Phytic-Acid-Modified Copper Foil as a Current Collector for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
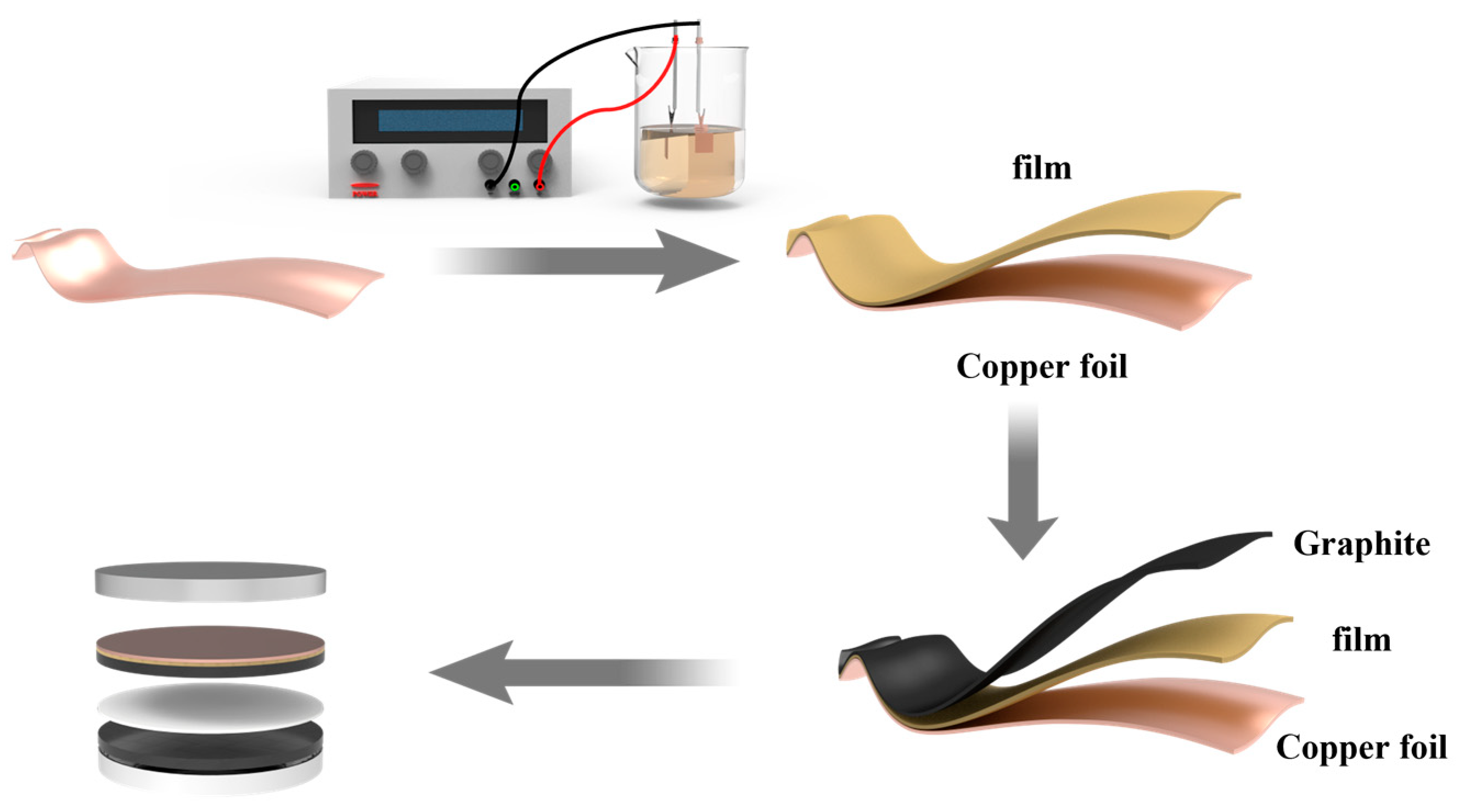
2.1. Preparation of PA-Treated Cu Foil
2.2. Preparation of Electrodes and Assembly of Batteries
2.3. Characterization and Electrochemical Tests
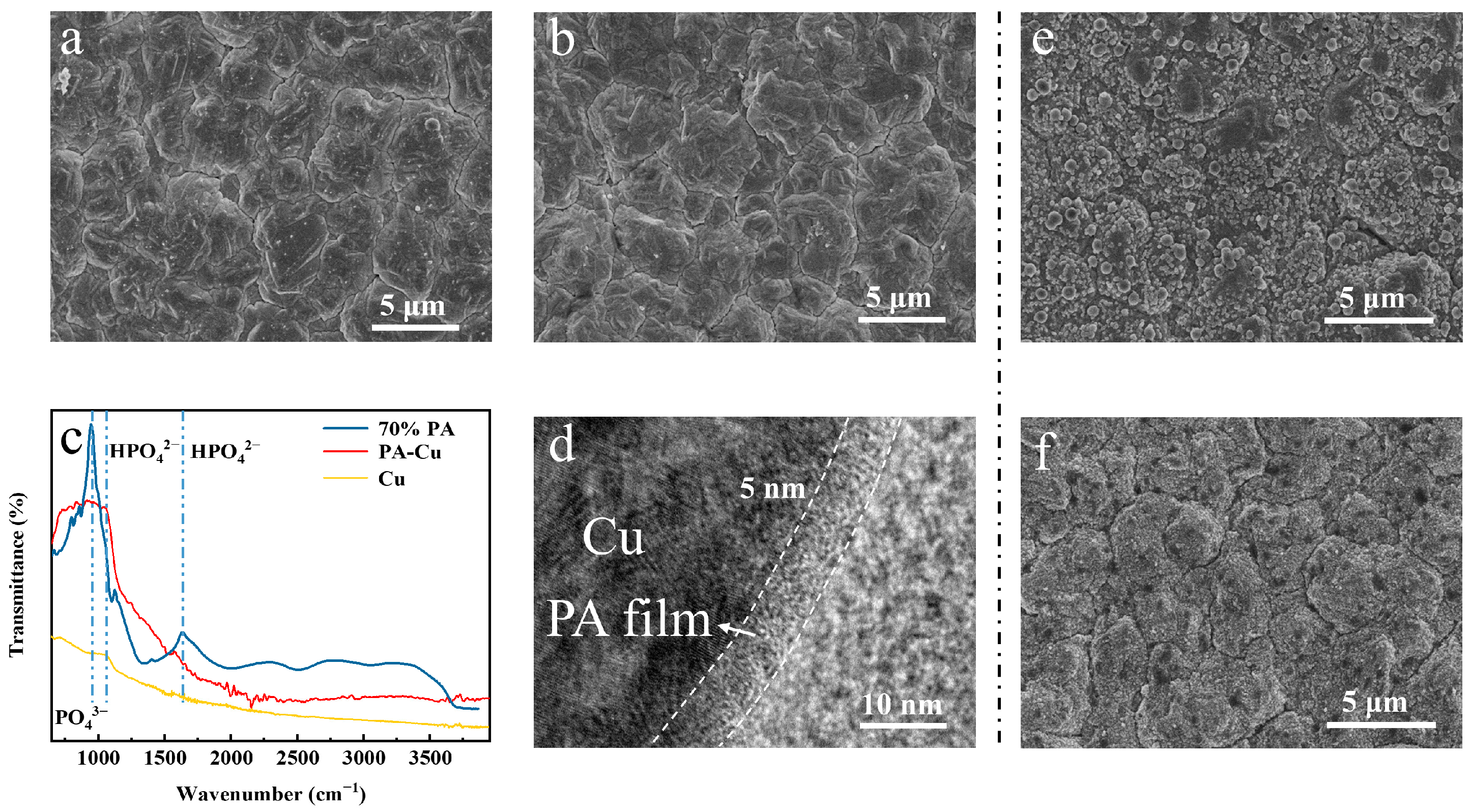
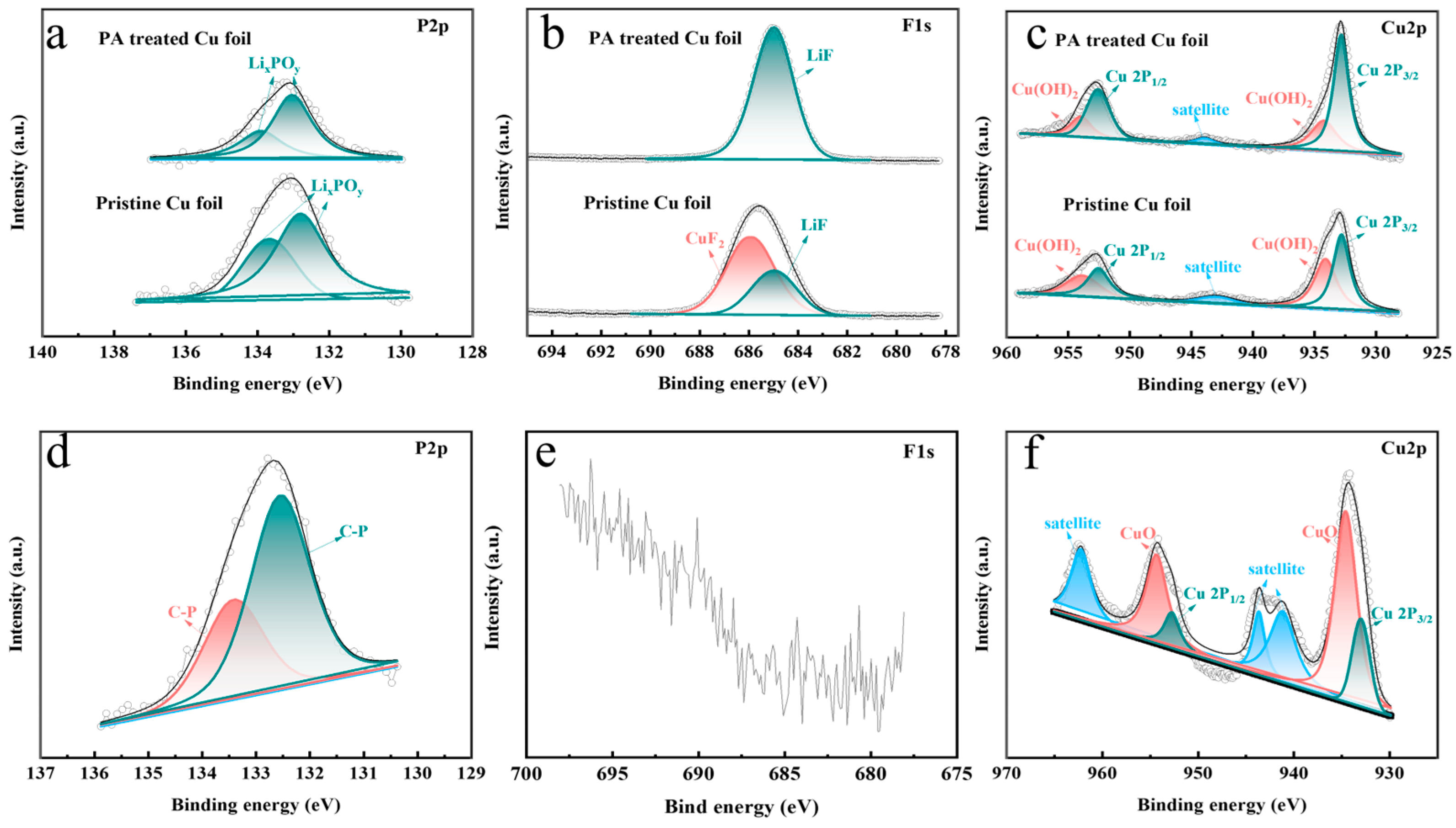
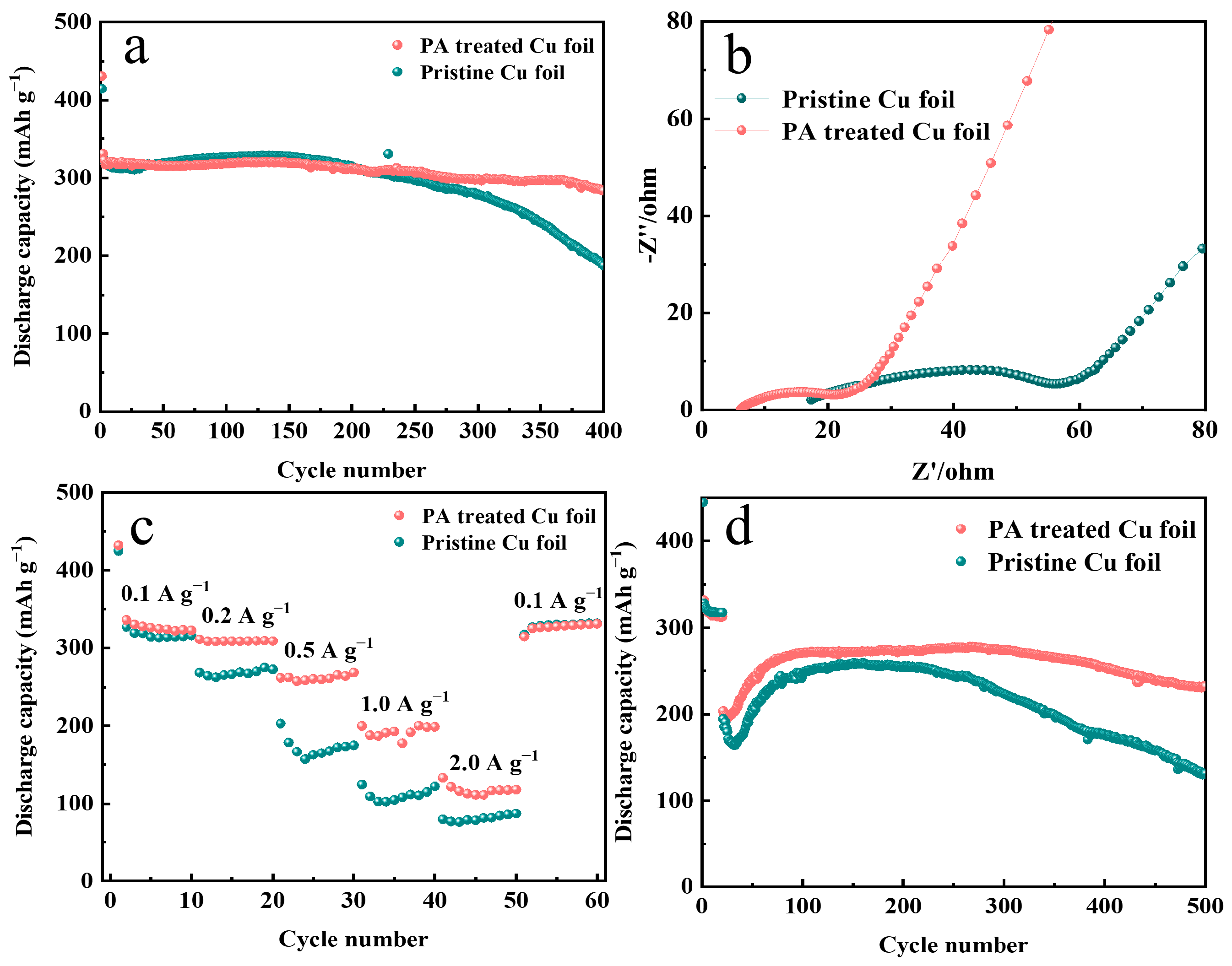
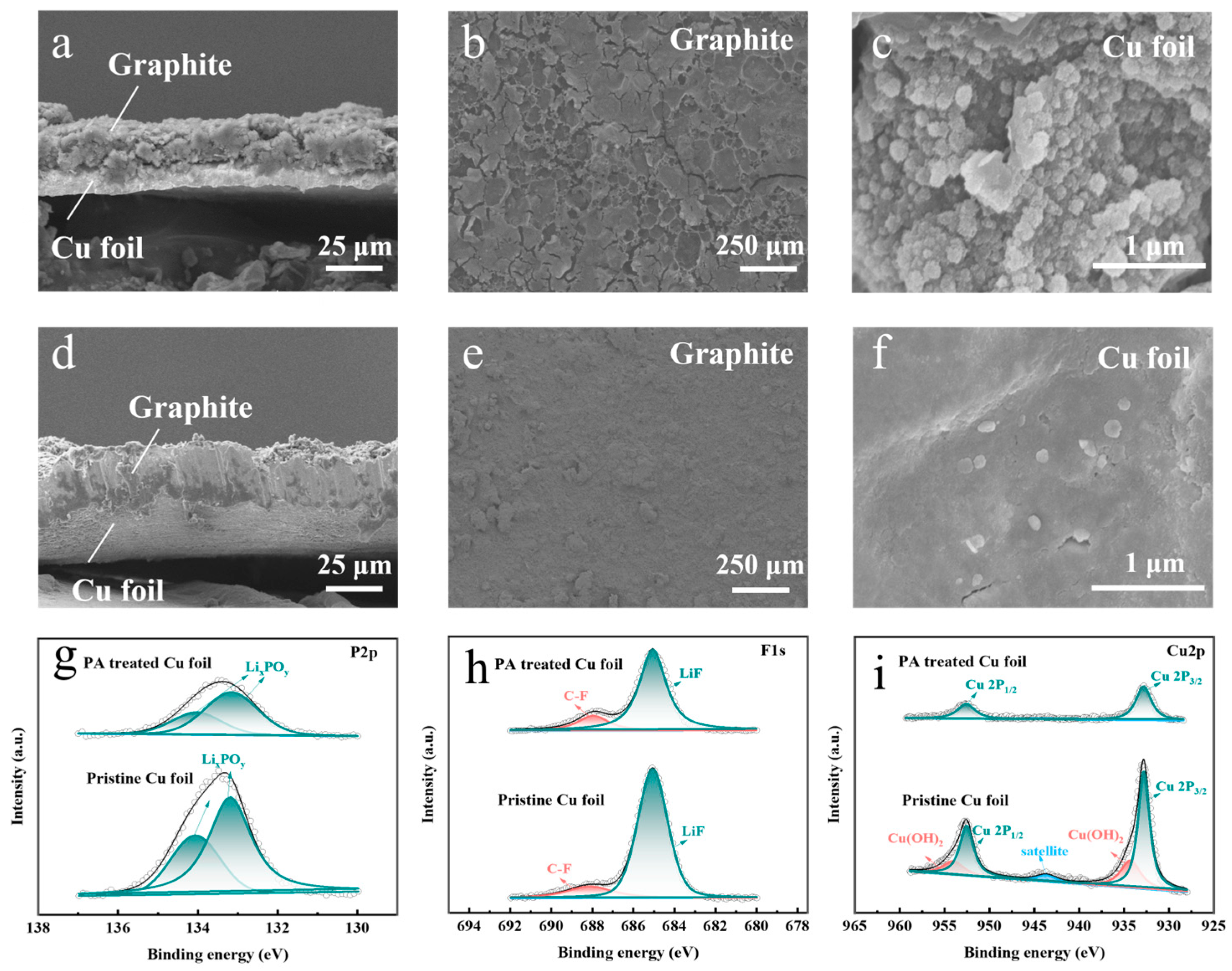
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Aravindan, V. An Urgent Call to Spent LIB Recycling: Whys and Wherefores for Graphite Recovery. Adv. Energy Mater. 2020, 10, 2002238. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, H.; Luo, L.; Zhao, B.; Luo, H.; Han, X.; Wang, J.; Wang, C.; Yang, Y.; Zhu, T.; et al. Harnessing the concurrent reaction dynamics in active Si and Ge to achieve high performance lithium-ion batteries. Energy Environ. Sci. 2018, 11, 669–681. [Google Scholar] [CrossRef]
- Sandstrom, S.K.; Ji, X. Reversible halogen cathodes for high energy lithium batteries. Joule 2023, 7, 13–14. [Google Scholar] [CrossRef]
- Fan, X.; Wang, C. High-voltage liquid electrolytes for Li batteries: Progress and perspectives. Chem. Soc. Rev. 2021, 50, 10486–10566. [Google Scholar] [CrossRef]
