Toughness of Bulk Metallic Glasses
Abstract
:1. Introduction
2. Techniques of Measuring Toughness
2.1. KIc/Notch Toughness Tests
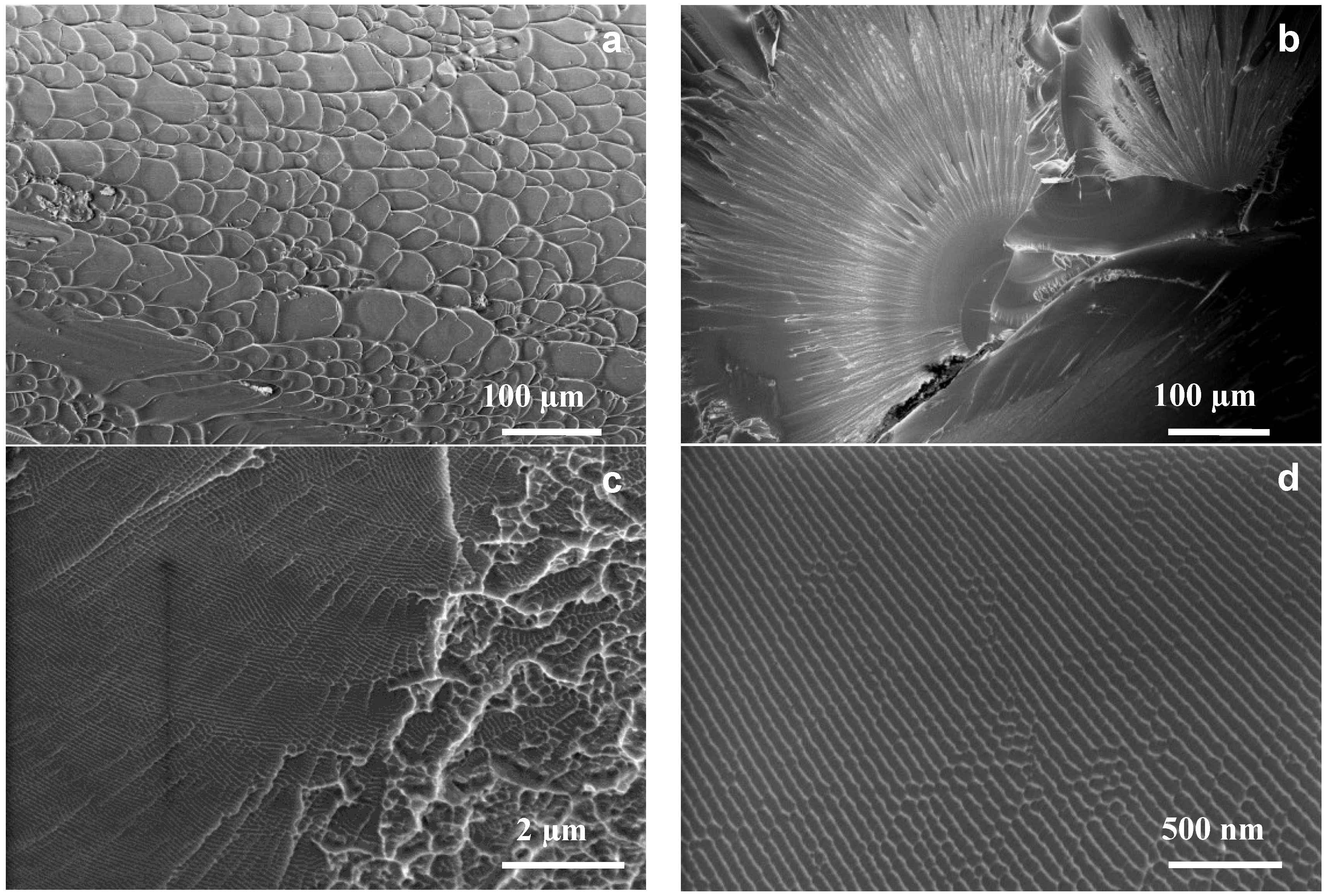
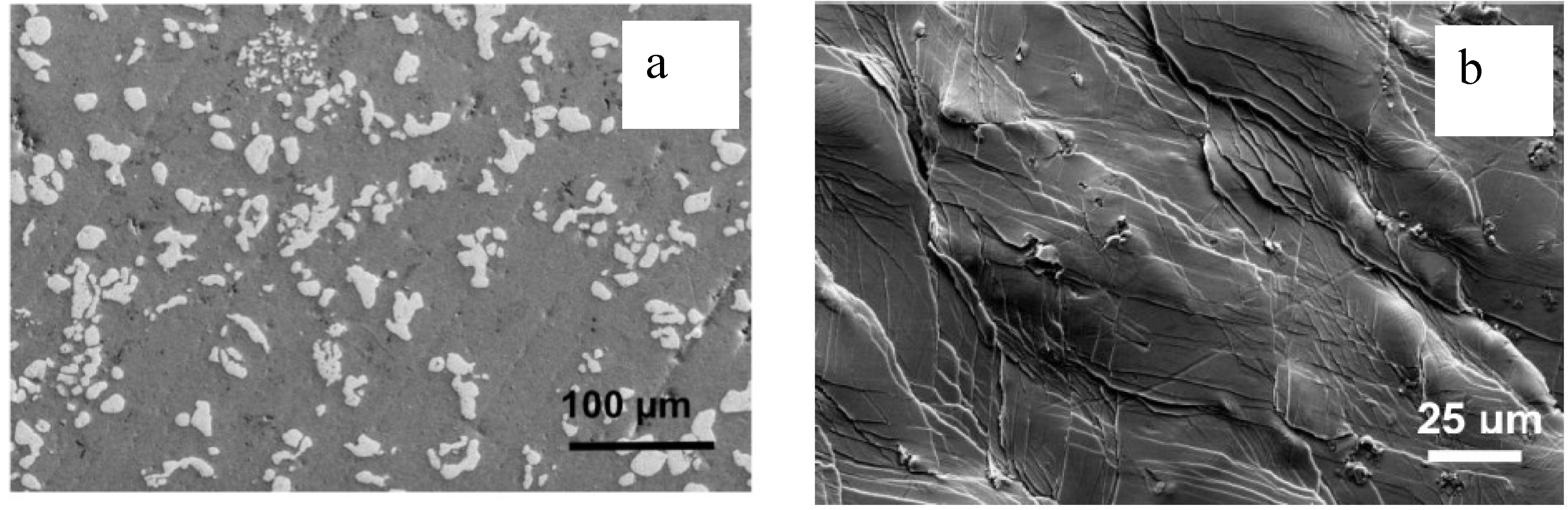
2.2. Toughness from Fracture Surfaces
2.3. Compression Testing
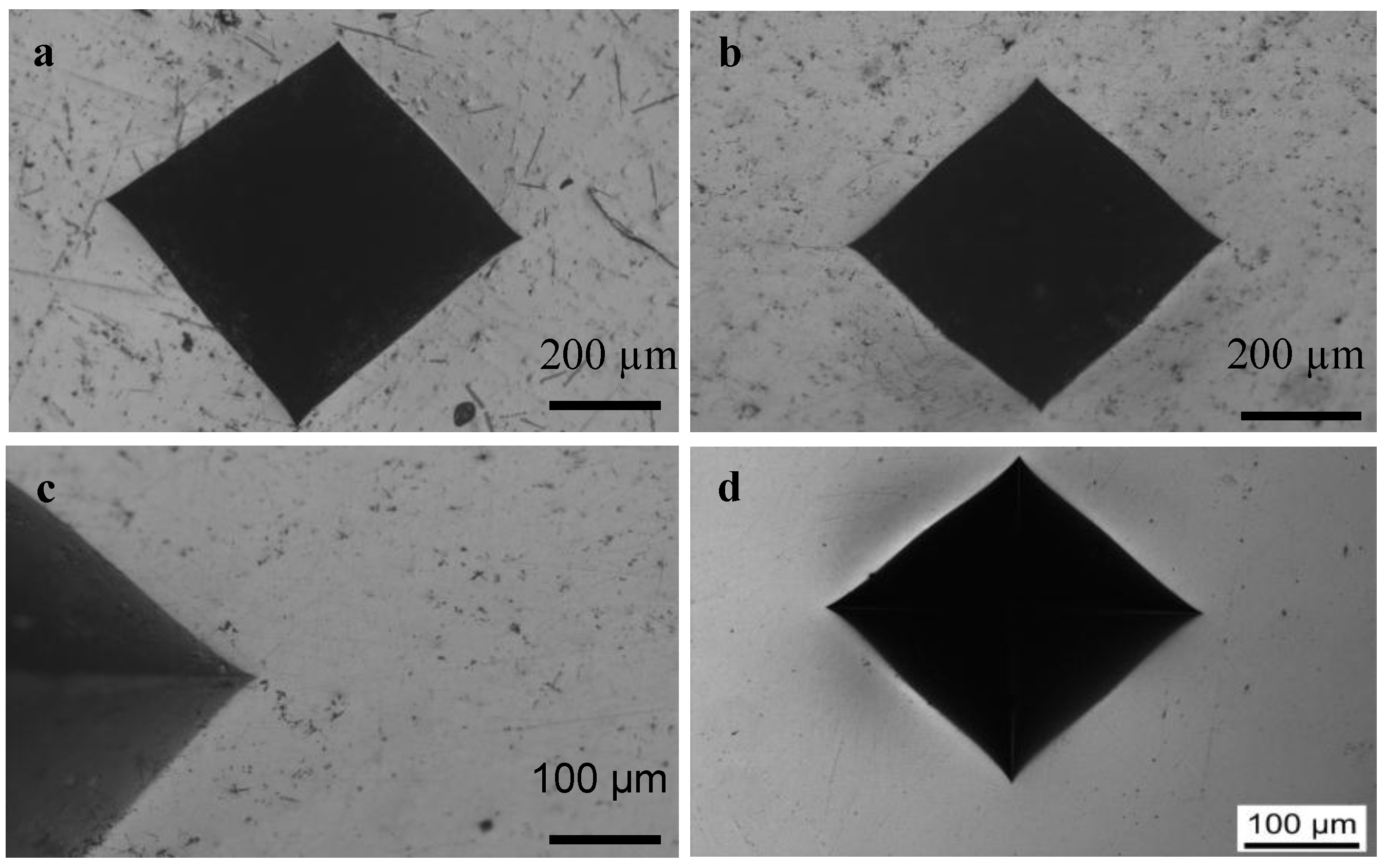
2.4. Indentation Fracture Toughness
| Alloy Composition (at. %) | Poisson’s Ratio (ν) | Yield Strength (σy) GPa | Shear Modulus (μ) GPa | STZ Barrier Energy Density. ρ (GJ/m3) | Indentation Toughness (MPa.m1/2) | Reference |
|---|---|---|---|---|---|---|
| Ce60Al20Cu10Ni10 * | 0.317 * | 0.8 | 15 * | 0.0259 | Tough | This work, [55] |
| La55Co5Cu10Ni10Al20 | 0.34 | 0.85 | 15.6 | 0.02815 | Tough | This work, [55] |
| Mg58Cu31Y11 | 0.318 | 0.986 | 20.4 | 0.02897 | 2.91 | [55,56] |
| Fe64Mo14C15B6Er1 | 0.316 | 3.9 | 75.4 ** | ~0.122 | Tough | This work, [55,57] |
