Influence of the Applied External Magnetic Field on the Deposition of Ni–Cu Alloys
Abstract
:1. Introduction
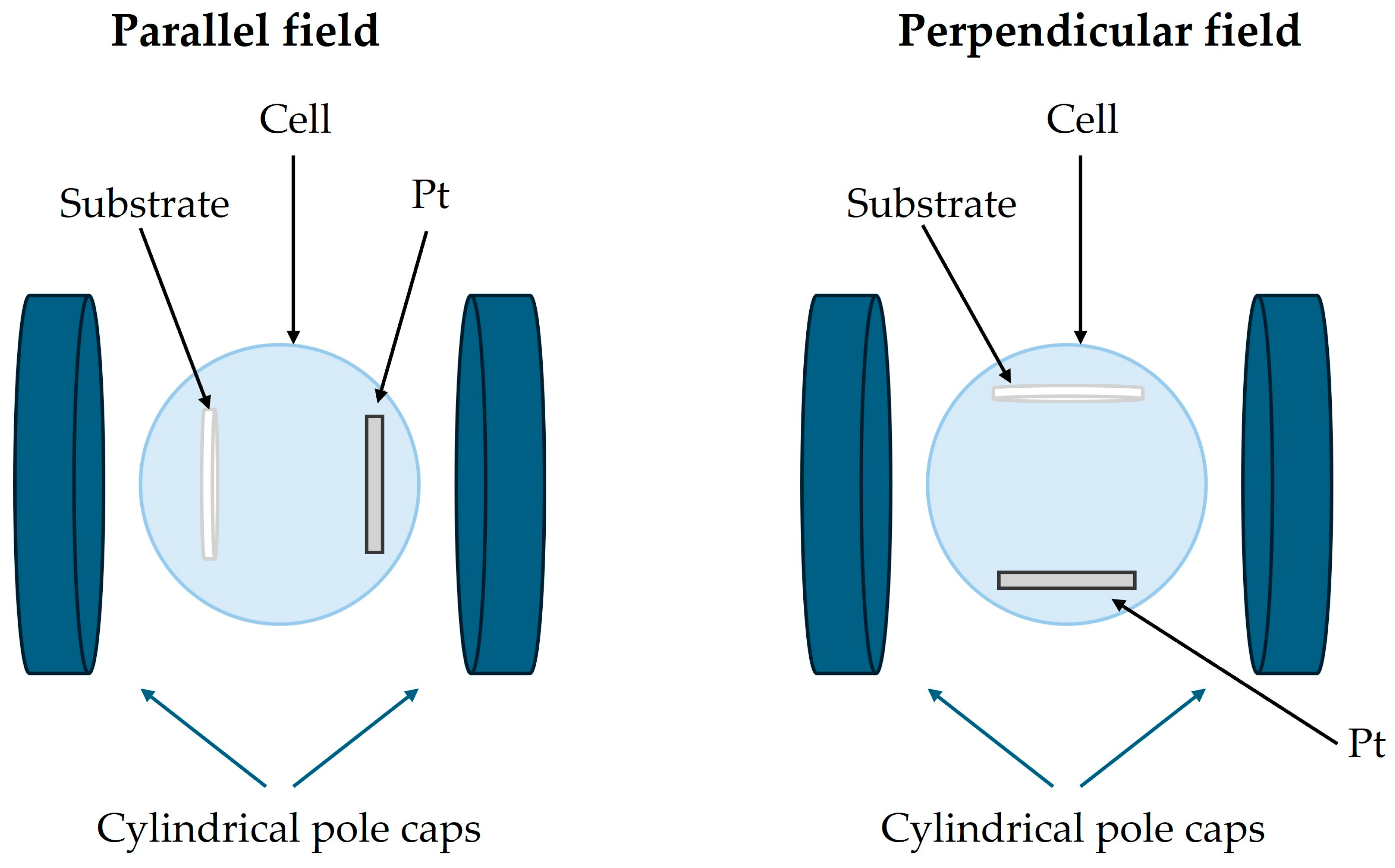
2. Materials and Methods
3. Results
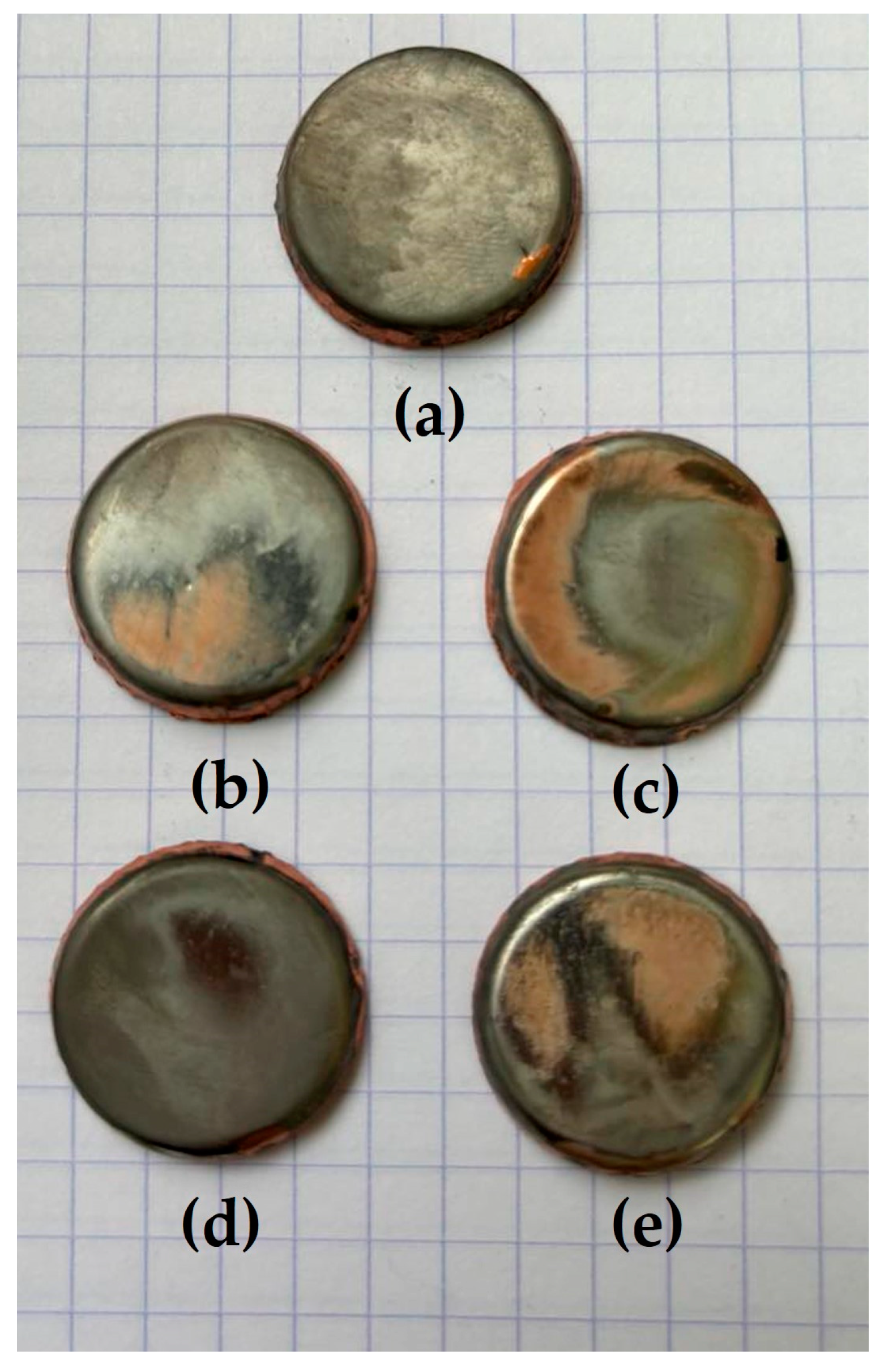
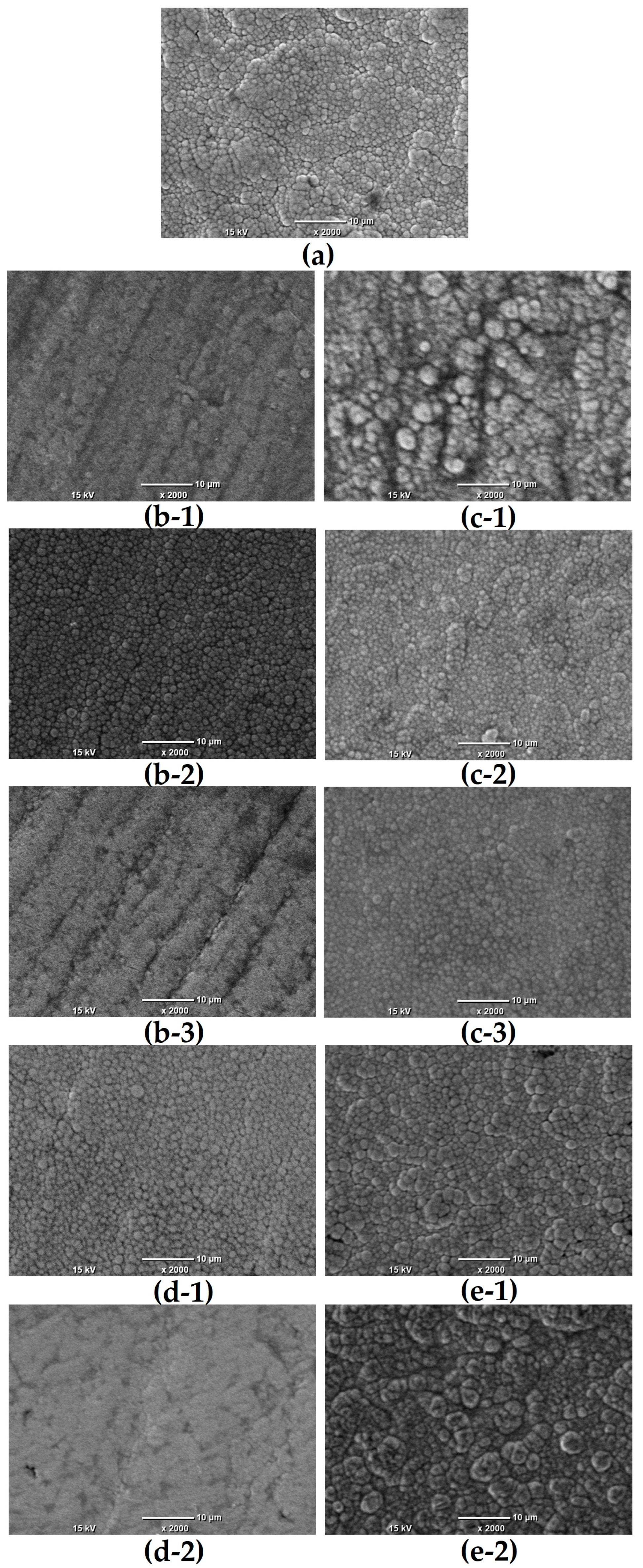
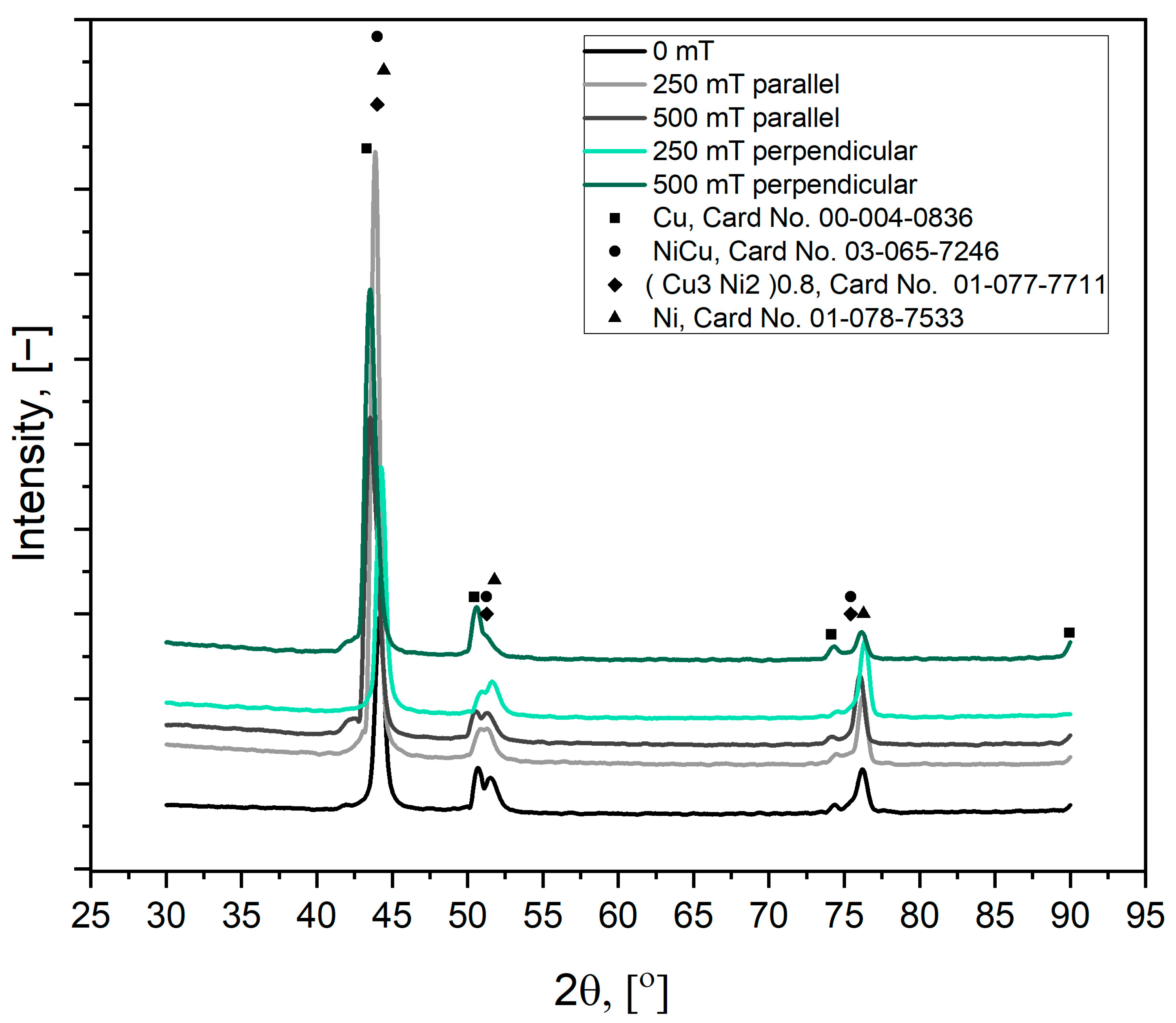
3.1. Morphology and Composition
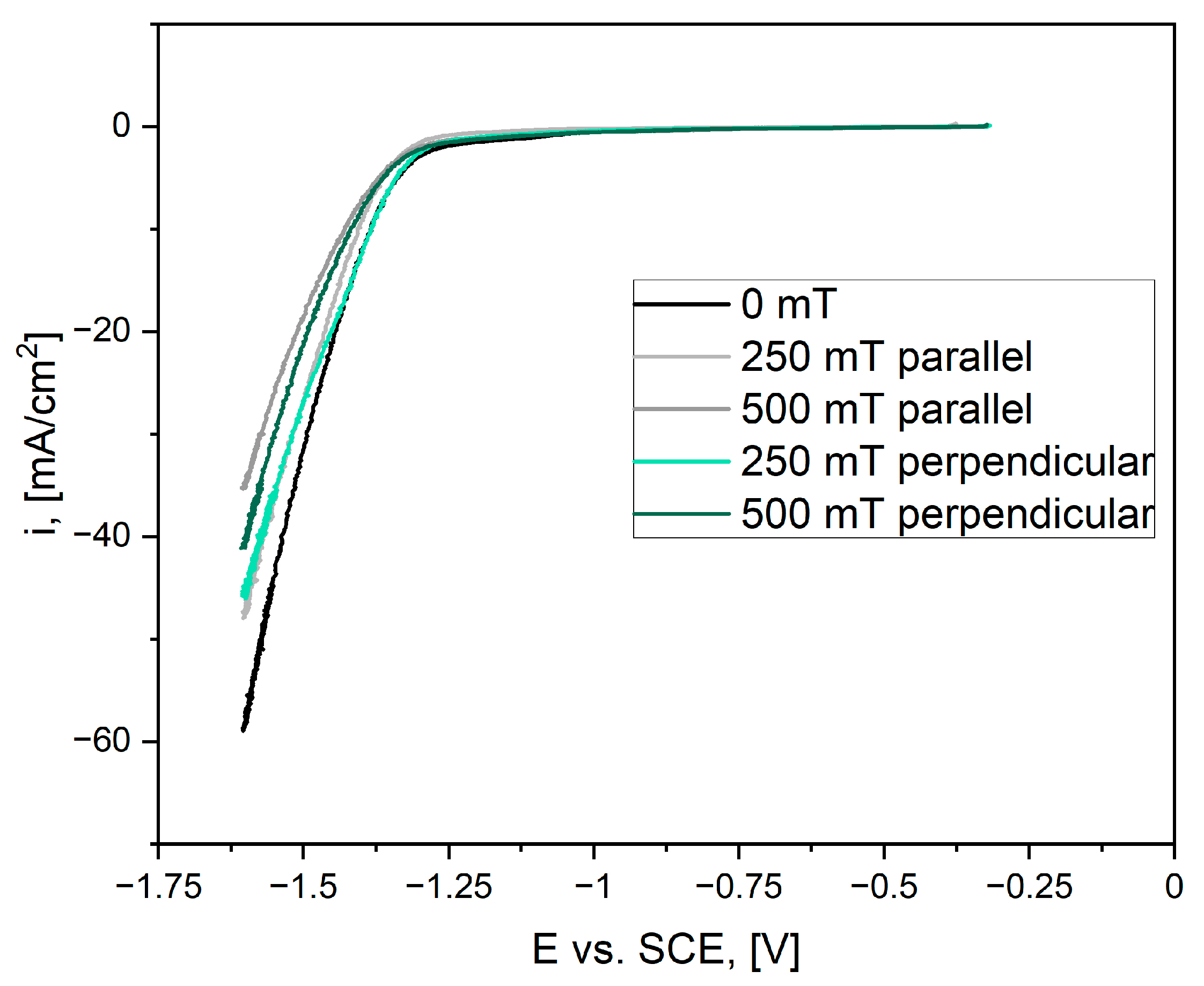
3.2. Catalytic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sarac, U.; Öksüzoğlu, R.M.; Baykul, M.C. Deposition potential dependence of composition, microstructure, and surface morphology of electrodeposited Ni–Cu alloy films. J. Mater. Sci. Mater. Electron. 2012, 23, 2110–2116. [Google Scholar] [CrossRef]
- Chassaing, E.; Quang, K.V.; Wiart, R. Mechanism of copper-nickel alloy electrodeposition. J. Appl. Electrochem. 1987, 17, 1267–1280. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Yang, H.; Dai, J.; Zhu, R.; Gong, J.; Peng, L.; Ding, W. Electrodeposition mechanism and characterization of Ni–Cu alloy coatings from a eutectic-based ionic liquid. Appl. Surf. Sci. 2014, 288, 530–536. [Google Scholar] [CrossRef]
- Staroń, S.; Ledwig, P.; Dubiel, B. Electrodeposited Ni-Cu Coatings with Hierarchical Surface Morphology. Metall. Mater. Trans. A 2022, 53, 2071–2085. [Google Scholar] [CrossRef]
- Goranova, D.; Avdeev, G.; Rashkov, R. Electrodeposition and characterization of Ni-Cu alloys. Surf. Coat. Technol. 2014, 240, 204–210. [Google Scholar] [CrossRef]
- Ngamlerdpokin, K.; Tantavichet, N. Electrodeposition of nickel-copper alloys to use as a cathode for hydrogen evolution in an alkaline media. Int. J. Hydrog. Energy 2014, 39, 2505–2515. [Google Scholar] [CrossRef]
- Brito, M.M.; Artisiani, R.A.; Carlos, I.A. The electrodeposition of Ni-Cu and Ni-Cu-P from aspartate-based baths. J. Alloys Compd. 2022, 890, 161761. [Google Scholar] [CrossRef]
- Kockar, H.; Bayirli, M.; Alper, M. A new example of the diffusion-limited aggregation: Ni–Cu film patterns. Appl. Surf. Sci. 2010, 256, 2995–2999. [Google Scholar] [CrossRef]
- Alper, M.; Baykul, M.C.; Péter, L.; Tóth, J.; Bakonyi, İ. Preparation and Characterisation of Electrodeposited Ni–Cu/Cu Multilayers. J. Appl. Electrochem. 2004, 34, 841–848. [Google Scholar] [CrossRef]