- Ma, Q.; Zheng, Y.; Luo, D.; Or, T.; Liu, Y.; Yang, L.; Dou, H.; Liang, J.; Nie, Y.; Wang, X.; et al. 2D Materials for All-Solid-State Lithium Batteries. Adv. Mater. 2022, 34, 2108079. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-H.; Wu, K.-J.; Zhang, T.-W.; Lu, L.-L.; Guan, Y.; Zhou, F.; Wang, X.-X.; Yin, Y.-C.; Tan, Y.-H.; Li, F.; et al. A Nacre-Inspired Separator Coating for Impact-Tolerant Lithium Batteries. Adv. Mater. 2019, 31, 1905711. [Google Scholar] [CrossRef]
- Liu, C.; Wu, X.; Wang, B. Performance modulation of energy storage devices: A case of Ni-Co-S electrode materials. Chem. Eng. J. 2020, 392, 123651. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R Rep. 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Li, W.; Guo, X.; Wang, Y.; Wang, G.; Zhao, D. Porous Carbon Composites for Next Generation Rechargeable Lithium Batteries. Adv. Energy Mater. 2017, 7, 1700283. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Zhu, C.; Zhang, P.; Jiang, G.; Wang, C.; Zhang, Q.; Ding, N.; Huang, Y.; Zhong, S. High-performance of sodium carboxylate-derived materials for electrochemical energy storage. Sci. China Mater. 2018, 61, 707–718. [Google Scholar] [CrossRef]
- Cho, H.; Kim, Y.; Yun, Y.J.; Lee, K.S.; Shim, J.; Lee, C.-H.; Seo, J.W.; Hong, W.G.; Kim, H.J.; Kim, H.Y.; et al. Versatile 3D porous recycled carbon garments with fully-loaded active materials in the current collector for advanced lithium-ion batteries. Compos. Part B Eng. 2019, 179, 107519. