. [Google Scholar] [CrossRef]
- Licht, S.; Naschitz, V.; Halperin, L.; Halperin, N.; Lin, L.; Chen, J.J.; Ghosh, S.; Liu, B. Analysis of ferrate(VI) compounds and super-iron Fe(VI) battery cathodes: FTIR, ICP, titrimetric, XRD, UV-VIS, and electrochemical characterization. J. Power Sources 2001, 101, 167–176. [Google Scholar] [CrossRef]
- Luo, Z.; Strouse, M.; Jiang, J.Q.; Sharma, V.K. Methodologies for the analytical determination of ferrate(VI): A Review. J. Environ. Sci. Heal. A 2011, 46, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Ressler, T. WinXAS: A program for X-ray absorption spectroscopy data analysis under MS-Windows. J. Synchrotron Radiat. 1998, 5, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Liu, S.Q.; Zhang, X.Y. Preparation and application of sustained release microcapsules of potassium ferrate(VI) for dinitro butyl phenol (DNBP) wastewater treatment. J. Hazard. Mater. 2009, 169, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.L.; Li, X.Z.; Graham, N. Reaction pathways of dimethyl phthalate degradation in TiO2–UV–O2 and TiO2–UV–Fe(VI) systems. Chemosphere 2008, 72, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Machala, L.; Zboril, R.; Sharma, V.K.; Filip, J.; Jancil, D.; Homonnay, Z. Transformation of solid potassium ferrate(VI) (K2FeO4): Mechanism and kinetic effect of air humidity. Eur. J. Inorg. Chem. 2009, 8, 1060–1067. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.-L.; Wang, Y.-S.; Liu, C.-H. Preparation of Potassium Ferrate from Spent Steel Pickling Liquid. Metals 2015, 5, 1770-1787. https://doi.org/10.3390/met5041770
Wei Y-L, Wang Y-S, Liu C-H. Preparation of Potassium Ferrate from Spent Steel Pickling Liquid. Metals. 2015; 5(4):1770-1787. https://doi.org/10.3390/met5041770
Chicago/Turabian StyleWei, Yu-Ling, Yu-Shun Wang, and Chia-Hung Liu. 2015. "Preparation of Potassium Ferrate from Spent Steel Pickling Liquid" Metals 5, no. 4: 1770-1787. https://doi.org/10.3390/met5041770