| Fe48Cr15Mo14Er2C15B6 | 0.318 | 3.75 | 80.8 | 0.1059 | 3.8 ± 0.3 | [52,57] |
| Fe41Co7Cr15Mo14C15B6Y2 | 0.334 | 3.5 | 84.1 | 0.0886 | 2.26 ± 0.4 | [53,58] |
2.5. Impact Toughness
2.6. Wear Resistance (An Indirect Indication of Toughness)
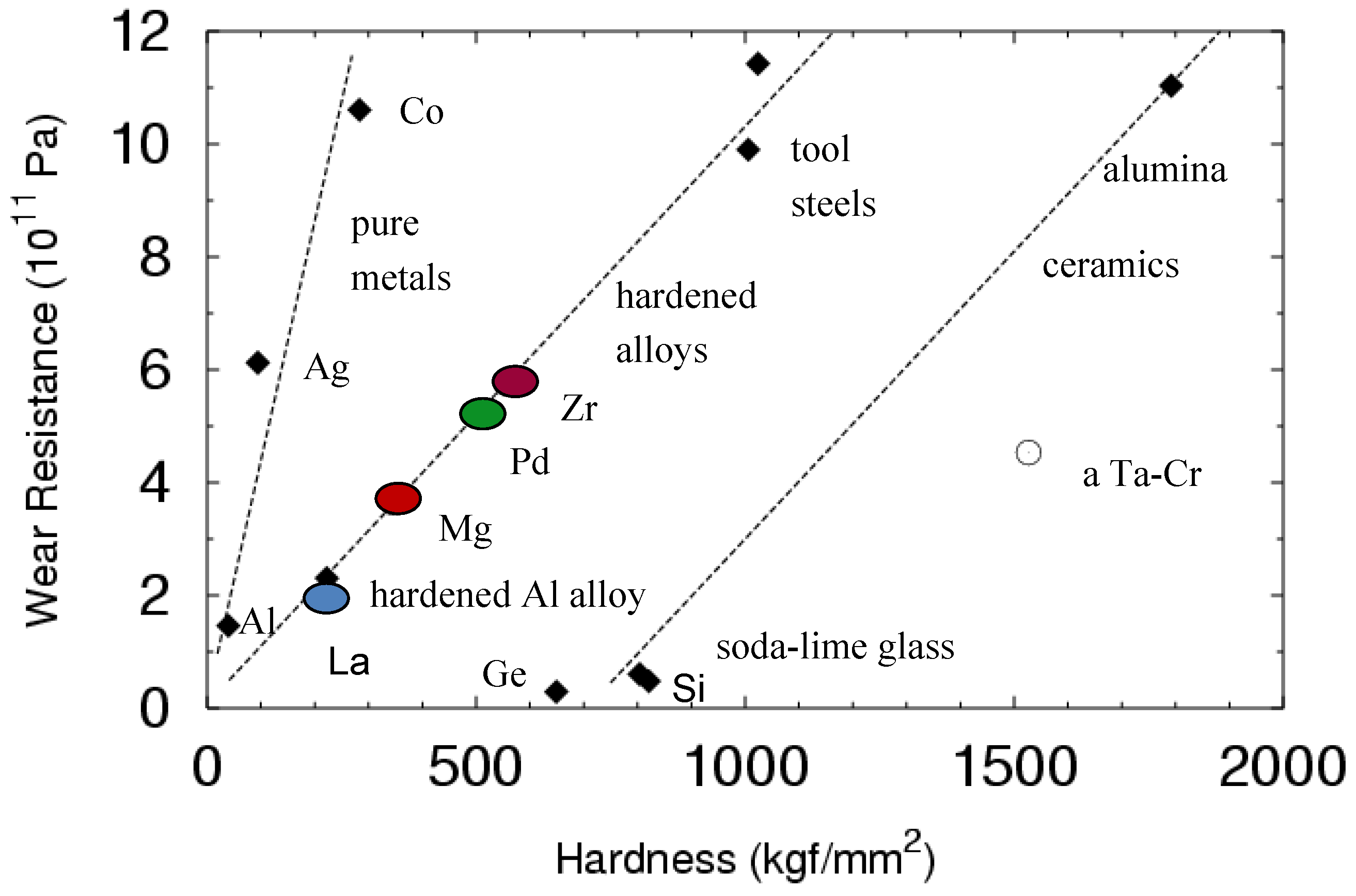
| Material | Wear Coefficient, K (3-body Abrasive Wear) |
|---|---|
| Zr–Cu–N–Al | 1.03 × 10−2 |
| Pd-BMG | 0.96 × 10−2 |
| Mg-BMG | 0.9 × 10−2 |
| La-BMG | 1.1 × 10−2 |
| Tool Steel | 0.98 × 10−2 |
| Hardened Al alloy | 0.93 × 10−2 |
| Pure Co | 0.2 × 10−2 |
| Pure Si | 0.15 |
3. What Controls Toughness of Bulk Metallic Glasses?
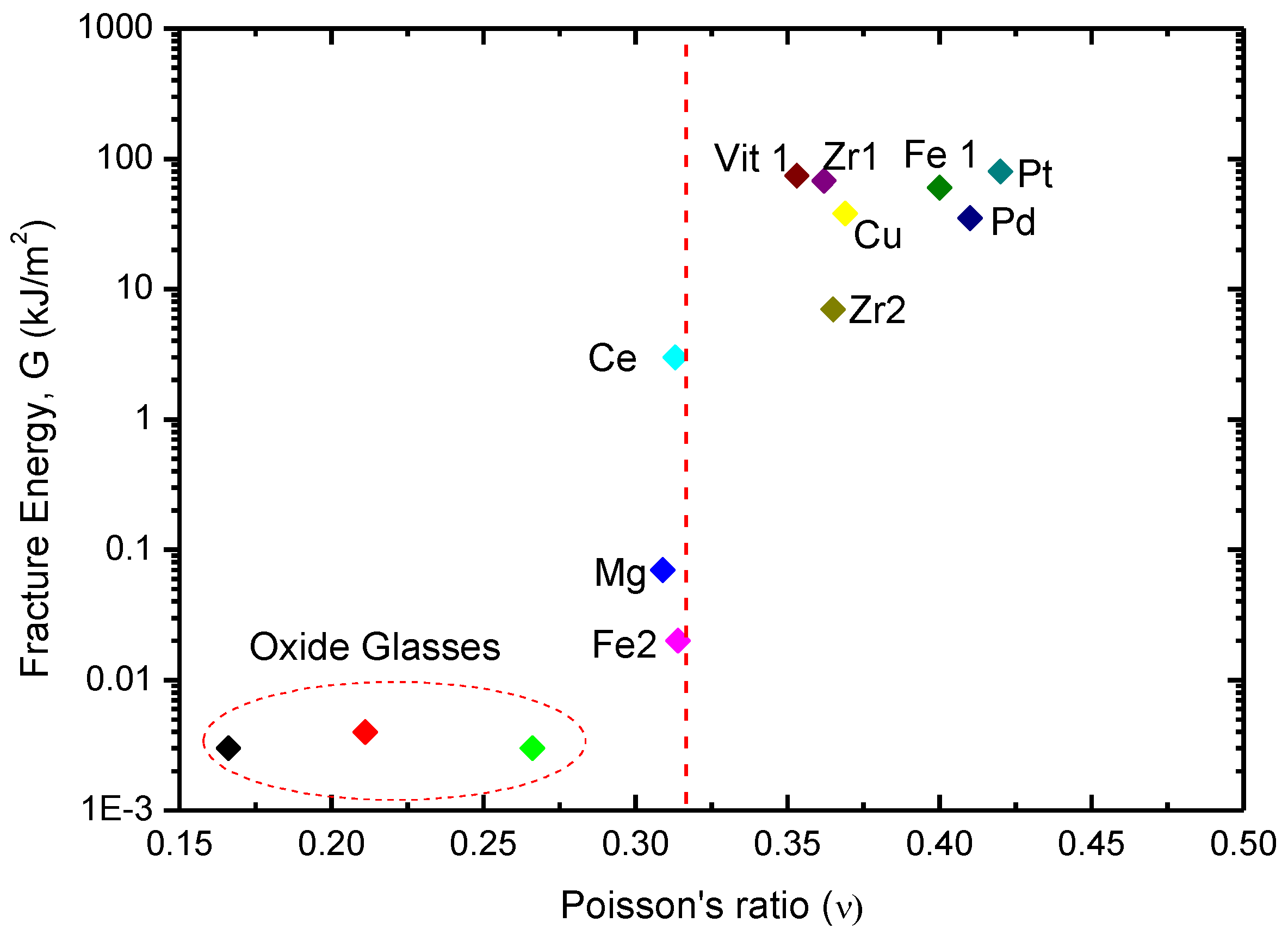
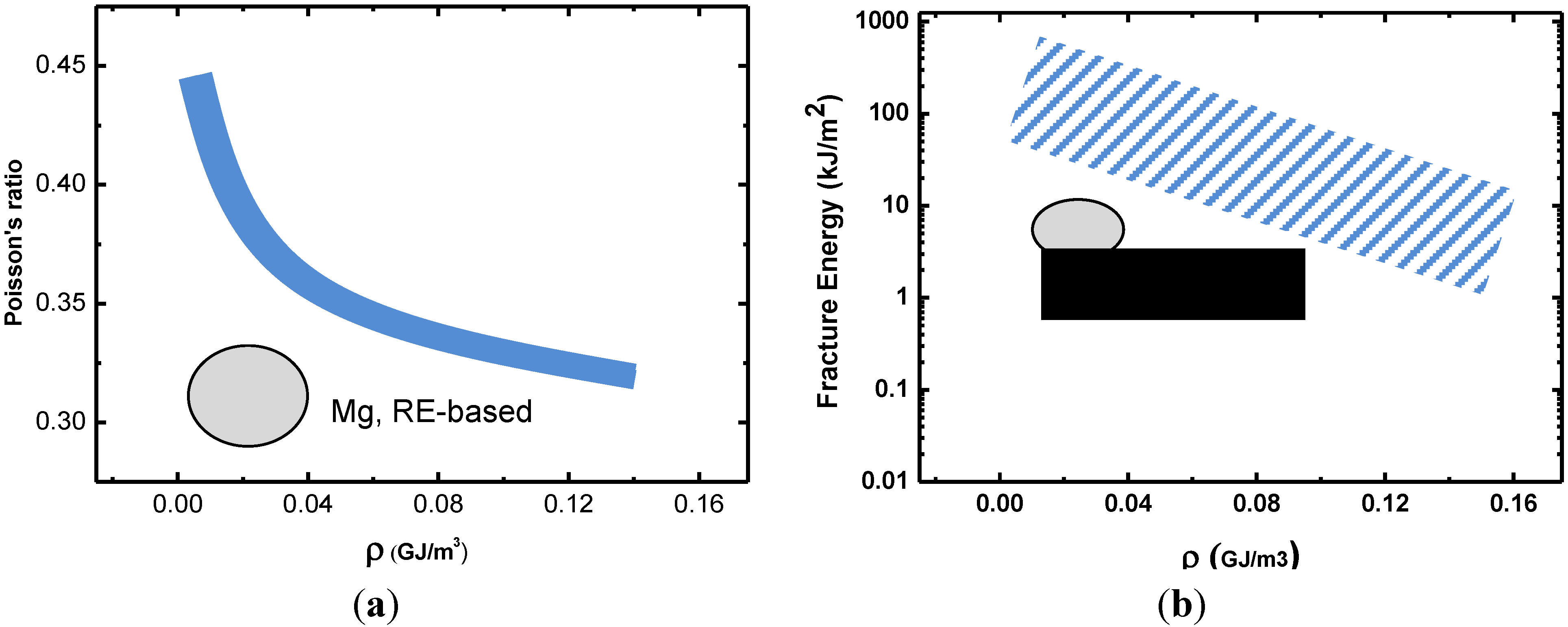
3.1. Toughness–Poisson’s Ratio Correlation

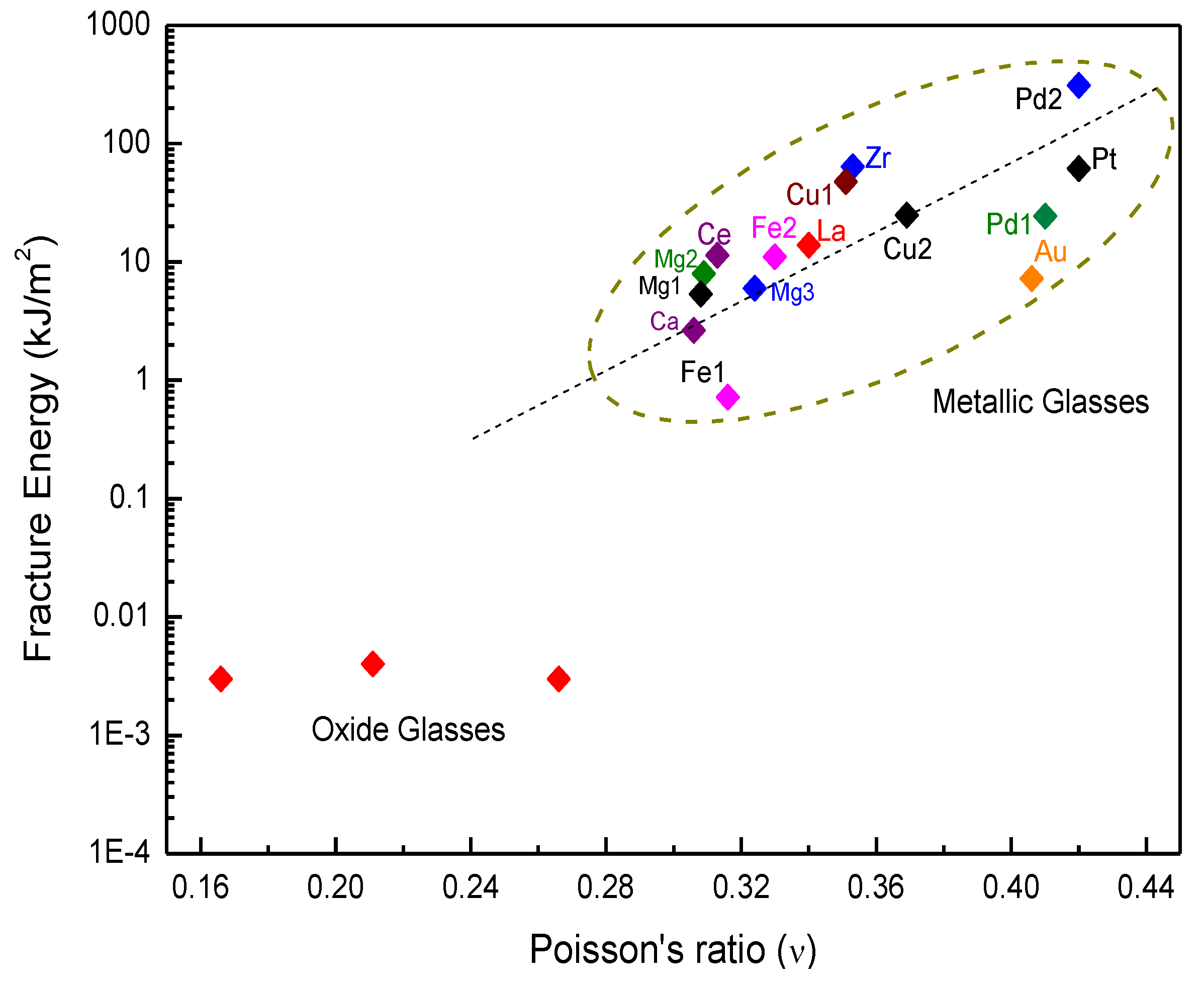
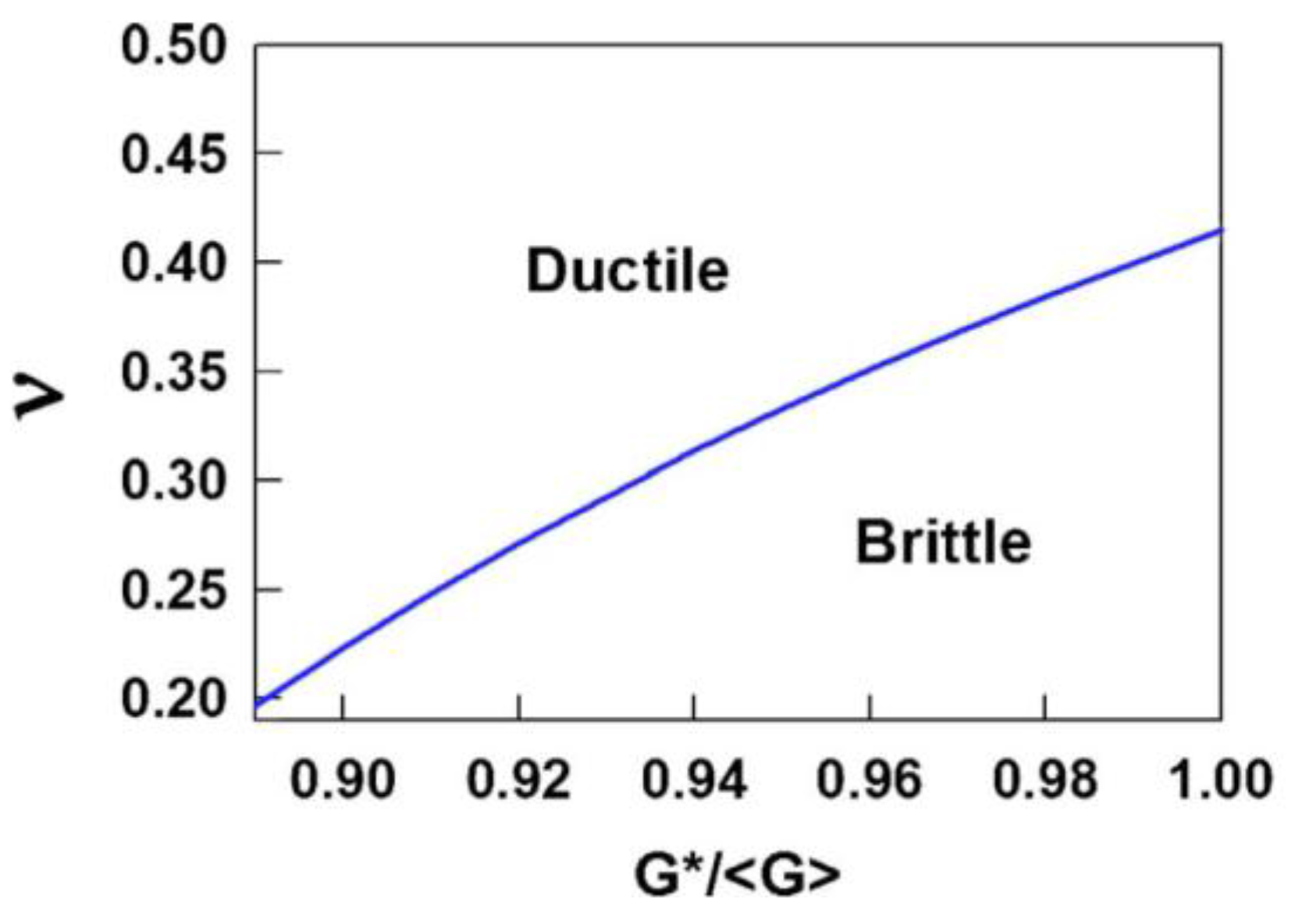
3.2. Toughness and Shear Transformation Zones (STZs)
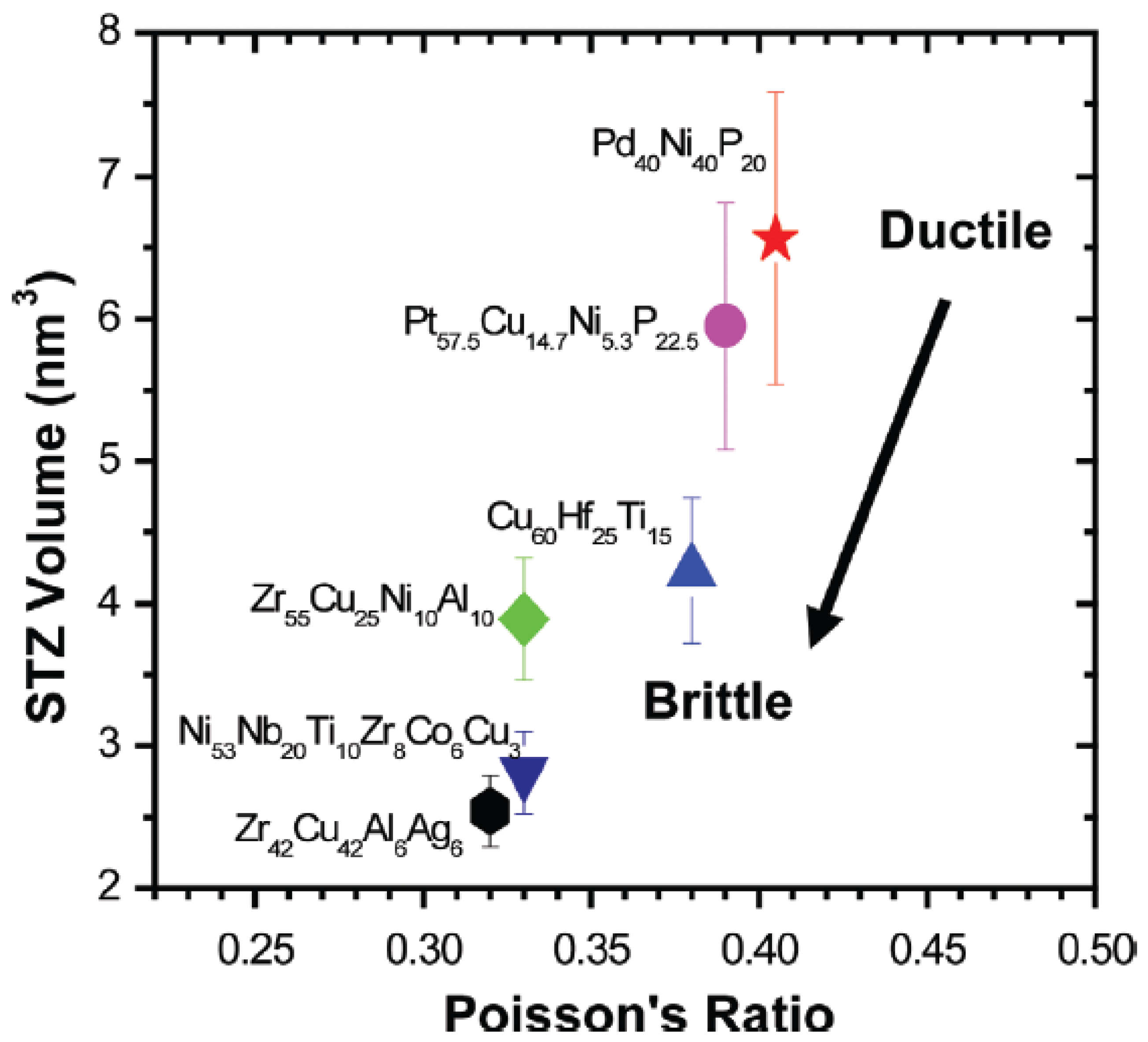
3.3. Toughness–Shear Modulus
3.4. Toughness and the Critical Fictive Temperature
4. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Peker, A.; Johnson, W.L. A highly processable metallic glass: Zr41.2Ti13.8Cu12.5Ni10Be22.5. Appl. Phys. Lett. 1993, 63, 2342–2344. [Google Scholar] [CrossRef]
- Conner, R.D.; Dandliker, R.B.; Johnson, W.L. Mechanical properties of tungsten and steel fiber reinforced Zr41.25Ti13.75Cu12.5Ni10Be22.5 metallic glass matrix composites. Acta Mater. 1998, 46, 6089–6102. [Google Scholar] [CrossRef]
- Choi-Yim, H.; Busch, R.; Köster, U.; Johnson, W.L. Synthesis and characterization of particulate reinforced Zr57Nb5Al10Cu15.4Ni12.6 bulk metallic glass composites. Acta Mater. 1999, 47, 2455–2462. [Google Scholar] [CrossRef]
- Tang, X.-P.; Geyer, U.; Busch, R.; Johnson, W.L.; Wu, Y. Diffusion mechanisms in metallic supercooled liquids and glasses. Nature 1999, 402, 160–162. [Google Scholar]
- Zumkley, T.; Naundorf, V.; Macht, M.-P.; Frohberg, G. Effect of reversible structural relaxation on diffusion in a ZrTiCuNiBe bulk glass. Scr. Mater. 2001, 45, 471–477. [Google Scholar] [CrossRef]