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, X.; Zou, P.; Fu, X.; Lin, Z.; Li, Y.; Liu, Y.; Yang, C.; Liu, M. Lithiated zinc oxide nanorod arrays on copper current collectors for robust Li metal anodes. Chem. Eng. J. 2019, 378, 122243. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Wu, Y.; Ying, D.; Yu, Y.; Chi, T.; Xia, S.; Zhou, X.; Liu, Z. Artificial Graphite Paper as a Corrosion-Resistant Current Collector for Long-Life Lithium Metal Batteries. Adv. Funct. Mater. 2023, 33, 2214523. [Google Scholar] [CrossRef]
- Lee, M.-j.; Chae, M.-r.; Jeong, J.-s.; Noh, J.-p.; Ahn, H.-j.; Cho, K.-k.; Choi, H.-k.; Nam, T.-h.; Kim, K.-w.; Cho, G.-b. Si film electrodes with surface-modified Cu current collectors for micro Li batteries. Mater. Res. Bull. 2016, 82, 87–91. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, S.; Jiang, X.; Yao, X.; Xu, X.; Peng, A.; Wang, L.; Xue, Q. High-performances of Li4Ti5O12 anodes for lithium-ion batteries via modifying the Cu current collector through magnetron sputtering amorphous carbon. J. Alloys Compd. 2020, 830, 154682. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Fan, B.; Shan, H.; Chen, Q.; Jiang, C.; Hou, G.; Tang, Y. Study on the relationship between crystal plane orientation and strength of electrolytic copper foil. J. Alloys Compd. 2021, 884, 161044. [Google Scholar] [CrossRef]
