Effect of Indium Content on the Microstructure, Mechanical Properties and Corrosion Behavior of Titanium Alloys
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Ti-xIn Alloys
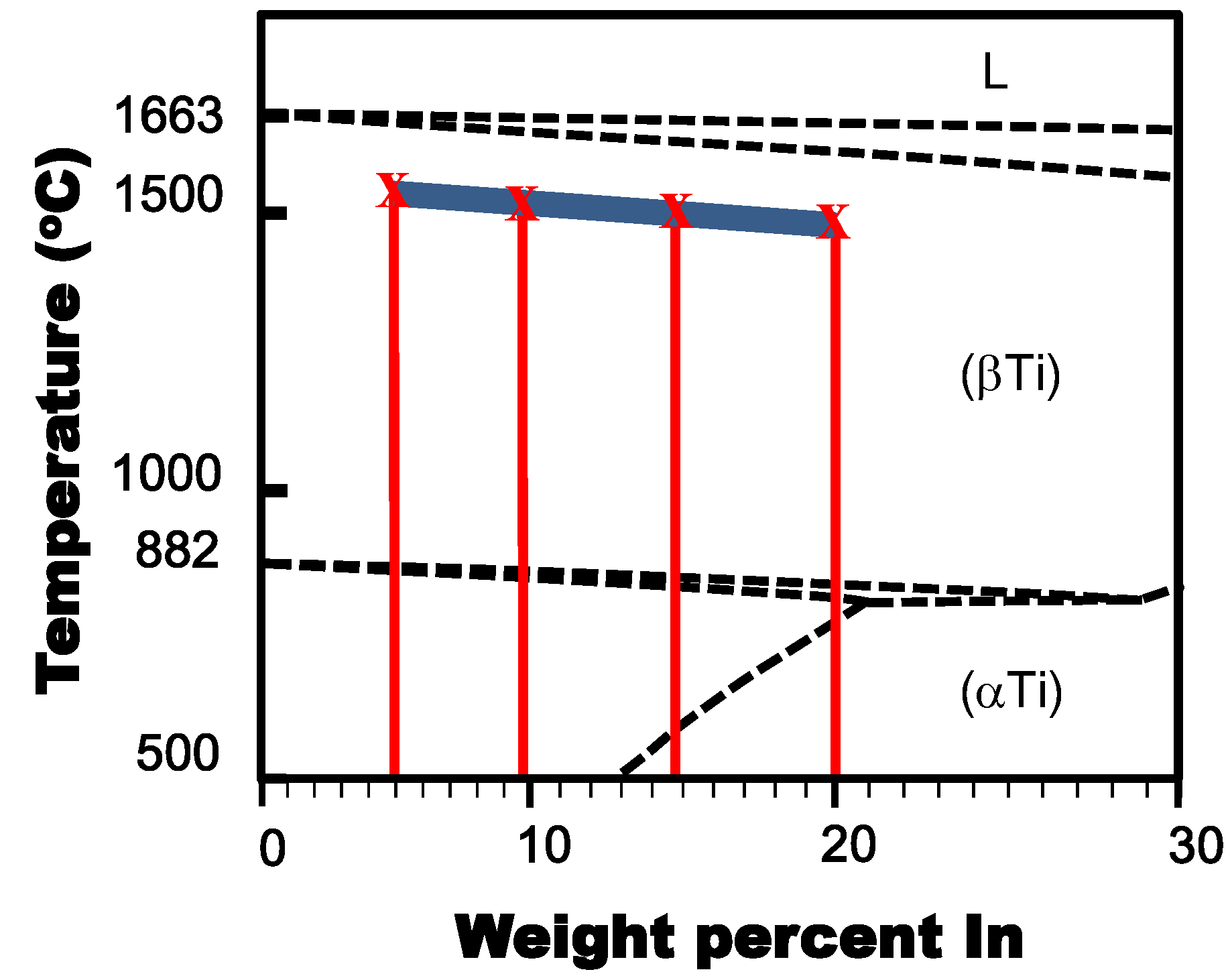
2.2. Material Characterization
2.3. Measurement of Mechanical Properties
2.4. Electrochemical Analysis
3. Results and Discussion
3.1. Phase and Microstructure
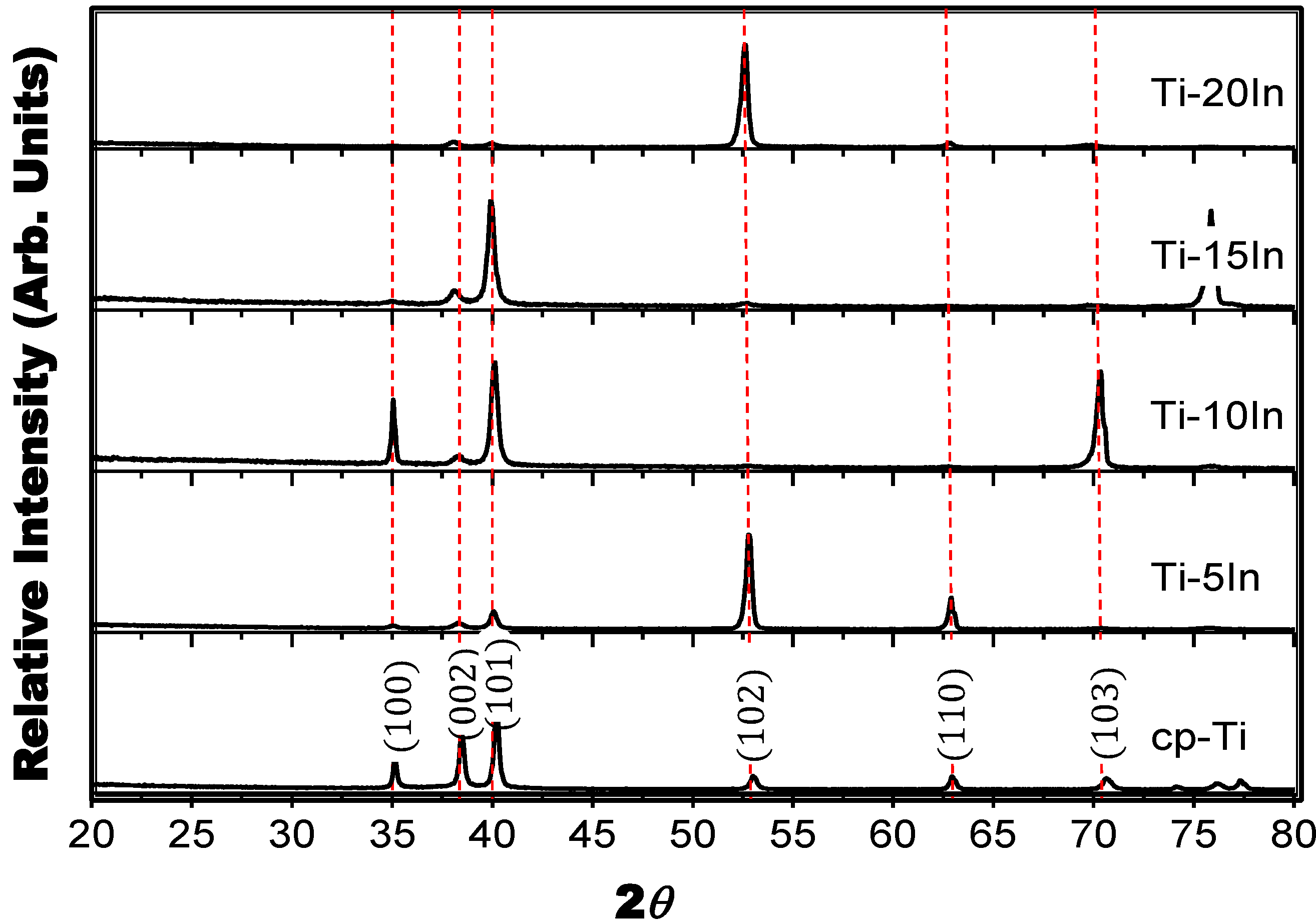
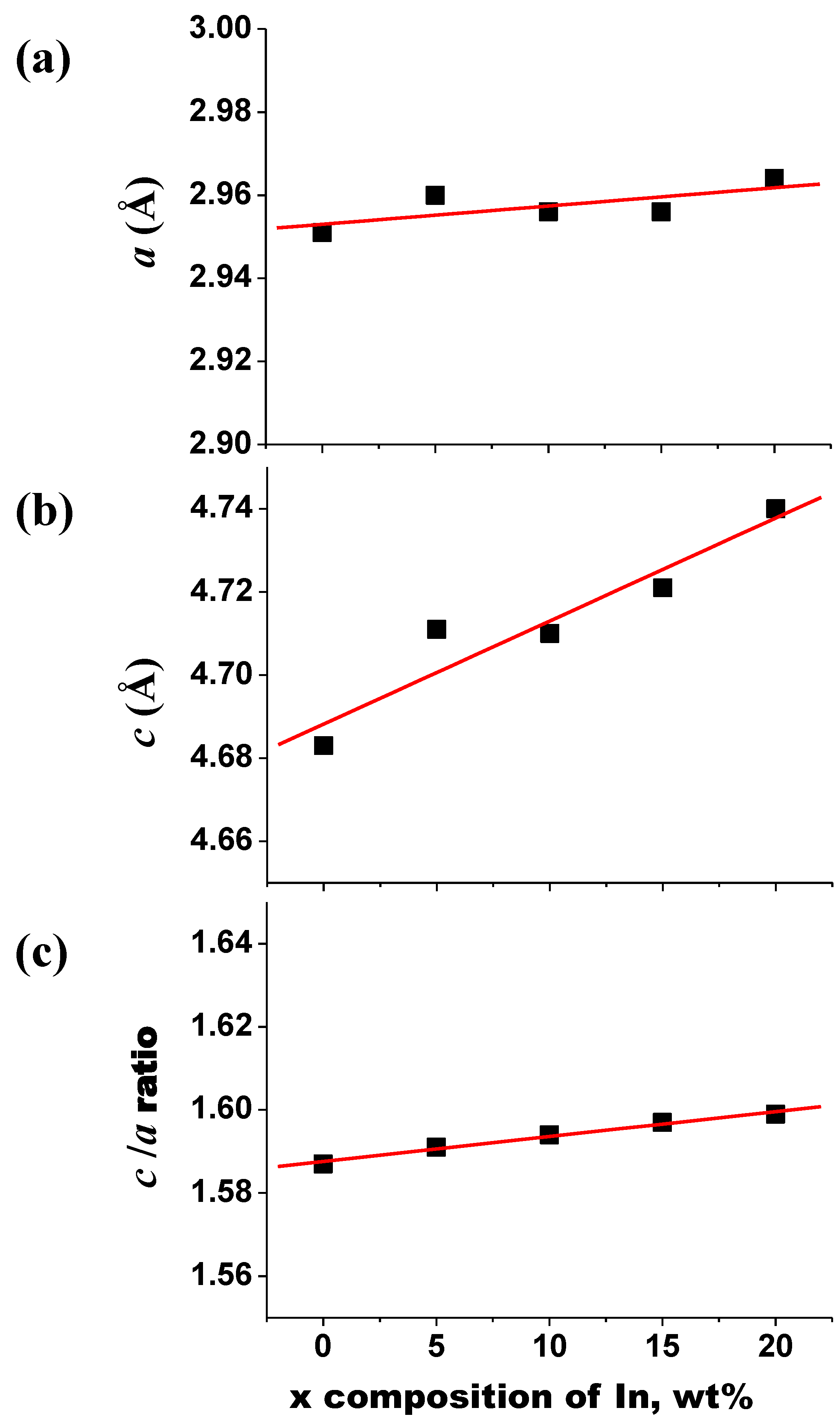


3.2. Mechanical Properties
| Alloy code | Hardness (VHN) | Elastic modulus (GPa) |
|---|---|---|
| cp-Ti | 164.54 (3.54) *,a | 132.35 (12.22) a |
| Ti-5In | 402.60 (128.12) b | 159.80 (2.57) c |
| Ti-10In | 522.40 (61.03) b | 124.10 (4.61) a |
| Ti-15In | 303.20 (89.38) c | 141.10 (4.01) b |
| Ti-20In | 332.00 (46.80) c | 124.43 (4.69) a |


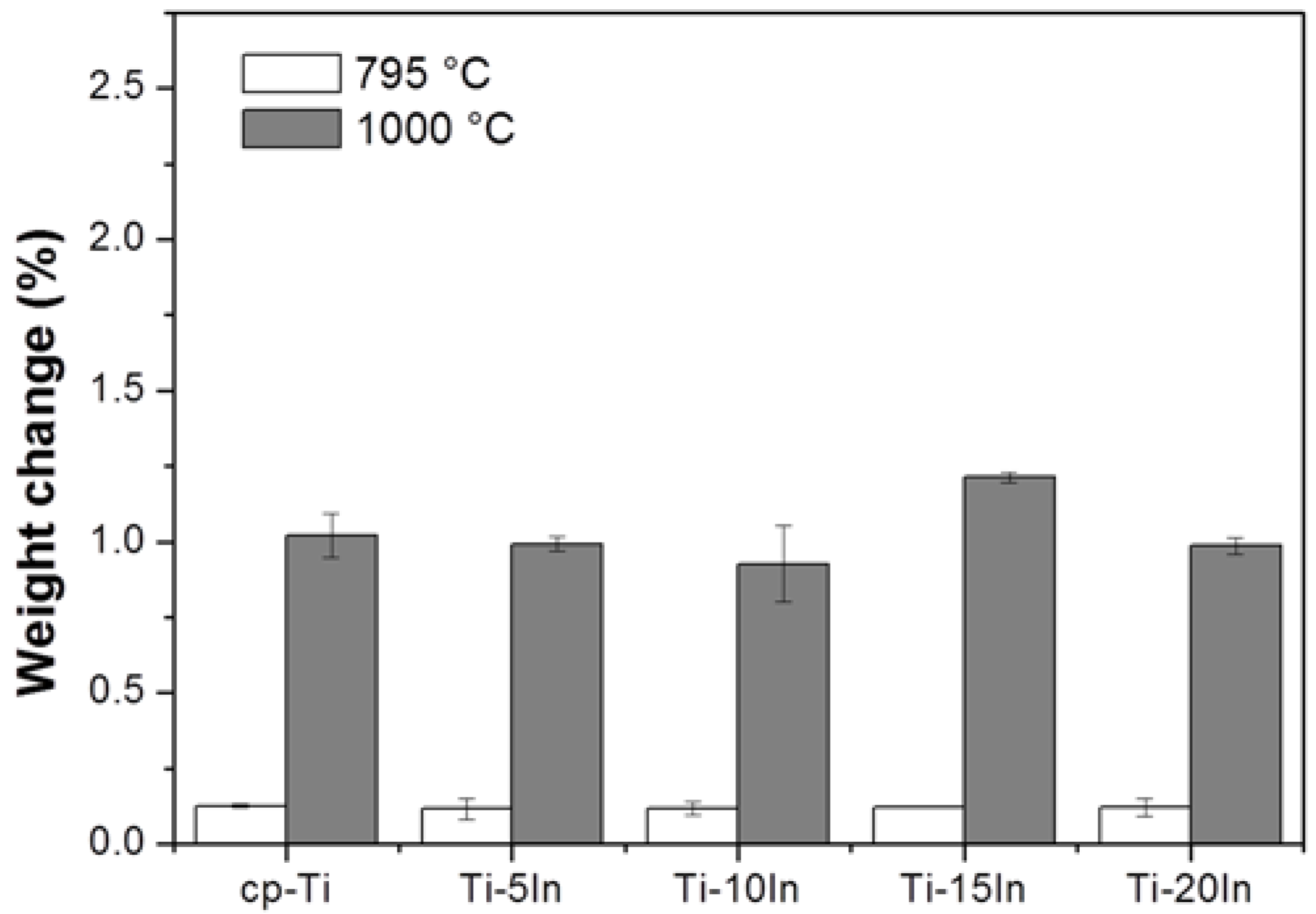
3.3. Corrosion Behavior
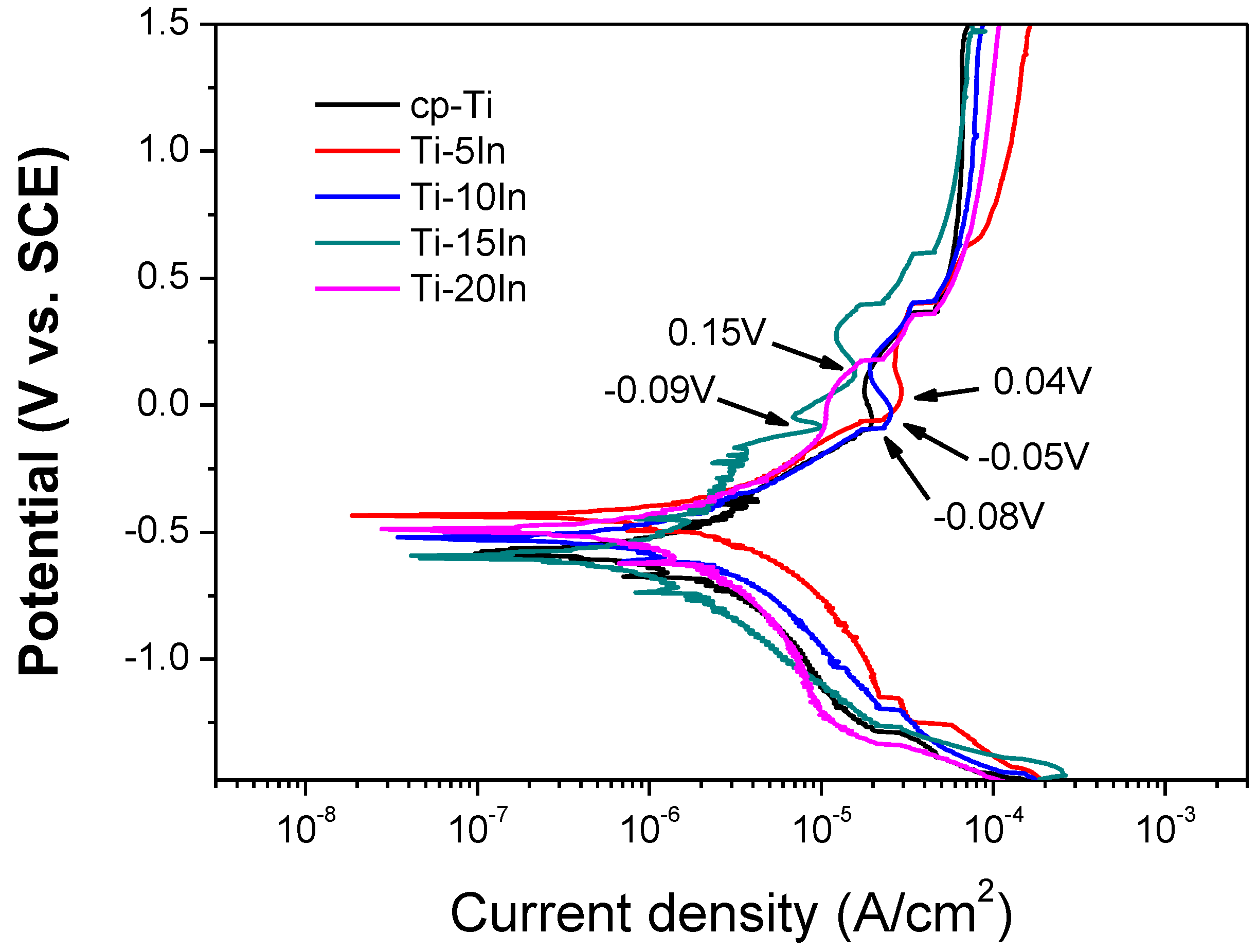
| Alloy code | Ecorr (±SD) (V) | Icorr (±SD) (μA/cm2) |
|---|---|---|
| cp-Ti | −0.58 (0.04) *,a | 0.61 (0.17) a,b |
| Ti-5In | −0.49 (0.05) a | 0.73 (0.36) b |
| Ti-10In | −0.59 (0.07) a | 0.42 (0.09) a,b |