- Myung, N.V.; Nobe, K. Electrodeposition of Ni/Cu multilayers. Trade J. 2000, 87, 125–134. [Google Scholar]
- Basori; Soegijono, B.; Susetyo, F.B. Magnetic field exposure on electroplating process of ferromagnetic nickel ion on copper substrate. J. Phys. Conf. Ser. 2022, 2377, 012002. [Google Scholar] [CrossRef]
- Bund, A.; Ispas, A.; Mutschke, G. Magnetic field effects on electrochemical metal depositions. Sci. Technol. Adv. Mater. 2008, 9, 024208. [Google Scholar] [CrossRef]
- Kołczyk-Siedlecka, K.; Kutyła, D.; Skibińska, K.; Jędraczka, A.; Żabiński, P. Catalytic Properties of Electroless Nickel-Based Coatings Modified by the Magnetic Field. Arch. Met. Mater 2024, 69, 17–24. [Google Scholar]
- Kołczyk-Siedlecka, K.; Skibińska, K.; Kutyła, D.; Kwiecińska, A.; Kowalik, R.; Żabiński, P. Influence of magnetic field on electroless metallization of 3D prints by copper and nickel. Arch. Metall. Mater. 2019, 64, 17–22. [Google Scholar] [CrossRef]
- Kovalyov, S.V.; Girin, O.B.; Debiemme-Chouvy, C.; Mishchenko, V.I. Copper electrodeposition under a weak magnetic field: Effect on the texturing and properties of the deposits. J. Appl. Electrochem. 2021, 51, 235–243. [Google Scholar] [CrossRef]
- Dobosz, I.; Kutyła, D.; Kac, M.; Włoch, G.; Żabiński, P. The influence of homogenous external magnetic field on morphology and magnetic properties of CoRu nanowire arrays. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 262, 114795. [Google Scholar] [CrossRef]
- Hang, T.; Hu, A.; Li, M.; Mao, D. Structural control of a cobalt nanocone array grown by directional electrodeposition. CrystEngComm 2010, 12, 2799–2802. [Google Scholar] [CrossRef]
- Huang, M.; Weber, N.; Mutschke, G. A Simulation Framework for Electrochemical Processes with Electrolyte Flow. J. Electrochem. Soc. 2023, 170, 073502. [Google Scholar] [CrossRef]
- Huang, M.; Uhlemann, M.; Eckert, K.; Mutschke, G. Pulse Reverse Plating of Copper Micro-Structures in Magnetic Gradient Fields. Magnetochemistry 2022, 8, 66. [Google Scholar] [CrossRef]
- Liang, P.; Li, Q.; Chen, L.; Tang, Z.; Li, Z.; Wang, Y.; Tang, Y.; Han, C.; Lan, Z.; Zhi, C.; et al. The magnetohydrodynamic effect enables a dendrite-free Zn anode in alkaline electrolytes. J. Mater. Chem. A 2022, 10, 11971–11979. [Google Scholar] [CrossRef]