- He, S.-Y.; Li, C.-C. Advantages of using carbon fabric over Cu foil as conductive matrix for anodes of micro- and nano-sized Si. Mater. Res. Bull. 2022, 148, 111690. [Google Scholar] [CrossRef]
- Park, H.; Um, J.H.; Choi, H.; Yoon, W.-S.; Sung, Y.-E.; Choe, H. Hierarchical micro-lamella-structured 3D porous copper current collector coated with tin for advanced lithium-ion batteries. Appl. Surf. Sci. 2017, 399, 132–138. [Google Scholar] [CrossRef]
- An, G.-H.; Cha, S.; Ahn, H.-J. Surface functionalization of the terraced surface-based current collector for a supercapacitor with an improved energy storage performance. Appl. Surf. Sci. 2019, 478, 435–440. [Google Scholar] [CrossRef]
- Fei, X.; Dong, Z.; Gong, B.; Zhao, X. Lightweight Through-Hole Copper Foil as a Current Collector for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 42266–42275. [Google Scholar] [CrossRef]
- Wojciechowski, J.; Kolanowski, Ł.; Bund, A.; Lota, G. The influence of current collector corrosion on the performance of electrochemical capacitors. J. Power Sources 2017, 368, 18–29. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; Gao, H.; Chen, S.; Li, W.; Liu, X.; Hu, X.; Yan, S. Rolled electrodeposited copper foil with modified surface morphology as anode current collector for high corrosion resistance in lithium-ion battery electrolyte. Surf. Coat. Technol. 2021, 421, 127369. [Google Scholar] [CrossRef]