| Ti-15In | −0.58 (0.12) a | 0.35 (0.02) a |
| Ti-20In | −0.54 (0.05) a | 0.44 (0.05) a,b |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Leyens, C.; Peters, M. Titanium and Titanium Aloys; Wiley Online Library: Weinheim, Germany, 2003. [Google Scholar]
- Lloyd, C.; Scrimgeour, S.; Brown, D.; Clarke, R.; Curtis, R.; Hatton, P.; Ireland, A.; McCabe, J.; Nicholson, J.; Setcos, J. Dental materials: 1995 literature review. J. Dent. 1997, 25, 173–208. [Google Scholar] [CrossRef] [PubMed]
- Ezugwu, E.; Wang, Z. Titanium alloys and their machinability—A review. J. Mater. Process. Technol. 1997, 68, 262–274. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Lee, C.; Ju, C.-P.; Chern Lin, J. Structure—Property relationship of cast Ti-Nb alloys. J. Oral Rehabil. 2002, 29, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Takahashi, M.; Okuno, O. Mechanical properties and grindability of dental cast Ti-Nb alloys. Dent. Mater. J. 2003, 22, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, B.; Qiu, K.; Lin, W.; Li, L.; Wang, Y.; Nie, F.; Zheng, Y. Microstructure, corrosion behavior and cytotoxicity of Zr–Nb alloys for biomedical application. Mater. Sci. Eng. C 2012, 32, 851–857. [Google Scholar] [CrossRef]