- Bau, H.H. Applications of Magneto Electrochemistry and Magnetohydrodynamics in Microfluidics. Magnetochemistry 2022, 8, 140. [Google Scholar] [CrossRef]
- Mitra, K.; Adalder, A.; Mandal, S.; Ghorai, U.K. Enhancing Electrochemical Reactivity with Magnetic Fields: Unraveling the Role of Magneto-Electrochemistry. Small Methods 2024, e2301132. [Google Scholar] [CrossRef]
- Luo, S.; Elouarzaki, K.; Xu, Z.J. Electrochemistry in Magnetic Fields. Angew. Chemie Int. Ed. 2022, 61, e202203564. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, M.; Guo, M.; Hong, A.; Yu, T.; Luo, X.; Yuan, C.; Lei, W.; Wang, S. Magnetic Enhancement for Hydrogen Evolution Reaction on Ferromagnetic MoS 2 Catalyst. Nano Lett. 2020, 20, 2923–2930. [Google Scholar] [CrossRef]
- Sambalova, O.; Billeter, E.; Yildirim, O.; Sterzi, A.; Bleiner, D.; Borgschulte, A. Magnetic field enhancement of electrochemical hydrogen evolution reaction probed by magneto-optics. Int. J. Hydrogen Energy 2021, 46, 3346–3353. [Google Scholar] [CrossRef]
- Salinas, G.; Lozon, C.; Kuhn, A. Unconventional applications of the magnetohydrodynamic effect in electrochemical systems. Curr. Opin. Electrochem. 2023, 38, 101220. [Google Scholar] [CrossRef]
- Deng, Y.; Lai, W.; Xu, B. A Mini Review on Doped Nickel-Based Electrocatalysts for Hydrogen Evolution Reaction. Energies 2020, 13, 4651. [Google Scholar] [CrossRef]
- Angeles-Olvera, Z.; Crespo-Yapur, A.; Rodríguez, O.; Cholula-Díaz, J.; Martínez, L.; Videa, M. Nickel-Based Electrocatalysts for Water Electrolysis. Energies 2022, 15, 1609. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, Y.; Cai, W.; Xia, T.; Jiang, W.-J.; Ding, W.; Cao, J.-P. Boosting hydrogen evolution activity and durability of Pd–Ni–P nanocatalyst via crystalline degree and surface chemical state modulations. Int. J. Hydrogen Energy 2019, 44, 31053–31061. [Google Scholar] [CrossRef]
- Mech, K.; Wróbel, M.; Wojnicki, M.; Mech-Piskorz, J.; Żabiński, P.; Kowalik, R. Electrodeposition of NiPd alloy from aqueous chloride electrolytes. Appl. Surf. Sci. 2016, 388, 809–816. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, X.; Zuo, Y.; Ju, P.; Tang, Y. Electrodeposited Pd-Ni-Mo film as a cathode material for hydrogen evolution reaction. Electrochim. Acta 2015, 174, 1041–1049. [Google Scholar] [CrossRef]