- Wennig, S.; Langklotz, U.; Prinz, G.M.; Schmidt, A.; Oberschachtsiek, B.; Lorke, A.; Heinzel, A. The influence of different pre-treatments of current collectors and variation of the binders on the performance of Li4Ti5O12 anodes for lithium ion batteries. J. Appl. Electrochem. 2015, 45, 1043–1055. [Google Scholar] [CrossRef]
- Nara, H.; Mukoyama, D.; Shimizu, R.; Momma, T.; Osaka, T. Systematic analysis of interfacial resistance between the cathode layer and the current collector in lithium-ion batteries by electrochemical impedance spectroscopy. J. Power Sources 2019, 409, 139–147. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, Z.; Zhang, H.; Fu, H.; Li, H.; Wang, A.; Zhang, H.; Hu, Z. Improved electrochemical performance of the Silicon/Graphite-Tin composite anode material by modifying the surface morphology of the Cu current collector. Electrochim. Acta 2014, 146, 322–327. [Google Scholar] [CrossRef]
- Wotango, A.S.; Su, W.-N.; Leggesse, E.G.; Haregewoin, A.M.; Lin, M.-H.; Zegeye, T.A.; Cheng, J.-H.; Hwang, B.-J. Improved Interfacial Properties of MCMB Electrode by 1-(Trimethylsilyl)imidazole as New Electrolyte Additive to Suppress LiPF6 Decomposition. ACS Appl. Mater. Interfaces 2017, 9, 2410–2420. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, A.; Lv, R.; Luo, J. A corrosion-resistant current collector for lithium metal anodes. Energy Storage Mater. 2019, 18, 199–204. [Google Scholar] [CrossRef]