- Gangopadhyay, A.K.; Croat, T.K.; Kelton, K.F. The effect of phase separation on subsequent crystallization in Al88Gd6La2Ni4. Acta Mater. 2000, 48, 4035–4043. [Google Scholar] [CrossRef]
- Madge, S.V.; Alexander, D.T.L.; Greer, A.L. An EFTEM study of compositional variations in Mg–Ni–Nd bulk metallic glasses. J. Non-Cryst. Solids 2003, 317, 23–29. [Google Scholar] [CrossRef]
- Park, E.S.; Kim, D.H. Phase separation and enhancement of plasticity in Cu–Zr–Al–Y bulk metallic glasses. Acta Mater. 2006, 54, 2597–2604. [Google Scholar] [CrossRef]
- Greer, A.L.; Rutherford, K.L.; Hutchings, I.M. Wear resistance of amorphous alloys and related materials. Int. Mater. Rev. 2002, 47, 87–112. [Google Scholar] [CrossRef]
- Madge, S.V.; Caron, A.; Gralla, R.; Wilde, G.; Mishra, S.K. Novel W-based metallic glass with high hardness and wear resistance. Intermetallics 2014, 47, 6–10. [Google Scholar] [CrossRef]
- Xu, T.; Pang, S.; Li, H.; Zhang, T. Corrosion resistant Cr-based bulk metallic glasses with high strength and hardness. J. Non-Cryst. Solids 2015, 410, 20–25. [Google Scholar] [CrossRef]
- Schuh, C.A.; Hufnagel, T.C.; Ramamurty, U. Mechanical behavior of amorphous alloys. Acta Meter. 2007, 55, 4067–4109. [Google Scholar] [CrossRef]
- Conner, R.D.; Rosakis, A.J.; Johnson, W.L.; Owen, D.M. Fracture toughness determination for a beryllium-bearing bulk metallic glass. Scr. Mater. 1997, 37, 1373–1378. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Shazly, M.; Shamimi Nouri, A. Intrinsic and extrinsic toughening of metallic glasses. Scr. Mater. 2006, 54, 337–341. [Google Scholar] [CrossRef]
- Nishiyama, N.; Amiya, K.; Inoue, A. Novel applications of bulk metallic glass for industrial products. J. Non-Cryst. Solids 2007, 353, 3615–3621. [Google Scholar] [CrossRef]
- Inoue, A.; Takeuchi, A. Recent development and application products of bulk glassy alloys. Acta Mater. 2011, 59, 2243–2267. [Google Scholar] [CrossRef]
- Liquidmetal Technologies. Available online: http://liquidmetal.com/ (accessed on 14 May 2015).