- Fukumoto, M.; Takahashi, H.; Kutyła, D.; Wojnicki, M.; Żabiński, P. Morphological investigation and electrochemical performance evaluation of novel porous Ni–Pt produced by Al-deposition/dissolution in molten salts for hydrogen and oxygen evolution reaction. Int. J. Hydrogen Energy 2023, 49, 754–765. [Google Scholar] [CrossRef]
- Fiameni, S.; Herraiz-Cardona, I.; Musiani, M.; Pérez-Herranz, V.; Vázquez-Gómez, L.; Verlato, E. The HER in alkaline media on Pt-modified three-dimensional Ni cathodes. Int. J. Hydrogen Energy 2012, 37, 10507–10516. [Google Scholar] [CrossRef]
- Eiler, K.; Krawiec, H.; Kozina, I.; Sort, J.; Pellicer, E. Electrochemical characterisation of multifunctional electrocatalytic mesoporous Ni-Pt thin films in alkaline and acidic media. Electrochim. Acta 2020, 359, 136952. [Google Scholar] [CrossRef]
- Kutyła, D.; Kołczyk-Siedlecka, K.; Kwiecińska, A.; Skibińska, K.; Kowalik, R.; Żabiński, P. Preparation and characterization of electrodeposited Ni-Ru alloys: Morphological and catalytic study. J. Solid State Electrochem. 2019, 23, 3089–3097. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Fo, Y.; Zhang, B.; Molochas, C.; Gao, J.; Li, W.; Cui, X.; Zhou, X.; Jiang, L.; et al. Engineering of unique Ni-Ru nano-twins for highly active and robust bifunctional hydrogen oxidation and hydrogen evolution electrocatalysis. Chem. Eng. J. 2023, 454, 139959. [Google Scholar] [CrossRef]
- Mao, J.; He, C.-T.; Pei, J.; Liu, Y.; Li, J.; Chen, W.; He, D.; Wang, D.; Li, Y. Isolated Ni Atoms Dispersed on Ru Nanosheets: High-Performance Electrocatalysts toward Hydrogen Oxidation Reaction. Nano Lett. 2020, 20, 3442–3448. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jin, P.; Liu, B.; Zhao, L.; Cai, J.; Wei, Z.; Zuo, S.; Zhang, J.; Feng, L. Ultrafine carbon encapsulated NiRu alloys as bifunctional electrocatalysts for boosting overall water splitting: Morphological and electronic modulation through minor Ru alloying. J. Mater. Chem. A 2020, 8, 9049–9057. [Google Scholar] [CrossRef]