- Ha, J.-K.; Haridas, A.K.; Cho, G.-B.; Ahn, H.-J.; Ahn, J.-H.; Cho, K.-K. Nano silicon encapsulated in modified copper as an anode for high performance lithium ion battery. Appl. Surf. Sci. 2019, 481, 307–312. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Li, G.; Zhang, C.; Liu, X.; Luo, J. Incorporating Ionic Paths into 3D Conducting Scaffolds for High Volumetric and Areal Capacity, High Rate Lithium-Metal Anodes. Adv. Mater. 2018, 30, 1801328. [Google Scholar] [CrossRef]
- Xia, T.; Liang, T.; Xiao, Z.; Chen, J.; Liu, J.; Zhong, S. Nanograined copper foil as a high-performance collector for lithium-ion batteries. J. Alloys Compd. 2020, 831, 154801. [Google Scholar] [CrossRef]
- Nourmohammadi Miankushki, H.; Sedghi, A.; Saeid, B. Comparison of copper compounds on copper foil as current collector for fabrication of graphene/polypyrrole electrode. J. Energy Storage 2018, 19, 201–212. [Google Scholar] [CrossRef]
- Xiao, Z.; Rao, X.; Chen, J.; Potapenko, H.; Zhang, Q.; Zhong, S. Organic carbonized copper foil facilitates the performance of the current collector for lithium-ion batteries. Mater. Chem. Front. 2022, 6, 2478–2490. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.-H.; Hu, J.