- Oh, K.-T.; Shim, H.-M.; Kim, K.-N. Properties of titanium-silver alloys for dental application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 74, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qiu, K.; Wang, B.; Li, L.; Zheng, Y. Surface characterization and cell response of binary ti-ag alloys with cp ti as material control. J. Mater. Sci. Technol. 2012, 28, 779–784. [Google Scholar] [CrossRef]
- Takahashi, M.; Kikuchi, M.; Okuno, O. Mechanical properties and grindability of experimental Ti-Au alloys. Dent. Mater. J. 2004, 23, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Takahashi, M.; Sato, H.; Okuno, O.; Nunn, M.E.; Okabe, T. Grindability of cast Ti–Hf alloys. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 77, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Weidmann, A.; Nebe, B.; Burkel, E. Preparation of TiMn alloy by mechanical alloying and spark plasma sintering for biomedical applications. J. Phys. Conf. Ser. 2009, 012007. [Google Scholar]
- Hsu, H.-C.; Wu, S.-C.; Chiang, T.-Y.; Ho, W.-F. Structure and grindability of dental Ti–Cr alloys. J. Alloys Compd. 2009, 476, 817–825. [Google Scholar] [CrossRef]
- Ho, W.-F.; Chiang, T.-Y.; Wu, S.-C.; Hsu, H.-C. Mechanical properties and deformation behavior of cast binary Ti–Cr alloys. J. Alloys Compd. 2009, 468, 533–538. [Google Scholar] [CrossRef]
- Ho, W.; Ju, C.; Chern Lin, J. Structure and properties of cast binary Ti–Mo alloys. Biomaterials 1999, 20, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-C.; Lin, H.-C.; Wu, S.-C.; Hong, Y.-S.; Ho, W.-F. Microstructure and grindability of as-cast Ti–Sn alloys. J. Mater. Sci. 2010, 45, 1830–1836. [Google Scholar] [CrossRef]
- Ho, W.-F.; Chen, W.-K.; Wu, S.-C.; Hsu, H.-C. Structure, mechanical properties, and grindability of dental Ti–Zr alloys. J. Mater. Sci. Mater. Med. 2008, 19, 3179–3186. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-C.; Wu, S.-C.; Hsu, S.-K.; Sung, Y.-C.; Ho, W.-F. Effects of heat treatments on the structure and mechanical properties of Zr–30Ti alloys. Mater. Charact. 2011, 62, 157–163. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Wu, S.-C.; Sung, Y.-C.; Ho, W.-F. The structure and mechanical properties of as-cast Zr-Ti alloys. J. Alloys Compd. 2009, 488, 279–283. [Google Scholar] [CrossRef]