- Kutyła, D.; Salcı, A.; Kwiecińska, A.; Kołczyk-Siedlecka, K.; Kowalik, R.; Żabiński, P.; Solmaz, R. Catalytic activity of electrodeposited ternary Co–Ni–Rh thin films for water splitting process. Int. J. Hydrogen Energy 2020, 45, 34805–34817. [Google Scholar] [CrossRef]
- Skibińska, K.; Kutyła, D.; Yang, X.; Krause, L.; Marzec, M.M.; Żabiński, P. Rhodium-decorated nanoconical nickel electrode synthesis and characterization as an electrochemical active cathodic material for hydrogen production. Appl. Surf. Sci. 2022, 592, 153326. [Google Scholar] [CrossRef]
- Nguyen, N.-A.; Choi, H.-S. Effect of Ni/Rh ratios on characteristics of NixRhy nanosponges towards high-performance hydrogen evolution reaction. Data Br. 2019, 24, 103941. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.-A.; Nguyen, V.-T.; Shin, S.; Choi, H.-S. NiRh nanosponges with highly efficient electrocatalytic performance for hydrogen evolution reaction. J. Alloys Compd. 2019, 789, 163–173. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Chang, Y.; Lu, Y.; Zhao, S.; Xu, D.; Dai, Z.; Han, M.; Bao, J. Component-Controlled Synthesis of Necklace-Like Hollow Ni X Ru y Nanoalloys as Electrocatalysts for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 17326–17336. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.J.; Franceschini, E.A.; Lacconi, G.I. Ni and Ni x Co y Alloys Electrodeposited on Stainless Steel AISI 316L for Hydrogen Evolution Reaction. Electrocatalysis 2018, 9, 459–470. [Google Scholar] [CrossRef]
- González-Buch, C.; Herraiz-Cardona, I.; Ortega, E.; García-Antón, J.; Pérez-Herranz, V. Synthesis and characterization of macroporous Ni, Co and Ni–Co electrocatalytic deposits for hydrogen evolution reaction in alkaline media. Int. J. Hydrogen Energy 2013, 38, 10157–10169. [Google Scholar] [CrossRef]
- Lupi, C.; Dell’Era, A.; Pasquali, M. Nickel–cobalt electrodeposited alloys for hydrogen evolution in alkaline media. Int. J. Hydrogen Energy 2009, 34, 2101–2106. [Google Scholar] [CrossRef]
- Vernickaite, E.; Tsyntsaru, N.; Sobczak, K.; Cesiulis, H. Electrodeposited tungsten-rich Ni-W, Co-W and Fe-W cathodes for efficient hydrogen evolution in alkaline medium. Electrochim. Acta 2019, 318, 597–606. [Google Scholar] [CrossRef]