- Nanosteel. Available online: https://nanosteelco.com/ (accessed on 14 May 2015).
- Greer, A.L.; Cheng, Y.Q.; Ma, E. Shear bands in metallic glasses. Mater. Sci. Eng. R 2013, 74, 71–132. [Google Scholar] [CrossRef]
- Xu, J.; Ramamurty, U.; Ma, E. The fracture toughness of bulk metallic glasses. JOM 2010, 62, 10–18. [Google Scholar] [CrossRef]
- Greer, J.R.; de Hosson, J.T.M. Plasticity in small-sized metallic systems: Intrinsic versus extrinsic size effect. Prog. Mater. Sci. 2011, 56, 654–724. [Google Scholar] [CrossRef]
- Gilbert, C.J.; Schroeder, V.; Ritchie, R.O. Mechanisms for fracture and fatigue-crack propagation in a bulk metallic glass. Metall. Mater. Trans. A 1999, 30, 1739–1753. [Google Scholar] [CrossRef]
- Lowhaphandu, P.; Lewandowski, J.J. Fracture toughness and notched toughness of bulk amorphous alloy: Zr–Ti–Cu–Ni–Be. Scr. Mater. 1998, 38, 1811–1817. [Google Scholar] [CrossRef]
- Kim, C.P.; Suh, J.Y.; Wiest, A.; Lind, M.L.; Conner, R.D.; Johnson, W.L. Fracture toughness study of new Zr-based Be-bearing bulk metallic glasses. Scr. Mater. 2009, 60, 80–83. [Google Scholar] [CrossRef]
- Launey, M.E.; Busch, R.; Kruzic, J.J. Effects of free volume changes and residual stresses on the fatigue and fracture behavior of a Zr–Ti–Ni–Cu–Be bulk metallic glass. Acta Mater. 2008, 56, 500–510. [Google Scholar] [CrossRef]
- Keryvin, V.; Nadot, Y.; Yokoyama, Y. Fatigue pre-cracking and toughness of the Zr55Cu30Al10Ni5 bulk metallic glass for two oxygen levels. Scr. Mater. 2007, 57, 145–148. [Google Scholar] [CrossRef]
- Flores, K.M.; Dauskardt, R.H. Fracture and deformation of bulk metallic glasses and their composites. Intermetallics 2004, 12, 1025–1029. [Google Scholar] [CrossRef]
- Flores, K.M.; Dauskardt, R.H. Mode II fracture behavior of a Zr-based bulk metallic glass. J. Mech. Phys. Solids 2006, 54, 2418–2435. [Google Scholar] [CrossRef]
- Gu, X.J.; Poon, S.J.; Shiflet, G.J.; Lewandowski, J.J. Compressive plasticity and toughness of a Ti-based bulk metallic glass. Acta Mater. 2010, 58, 1708–1720. [Google Scholar] [CrossRef]
- Demetriou, M.D.; Launey, M.E.; Garrett, G.; Schramm, J.P.; Hofmann, D.C.; Johnson, W.L.; Ritchie, R.O. A damage-tolerant glass. Nat. Mater. 2011, 10, 123–128. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shang, J.K.; Ma, E.; Xu, J. Crack-resistance curve of a Zr–Ti–Cu–Al bulk metallic glass with extraordinary fracture toughness. Acta Mater. 2012, 60, 4940–4949. [Google Scholar] [CrossRef]
- Varadarajan, R.; Thurston, A.K.; Lewandowski, J.J. Increased toughness of zirconium-based bulk metallic glasses tested under mixed mode conditions. Metall. Mater. Trans. A 2010, 41, 149–158. [Google Scholar] [CrossRef]
- Xu, J.; Ma, E. Damage-tolerant Zr–Cu–Al bulk metallic glasses with record-breaking fracture toughness. J. Mater. Res. 2014, 29, 1489–1499. [Google Scholar] [CrossRef]
- Gludovatz, B.; Naleway, S.E.; Ritchie, R.O.; Kruzic, J.J. Size-dependent fracture toughness of bulk metallic glasses. Acta Mater. 2014, 70, 198–207. [Google Scholar] [CrossRef]
- Conner, R.D.; Li, Y.; Nix, W.D.; Johnson, W.L. Shear band spacing under bending of Zr-based metallic glass plates. Acta Mater. 2004, 52, 2429–2434. [Google Scholar] [CrossRef]
- Argon, A.S.; Salama, M. The mechanism of fracture in glassy materials capable of some inelastic deformation. Mater. Sci. Eng. 1976, 23, 219–230. [Google Scholar] [CrossRef]
- Narasimhan, R.; Tandaiya, P.; Singh, I.; Narayan, R.L.; Ramamurty, U. Fracture in metallic glasses: Mechanics and mechanisms. Int. J. Fract. 2015, 191, 53–75. [Google Scholar] [CrossRef]
- Jiang, M.Q.; Ling, Z.; Meng, J.X.; Dai, L.H. Energy dissipation in fracture of bulk metallic glasses via inherent competition between local softening and quasi-cleavage. Philos. Mag. 2008, 88, 407–426. [Google Scholar] [CrossRef]
- Wang, G.; Chan, K.C.; Xu, X.H.; Wang, W.H. Instability of crack propagation in brittle bulk metallic glass. Acta Mater. 2008, 56, 5845–5860. [Google Scholar] [CrossRef]
- Narayan, R.L.; Tandaiya, P.; Narasimhan, R.; Ramamurty, U. Wallner lines, crack velocity and mechanisms of crack nucleation and growth in a brittle bulk metallic glass. Acta Mater. 2014, 80, 407–420. [Google Scholar] [CrossRef]
- Murali, P.; Guo, T.F.; Zhang, Y.W.; Narasimhan, R.; Li, Y.; Gao, H.J. Atomic scale fluctuations govern brittle fracture and cavitation behaviour in metallic glasses. Phys. Rev. Lett. 2011, 107, 215501. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Masumoto, T. Deformation and fracture of an amorphous Pd–Cu–Si alloy in V-notch bending tests-II. Ductile-brittle transition. Acta Metall. 1980, 28, 1677–1693. [Google Scholar] [CrossRef]
- Xi, X.K.; Zhao, D.Q.; Pan, M.X.; Wang, W.H.; Wu, Y.; Lewandowski, J.J. Fracture of Brittle metallic glasses: Brittleness or plasticity. Phys. Rev. Lett. 2005, 94, 125510. [Google Scholar] [CrossRef] [PubMed]
- Madge, S.V.; Louzguine-Luzgin, D.V.; Lewandowski, J.J.; Greer, A.L. Toughness, extrinsic effects and Poisson’s ratio of bulk metallic glasses. Acta Mater. 2012, 60, 4800–4809. [Google Scholar] [CrossRef]
- Ghidelli, M.; Gravier, S.; Blandin, J.-J.; Raskin, J.-P.; Lani, F.; Pardoen, T. Size-dependent failure mechanisms in ZrNi thin metallic glass films. Scr. Mater. 2014, 89, 9–12. [Google Scholar] [CrossRef]