- Mareci, D.; Chelariu, R.; Gordin, D.-M.; Ungureanu, G.; Gloriant, T. Comparative corrosion study of Ti–Ta alloys for dental applications. Acta Biomater. 2009, 5, 3625–3639. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-L.; Niinomi, M. Ti–25Ta alloy with the best mechanical compatibility in Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. C 2009, 29, 1061–1065. [Google Scholar] [CrossRef]
- Wang, R.; Welsch, G. Evaluation of an experimental Ti-Co alloy for dental restorations. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.; Lin, J.C.; Taira, M.; Greener, E. Development of dental Pd−Ti alloys. Dent. Mater. 1985, 1, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-J.; Wang, B.-L.; Qiu, K.-J.; Zhou, F.-Y.; Li, L.; Lin, J.-P.; Wang, Y.-B.; Zheng, Y.-F. Ti–Ge binary alloy system developed as potential dental materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2239–2250. [Google Scholar] [CrossRef] [PubMed]
- Okabe, T.; Kikuchi, M.; Ohkubo, C.; Koike, M.; Okuno, O.; Oda, Y. The grindability and wear of Ti-Cu alloys for dental applications. JOM 2004, 56, 46–48. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Lin, J.; Zheng, Y. Development and properties of Ti-In binary alloys as dental biomaterials. Mater. Sci. Eng. C 2012, 33, 1601–1606. [Google Scholar] [CrossRef]
- Kim, T.-I.; Han, J.-H.; Lee, I.-S.; Lee, K.-H.; Shin, M.-C.; Choi, B.-B. New titanium alloys for biomaterials: A study of mechanical and corrosion properties and cytotoxicity. Bio-Med. Mater. Eng. 1997, 7, 253–263. [Google Scholar]
- Gulay, L.; Schuster, J. Investigation of the titanium–indium system. J. Alloys Compd. 2003, 360, 137–142. [Google Scholar] [CrossRef]
- Hunter, B.A.; Howard, C.J. Australian Nuclear Science and Technology Organization. Available online: ftp://ftp.ansto.gov.au/pub/physics/neutron/rietveld/Rietica_LHPM95/MANUAL.PDF (accessed on 18 May 2015).
- ASTM International. Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements; ASTM G5-94; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Huheey, J.E.; Keiter, E.A.K.; Keiter, R.L.; Medhi, O.K. Inorganic Chemistry: Principles of Structure and Reactivity; Harper & Row: New York, NY, USA, 1983. [Google Scholar]