- Żabiński, P.; Mech, K.; Kowalik, R. Electrocatalytically active Co–W and Co–W–C alloys electrodeposited in a magnetic field. Electrochim. Acta 2013, 104, 542–548. [Google Scholar] [CrossRef]
- Laszczyńska, A. Electrodeposited alloy electrodes in the Co–W–Mo system as highly efficient catalysts for hydrogen production from alkaline water electrolysis. Mater. Chem. Phys. 2024, 314, 128876. [Google Scholar] [CrossRef]
- Laszczyńska, A.; Szczygieł, I. Electrocatalytic activity for the hydrogen evolution of the electrodeposited Co–Ni–Mo, Co–Ni and Co–Mo alloy coatings. Int. J. Hydrogen Energy 2020, 45, 508–520. [Google Scholar] [CrossRef]
- Goranova, D.; Lefterova, E.; Rashkov, R. Electrocatalytic activity of Ni-Mo-Cu and Ni-Co-Cu alloys for hydrogen evolution reaction in alkaline medium. Int. J. Hydrogen Energy 2017, 42, 28777–28785. [Google Scholar] [CrossRef]
- Negem, M.; Nady, H. Electroplated Ni-Cu nanocrystalline alloys and their electrocatalytic activity for hydrogen generation using alkaline solutions. Int. J. Hydrogen Energy 2017, 42, 28386–28396. [Google Scholar] [CrossRef]
- Cui, X.; Xiao, P.; Wang, J.; Zhou, M.; Guo, W.; Yang, Y.; He, Y.; Wang, Z.; Yang, Y.; Zhang, Y.; et al. Highly Branched Metal Alloy Networks with Superior Activities for the Methanol Oxidation Reaction. Angew. Chemie Int. Ed. 2017, 56, 4488–4493. [Google Scholar] [CrossRef]
- Koza, J.A.; Uhlemann, M.; Gebert, A.; Schultz, L. The effect of magnetic fields on the electrodeposition of CoFe alloys. Electrochim. Acta 2008, 53, 5344–5353. [Google Scholar] [CrossRef]
- Huang, M.; Skibinska, K.; Zabinski, P.; Wojnicki, M.; Włoch, G.; Eckert, K.; Mutschke, G. On the prospects of magnetic-field-assisted electrodeposition of nano-structured ferromagnetic layers. Electrochim. Acta 2022, 420, 140422. [Google Scholar] [CrossRef]
- Rode, S.; Henninot, C.; Matlosz, M. Complexation Chemistry in Nickel and Copper-Nickel Alloy Plating from Citrate Baths. J. Electrochem. Soc. 2005, 152, C248. [Google Scholar] [CrossRef]
- Goranova, D.; Rashkov, R.; Avdeev, G.; Tonchev, V. Electrodeposition of Ni–Cu alloys at high current densities: Details of the elements distribution. J. Mater. Sci. 2016, 51, 8663–8673. [Google Scholar] [CrossRef]