-M. A novel and facile method for constructing micro-nano porous phytic acid pretreatment layer on metal surface. Corros. Sci. 2021, 186, 109464. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, C.; Zeng, Z.; Zhang, X.; Zhang, L.; Ma, Y.; Wang, Z. Sustainable Phytic Acid–Zinc Anticorrosion Interface for Highly Reversible Zinc Metal Anodes. ACS Appl. Mater. Interfaces 2022, 14, 10419–10427. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhao, C.; Lu, H.; Gao, F.; Ma, H. Influence of phytic acid on the corrosion behavior of iron under acidic and neutral conditions. Electrochim. Acta 2014, 150, 188–196. [Google Scholar] [CrossRef]
- Yan, R.; Gao, X.; He, W.; Guo, R.; Wu, R.; Zhao, Z.; Ma, H. A simple and convenient method to fabricate new types of phytic acid–metal conversion coatings with excellent anti-corrosion performance on the iron substrate. RSC Adv. 2017, 7, 41152–41162. [Google Scholar] [CrossRef]
- Shen, S.; Guo, X.-y.; Song, P.; Pan, Y.-C.; Wang, H.-q.; Wen, Y.; Yang, H.-F. Phytic acid adsorption on the copper surface: Observation of electrochemistry and Raman spectroscopy. Appl. Surf. Sci. 2013, 276, 167–173. [Google Scholar] [CrossRef]
- Wang, W.-j.; Liu, J.; Liu, X.-f.; Li, Q.-w. Electrodeposited chromate-free organic passive film on the rolled copper foil. Prog. Org. Coat. 2022, 163, 106663. [Google Scholar] [CrossRef]