- Joint Committee on Powder Diffraction Standards (JCPDS)-International Centre for Diffraction Data (ICDD). Card No. 44-1294; Joint Committee on Powder Diffraction Standards (JCPDS)-International Centre for Diffraction Data (ICDD): Swarthmore, PA, USA, 1995. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.-K.; Im, J.-B.; Hwang, M.-J.; Kim, B.-J.; Kim, H.-Y.; Park, Y.-J. Effect of Indium Content on the Microstructure, Mechanical Properties and Corrosion Behavior of Titanium Alloys. Metals 2015, 5, 850-862. https://doi.org/10.3390/met5020850
Han M-K, Im J-B, Hwang M-J, Kim B-J, Kim H-Y, Park Y-J. Effect of Indium Content on the Microstructure, Mechanical Properties and Corrosion Behavior of Titanium Alloys. Metals. 2015; 5(2):850-862. https://doi.org/10.3390/met5020850
Chicago/Turabian StyleHan, Mi-Kyung, Jae-Bong Im, Moon-Jin Hwang, Bong-Jun Kim, Hae-Young Kim, and Yeong-Joon Park. 2015. "Effect of Indium Content on the Microstructure, Mechanical Properties and Corrosion Behavior of Titanium Alloys" Metals 5, no. 2: 850-862. https://doi.org/10.3390/met5020850