- Ghidelli, M.; Gravier, S.; Blandin, J.-J.; Djemia, P.; Mompiou, F.; Abadias, G.; Raskin, J.-P.; Pardoen, T. Extrinsic mechanical size effects in thin ZrNi metallic glass films. Acta Mater. 2015, 90, 232–241. [Google Scholar] [CrossRef]
- Han, Z.; Wu, W.F.; Li, Y.; Wei, Y.J.; Gao, H.J. An instability index of shear band for plasticity in metallic glasses. Acta Mater. 2009, 57, 1367–1372. [Google Scholar] [CrossRef]
- Mondal, K.; Kumar, G.; Ohkubo, T.; Oishi, K.; Mukai, T.; Hono, K. Large apparent compressive strain of metallic glasses. Philos. Mag. Lett. 2007, 87, 625–635. [Google Scholar] [CrossRef]
- Li, G.; Jiang, M.Q.; Jiang, F.; He, L.; Sun, J. The ductile to brittle transition behavior in a Zr-based bulk metallic glass. Mater. Sci. Eng. A 2015, 625, 393–402. [Google Scholar] [CrossRef]
- Madge, S.V.; Wada, T.; Louzguine-Luzgin, D.V.; Greer, A.L.; Inoue, A. Oxygen embrittlement in a Cu–Hf–Al bulk metallic glass. Scr. Mater. 2009, 61, 540–543. [Google Scholar] [CrossRef]
- Hess, P.A.; Poon, S.J.; Shiflet, G.J.; Dauskardt, R.H. Indentation fracture toughness of amorphous steel. J. Mater. Res. 2005, 20, 783–786. [Google Scholar] [CrossRef]
- Keryvin, V.; Hoang, V.H.; Shen, J. Hardness, toughness, brittleness and cracking systems in an iron-based bulk metallic glass by indentation. Intermetallics 2009, 17, 211–217. [Google Scholar] [CrossRef]
- Kruzic, J.J.; Kim, D.K.; Koester, K.J.; Ritchie, R.O. Indentation techniques for evaluating the fracture toughness of biomaterials and hard tissues. J. Mech. Behav. Biomed. Mater. 2009, 2, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Wang, K.; Inoue, A.; Sakurai, T.; Chen, M.W. Energetic criterion on the intrinsic ductility of bulk metallic glasses. Scr. Mater. 2010, 62, 586–589. [Google Scholar] [CrossRef]
- Hsieh, P.J.; Lin, S.C.; Su, H.C.; Jang, J.S.C. Glass forming ability and mechanical properties characterization on Mg58Cu31Y11−xGdx bulk metallic glasses. J. Alloys Compd. 2009, 483, 40–43. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Gu, X.J.; Shamimi Nouri, A.; Poon, S.J.; Shiflet, G.J. Tough Fe-based bulk metallic glasses. Appl. Phys. Lett. 2008, 92, 091918. [Google Scholar] [CrossRef]
- Wang, W.H. The elastic properties, elastic models and elastic perspectives of metallic glasses. Prog. Mater. Sci. 2012, 57, 487–656. [Google Scholar] [CrossRef]
- Zhang, Y.; Greer, A.L. Correlations for predicting plasticity or brittleness of metallic glasses. J. Alloys. Compd. 2007, 434–435, 2–5. [Google Scholar] [CrossRef]
- Nagendra, N.; Ramamurty, U.; Goh, T.T.; Li, Y. Effect of crystallinity on the impact toughness of a La-based bulk metallic glass. Acta Mater. 2000, 48, 2603–2615. [Google Scholar] [CrossRef]
- Raghavan, R.; Shastry, V.V.; Kumar, A.; Jayakumar, T.; Ramamurty, U. Toughness of as-cast and partially crystallized composites of a bulk metallic glass. Intermetallics 2009, 17, 835–839. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Yamasaki, T.; Liaw, P.K.; Inoue, A. Study of the structural relaxation-induced embrittlement of hypoeutectic Zr–Cu–Al ternary bulk glassy alloys. Acta Mater. 2008, 56, 6097–6108. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Yamasaki, T.; Nishijima, M.; Inoue, A. Drastic increase in the toughness of structural relaxed hypoeutectic Zr59Cu31Al10 bulk glassy alloy. Mater. Trans. JIM 2007, 48, 1276–1281. [Google Scholar] [CrossRef]
- Madge, S.V. Mg-based Bulk Metallic Glasses. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2003. [Google Scholar]
- Wu, T.; Spaepen, F. The relation between embrittlement and structural relaxation of an amorphous metal. Philos. Mag. B 1990, 61, 739–750. [Google Scholar] [CrossRef]
- Raghavan, R.; Murali, P.; Ramamurty, U. On the factors influencing the ductile-to-brittle transition in a bulk metallic glass. Acta Mater. 2009, 57, 3332–3340. [Google Scholar] [CrossRef]
- Chen, H.S.; Krause, J.T.; Coleman, E. Elastic constants, hardness and their implications to flow properties of metallic glasses. J. Non-Cryst. Solids 1975, 18, 157–171. [Google Scholar] [CrossRef]
- Schroers, J.; Johnson, W.L. Ductile bulk metallic glass. Phys. Rev. Lett. 2004, 93, 255506. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, J.J.; Wang, W.H.; Greer, A.L. Intrinsic plasticity or brittleness of metallic glasses. Philos. Mag. Lett. 2005, 85, 77–87. [Google Scholar] [CrossRef]
- Greaves, G.N.; Greer, A.L.; Lakes, R.S.; Rouxel, T. Poisson’s ratio and modern materials. Nat. Mater. 2011, 10, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Wang, W.H.; Jiang, M.Q.; Zhang, Z.F. Intrinsic factor controlling the deformation and ductile-to-brittle transition of metallic glasses. Philos. Mag. Lett. 2014, 94, 658–668. [Google Scholar] [CrossRef]
- Kelly, A.; Tyson, W.R.; Cottrell, A.H. Ductile and brittle crystals. Philos. Mag. 1967, 15, 567–586. [Google Scholar] [CrossRef]
- Gu, X.J.; McDermott, A.G.; Poon, S.J.; Shiflet, S.J. Critical Poisson’s ratio for plasticity in Fe–Mo–C–B–Ln bulk amorphous steel. Appl. Phys. Lett. 2006, 88, 211905. [Google Scholar] [CrossRef]
- Jia, P.; Zhu, Z.; Ma, E.; Xu, J. Notch toughness of Cu-based bulk metallic glasses. Scr. Mater. 2009, 61, 137–140. [Google Scholar] [CrossRef]
- Kumar, G.; Rector, D.; Conner, R.D.; Schroers, J. Embrittlement of Zr-based bulk metallic glasses. Acta Mater. 2009, 57, 3572–3583. [Google Scholar] [CrossRef]