- Xu, X.; Su, H.; Zhang, J.; Zhong, Y.; Xu, Y.; Qiu, Z.; Wu, H.B.; Wang, X.; Gu, C.; Tu, J. Sulfamate-Derived Solid Electrolyte Interphase for Reversible Aqueous Zinc Battery. ACS Energy Lett. 2022, 7, 4459–4468. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Zhang, J.; Zhong, Y.; Li, Z.; Qiu, H.; Wu, H.B.; Wang, J.; Wang, X.; Gu, C.; et al. Quasi-Solid Electrolyte Interphase Boosting Charge and Mass Transfer for Dendrite-Free Zinc Battery. Nano-Micro Lett. 2023, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, H.; Jia, H.; Engelhard, M.H.; Zhang, J.-G.; Xu, W.; Wang, C. Sweeping potential regulated structural and chemical evolution of solid-electrolyte interphase on Cu and Li as revealed by cryo-TEM. Nano Energy 2020, 76, 105040. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, M.; Zhu, M.; Tu, J.; Wang, X.; Gu, C. Phytic-Acid-Modified Copper Foil as a Current Collector for Lithium-Ion Batteries. Metals 2024, 14, 247. https://doi.org/10.3390/met14020247
Gan M, Zhu M, Tu J, Wang X, Gu C. Phytic-Acid-Modified Copper Foil as a Current Collector for Lithium-Ion Batteries. Metals. 2024; 14(2):247. https://doi.org/10.3390/met14020247
Chicago/Turabian StyleGan, Mingtao, Mengjun Zhu, Jiangping Tu, Xiuli Wang, and Changdong Gu. 2024. "Phytic-Acid-Modified Copper Foil as a Current Collector for Lithium-Ion Batteries" Metals 14, no. 2: 247. https://doi.org/10.3390/met14020247