- Watanabe, T. Control of Macrostructure in Plated Films and Fabrication of Three-Dimensional Microstructure. In Nano Plating—Microstructure Formation Theory of Plated Films and a Database of Plated Films; Elsevier: Amsterdam, The Netherlands, 2004; pp. 195–205. [Google Scholar]
- Landolt, D. Electrochemical and materials science aspects of alloy deposition. Electrochim. Acta 1994, 39, 1075–1090. [Google Scholar] [CrossRef]
- Krause, L.; Skibińska, K.; Rox, H.; Baumann, R.; Marzec, M.M.; Yang, X.; Mutschke, G.; Żabiński, P.; Lasagni, A.F.; Eckert, K. Hydrogen Bubble Size Distribution on Nanostructured Ni Surfaces: Electrochemically Active Surface Area Versus Wettability. ACS Appl. Mater. Interfaces 2023, 15, 18290–18299. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, S.; Kutyła, D.; Zabinski, P. The Influence of the Magnetic Field on Ni Thin Film Preparation by Electrodeposition Method and Its Electrocatalytic Activity towards Hydrogen Evolution Reaction. Coatings 2023, 13, 1816. [Google Scholar] [CrossRef]
- Solmaz, R.; Döner, A.; Kardaş, G. The stability of hydrogen evolution activity and corrosion behavior of NiCu coatings with long-term electrolysis in alkaline solution. Int. J. Hydrog. Energy 2009, 34, 2089–2094. [Google Scholar] [CrossRef]
- Lee, J.M.; Bae, K.M.; Jung, K.K.; Jeong, J.H.; Ko, J.S. Creation of microstructured surfaces using Cu–Ni composite electrodeposition and their application to superhydrophobic surfaces. Appl. Surf. Sci. 2014, 289, 14–20. [Google Scholar] [CrossRef]
- Eugénio, S.; Silva, T.M.; Carmezim, M.J.; Duarte, R.G.; Montemor, M.F. Electrodeposition and characterization of nickel–copper metallic foams for application as electrodes for supercapacitors. J. Appl. Electrochem. 2014, 44, 455–465. [Google Scholar] [CrossRef]
- Mao, Y.-H.; Chen, C.-Y.; Fu, J.-X.; Lai, T.-Y.; Lu, F.-H.; Tsai, Y.-C. Electrodeposition of nickel-copper on titanium nitride for methanol electrooxidation. Surf. Coat. Technol. 2018, 350, 949–953. [Google Scholar] [CrossRef]


| Sample | Point | Chemical Composition, [% at.] | ||
|---|---|---|---|---|
| Cu | Ni | O | ||
| 0 mT | - | 47.7 | 49.6 | 2.7 |