- Leonhard, A.; Xing, L.Q.; Heilmaier, M.; Gebert, A.; Eckert, J.; Schultz, L. Effect of crystalline precipitates on the mechanical behavior of bulk glass forming Zr-based alloys. Nanostr. Mater. 1998, 10, 805–817. [Google Scholar] [CrossRef]
- Keryvin, V.; Bernard, C.; Sanglebœuf, J.-C.; Yokoyama, Y.; Rouxel, T. Toughness of Zr55Cu30Al10Ni5 bulk metallic glass for two oxygen levels. J. Non-Cryst. Solids 2006, 352, 2863–2868. [Google Scholar] [CrossRef]
- Madge, S.V.; Sharma, P.; Louzguine-Luzgin, D.V.; Greer, A.L.; Inoue, A. Mechanical behaviour of Zr-La-Cu-Ni-Al glass-based composites. Intermetallics 2011, 19, 1474. [Google Scholar] [CrossRef]
- Granata, D.; Fischer, E.; Wessels, V.; Loeffler, J.F. Fluxing of Pd–Si–Cu bulk metallic glass and the role of cooling rate and purification. Acta Mater. 2014, 71, 145–152. [Google Scholar] [CrossRef]
- Kinaka, M.; Kato, H.; Hasegawa, M.; Inoue, A. High specific strength Mg-based bulk metallic glass matrix composite highly ductilized by Ti dispersoid. Mater. Sci. Eng. A 2008, 494, 299–303. [Google Scholar] [CrossRef]
- Madge, S.V.; Sharma, P.; Louzguine-Luzgin, D.V.; Greer, A.L.; Inoue, A. New La-based glass-crystal ex situ composites with enhanced toughness. Scr. Mater. 2010, 62, 210–213. [Google Scholar] [CrossRef]
- Madge, S.V.; Louzguine-Luzgin, D.V.; Inoue, A.; Greer, A.L. Large compressive plasticity in a la-based glass-crystal composite. Metals 2013, 3, 41–48. [Google Scholar] [CrossRef]
- Lee, M.L.; Li, Y.; Schuh, C.A. Effect of a controlled volume fraction of dendritic phases on tensile and compressive ductility in La-based metallic glass matrix composites. Acta Mater. 2004, 52, 4121–4131. [Google Scholar] [CrossRef]
- Lewandowski, J.J. Modern fracture mechanics. Philos. Mag. 2013, 93, 3893–3906. [Google Scholar] [CrossRef]
- Poon, S.J.; Zhu, A.; Shiflet, G.J. Poisson’s ratio and intrinsic plasticity of metallic glasses. Appl. Phys. Lett. 2008, 92, 261902. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, J.; Yuan, F.; Huang, L. Intrinsic ductility of solids. J. Appl. Phys. 2014, 115, 043528. [Google Scholar] [CrossRef]
- Spaepen, F. A microscopic mechanism for steady state inhomogeneous flow in metallic glasses. Acta Metall. 1977, 25, 407–415. [Google Scholar] [CrossRef]
- Pan, D.; Inoue, A.; Sakurai, T.; Chen, M.W. Experimental characterization of shear transformation zones for plastic flow of bulk metallic glasses. Proc. Natl. Acad. Sci. 2008, 105, 14769–14772. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.L.; Samwer, K. A universal criterion for plastic yielding of metallic glasses with a (T/Tg)2/3 temperature dependence. Shear transformation zone volume dertermining ductile-brittle transition of bulk metallic glasses. Phys. Rev. Lett. 2005, 95, 195501. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Jiang, M.Q.; Wang, H.F.; Zhao, Y.L.; He, L.; Sun, J. Shear transformation zone volume determining ductile-brittle transition of bulk metallic glasses. Acta Mater. 2011, 59, 2057–2068. [Google Scholar] [CrossRef]
- Jiang, M.Q.; Wilde, G.; Chen, J.H.; Qu, C.B.; Fu, S.Y.; Jiang, F.; Dai, L.H. Cryogenic-temperature-induced transition from shear to dilatational failure in metallic glasses. Acta Mater. 2014, 77, 248–257. [Google Scholar] [CrossRef]
- Demetriou, M.D.; Kaltenboech, G.; Suh, J.; Garrett, G.; Floyd, M.; Crewdson, C.; Hofmann, D.C.; Kozachkov, H.; Wiest, A.; Schramm, J.P.; et al. Glassy steel optimized for glass-forming ability and toughness. Appl. Phys. Lett. 2009, 95, 041907. [Google Scholar] [CrossRef]
- He, Q.; Cheng, Y.Q.; Ma, E.; Xu, J. Locating bulk metallic glasses with high fracture toughness: Chemical effects and composition optimization. Acta Mater. 2011, 59, 202–215. [Google Scholar] [CrossRef]
- Egami, T. Formation and deformation of metallic glasses: Atomistic theory. Intermetallics 2006, 14, 882–887. [Google Scholar] [CrossRef]
- Badrinarayanan, P.; Zheng, W.; Li, Q.; Simon, S.L. The glass transition temperature versus the fictive temperature. J. Non-Cryst. Solids 2007, 353, 2603–2612. [Google Scholar] [CrossRef]
- Cahn, R.W.; Greer, A.L. Metastable states of alloys. In Physical Metallurgy, 4th ed.; Cahn, R.W., Haasen, P., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1996; Volume 2, pp. 1724–1830. [Google Scholar]
- Kumar, G.; Neibecker, P.; Liu, Y.H.; Schroers, J. Critical fictive temperature for plasticity in metallic glasses. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef]
- Angell, C.A. Formation of glasses from liquids and biopolymers. Science 1995, 267, 1924–1935. [Google Scholar] [CrossRef] [PubMed]
- Busch, R.; Liu, W.; Johnson, W.L. Thermodynamics and kinetics of the Mg65Cu25Y10 bulk metallic glass forming liquid. J. Appl. Phys. 1998, 83, 4134–4141. [Google Scholar] [CrossRef]
- Bossuyt, S.; Madge, S.V.; Chen, G.Z.; Castellero, A.; Deledda, S.; Eckert, J.; Fray, D.J.; Greer, A.L. Electrochemical removal of oxygen for processing glass-forming alloys. Mater. Sci. Eng. A 2004, 375–377, 240–243. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madge, S.V. Toughness of Bulk Metallic Glasses. Metals 2015, 5, 1279-1305. https://doi.org/10.3390/met5031279
Madge SV. Toughness of Bulk Metallic Glasses. Metals. 2015; 5(3):1279-1305. https://doi.org/10.3390/met5031279
Chicago/Turabian StyleMadge, Shantanu V. 2015. "Toughness of Bulk Metallic Glasses" Metals 5, no. 3: 1279-1305. https://doi.org/10.3390/met5031279