| 250 mT parallel | 1 | 80.1 | 16.7 | 3.2 |
| 2 | 35.5 | 61.3 | 3.2 | |
| 3 | 62.7 | 35.1 | 2.2 | |
| 500 mT parallel | 1 | 82.2 | 3.6 | 14.2 |
| 2 | 34.1 | 61.3 | 4.6 | |
| 3 | 29.0 | 65.8 | 5.2 | |
| 250 mT perpendicular | 1 | 50.1 | 46.8 | 3.1 |
| 2 | 66.1 | 31.8 | 2.1 | |
| 500 mT perpendicular | 1 | 36.1 | 59.9 | 4.0 |
| 2 | 77.0 | 8.2 | 14.8 | |
| Sample | Chemical Composition [% Mass] | |
|---|---|---|
| Cu | Ni | |
| 0 mT | 54.15 | 45.85 |
| 250 mT parallel | 70.69 | 29.31 |
| 500 mT parallel | 77.38 | 22.62 |
| 250 mT perpendicular | 54.75 | 45.25 |
| 500 mT perpendicular | 76.69 | 23.31 |
| Sample | Crystallite Size [nm] |
|---|---|
| 0 mT | 12 |
| 250 mT parallel | 13 |
| 500 mT parallel | 13 |
| 250 mT perpendicular | 12 |
| 500 mT perpendicular | 15 |
| Sample | Contact Angle [°] |
|---|---|
| 0 mT | 71 ± 4 |
| 250 mT parallel | 73 ± 8 |
| 500 mT parallel | 71 ± 8 |
| 250 mT perpendicular | 68 ± 7 |
| 500 mT perpendicular | 65 ± 7 |
| Sample | EONSET [V] |
|---|---|
| 0 mT | −1.38 |
| 250 mT parallel | −1.35 |
| 500 mT parallel | −1.37 |
| 250 mT perpendicular | −1.33 |
| 500 mT perpendicular | −1.38 |
| Sample | Tafel Slope [mV/dec] |
|---|---|
| 0 mT | 172 |
| 250 mT parallel | 143 |
| 500 mT parallel | 181 |
| 250 mT perpendicular | 138 |
| 500 mT perpendicular | 173 |
| Sample | Time [s] |
|---|---|
| 0 mT | 192 |
| 250 mT parallel | 188 |
| 500 mT parallel | 192 |
| 250 mT perpendicular | 190 |
| 500 mT perpendicular | 192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skibińska, K.; Elsharkawy, S.; Kołczyk-Siedlecka, K.; Kutyła, D.; Żabiński, P. Influence of the Applied External Magnetic Field on the Deposition of Ni–Cu Alloys. Metals 2024, 14, 281. https://doi.org/10.3390/met14030281
Skibińska K, Elsharkawy S, Kołczyk-Siedlecka K, Kutyła D, Żabiński P. Influence of the Applied External Magnetic Field on the Deposition of Ni–Cu Alloys. Metals. 2024; 14(3):281. https://doi.org/10.3390/met14030281
Chicago/Turabian StyleSkibińska, Katarzyna, Safya Elsharkawy, Karolina Kołczyk-Siedlecka, Dawid Kutyła, and Piotr Żabiński. 2024. "Influence of the Applied External Magnetic Field on the Deposition of Ni–Cu Alloys" Metals 14, no. 3: 281. https://doi.org/10.3390/met14030281






