First-Principles Study of Cu Addition on Mechanical Properties of Ni3Sn4-Based Intermetallic Compounds
Abstract
:1. Introduction
2. First-Principles Calculation Details
3. Results and Discussion
3.1. Structure Optimisation
3.2. Mechanical Properties
3.3. Electronic Structures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le, F.; Lee, S.R.; Zhang, Q. 3D chip stacking with through silicon-vias (TSVs) for vertical interconnect and underfill dispensing. J. Micromech. Microeng. 2017, 27, 45012. [Google Scholar] [CrossRef]
- Cao, K.; Zhou, J.; Wei, T.; Chen, M.; Hu, S.; Li, K. A survey of optimization techniques for thermal-aware 3D processors. J. Syst. Archit. 2019, 97, 397–415. [Google Scholar] [CrossRef]
- Ma, H.R.; Kunwar, A.; Shang, S.Y.; Jiang, C.R.; Wang, Y.P.; Ma, H.T.; Zhao, N. Evolution behavior and growth kinetics of intermetallic compounds at Sn/Cu interface during multiple reflows. Intermetallics 2018, 96, 1–12. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Zhang, Z.; Zhang, P.; Yu, Z.; Zhang, S. Effects of doping trace Ni element on interfacial behavior of Sn/Ni (polycrystal/single-crystal) joints. Solder. Surf. Mt. Technol. 2021, 34, 124–133. [Google Scholar] [CrossRef]
- Tu, K. Solder Joint Technology; Springer: Berlin/Heidelberg, Germany, 2007; Volume 117, pp. 7–11. [Google Scholar]
- Tu, K.; Hsiao, H.; Chen, C. Transition from flip chip solder joint to 3D IC microbump: Its effect on microstructure anisotropy. Microelectron. Reliab. 2013, 53, 2–6. [Google Scholar] [CrossRef]
- Pan, H.; Wang, Y.; Luo, W.; Gao, L.; Li, M. Effect of Ni thickness on the IMC and reliability of ultrathin ENEPIG. In Proceedings of the 2018 19th International Conference on Electronic Packaging Technology (ICEPT), Shanghai, China, 8–11 August 2018; pp. 667–671. [Google Scholar]
- Jen, Y.; Chiou, Y.; Yu, C. Fracture mechanics study on the intermetallic compound cracks for the solder joints of electronic packages. Eng. Fail. Anal. 2011, 18, 797–810. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, W.; Zhang, W.; Chen, W.; Liu, Z. Research on thermal fatigue failure mechanism of BGA solder joints based on microstructure evolution. Int. J. Fatigue 2023, 167, 107356. [Google Scholar] [CrossRef]
- Yang, C.; Le, F.; Lee, S.R. Experimental investigation of the failure mechanism of Cu–Sn intermetallic compounds in SAC solder joints. Microelectron. Reliab. 2016, 62, 130–140. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C. Kinetic study of the intermetallic compound formation between eutectic Sn–3.5 Ag alloys and electroplated Ni metallization in flip-chip solder joints. J. Mater. Res. 2012, 27, 1169–1177. [Google Scholar] [CrossRef]
- Huang, C.; Jang, G.; Duh, J. Interfacial reactions and compound formation in the edge of PbSn flip-chip solder bumps on Ni/Cu under-bump metallization. J. Electron. Mater. 2003, 32, 1273–1277. [Google Scholar] [CrossRef]
- Kumar, A.; Chen, Z.; Mhaisalkar, S.G.; Wong, C.C.; Teo, P.S.; Kripesh, V. Effect of Ni–P thickness on solid-state interfacial reactions between Sn–3.5 Ag solder and electroless Ni–P metallization on Cu substrate. Thin Solid Film. 2006, 504, 410–415. [Google Scholar] [CrossRef]
- Hai, S.; Oh, S.; Yu, H. Evaluation of creep properties for aged Pb-free solder joints/(Ni-P/Au) UBM with small addition Cu using shear punch creep testing method. Eng. Fail. Anal. 2020, 113, 104558. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, F.; Li, J.; Chen, Z. Comparison of interfacial reactions and isothermal aging of cone Ni-P and flat Ni-P with Sn3. 5Ag solders. Appl. Surf. Sci. 2023, 625, 157219. [Google Scholar] [CrossRef]
- Mitchell, D.; Guo, Y.; Sarihan, V. Methodology for studying the impact of intrinsic stress on the reliability of the electroless Ni UBM structure. IEEE Trans. Compon. Packag. Technol. 2001, 24, 667–672. [Google Scholar] [CrossRef]
- Jeon, Y.; Paik, K.; Bok, K.; Choi, W.; Cho, C. Studies on Ni-Sn intermetallic compound and P-rich Ni layer at the electroless nickel UBM-solder interface and their effects on flip chip solder joint reliability. In Proceedings of the 2001 Proceedings, 51st Electronic Components and Technology Conference (Cat. No. 01CH37220), Orlando, FL, USA, 29 May–1 June 2001; pp. 1326–1332. [Google Scholar]
- Chen, P.; Li, C.; Yang, C.; Hu, A.; Li, M.; Gao, L.; Ling, H.; Hang, T.; Wu, Y. Growth behavior of Ni-Sn intermetallic compounds in microbumps during long-term aging process. Mater. Lett. 2022, 313, 131743. [Google Scholar] [CrossRef]
- Ji, H.; Li, M.; Ma, S.; Li, M. Ni3Sn4-composed die bonded interface rapidly formed by ultrasonic-assisted soldering of Sn/Ni solder paste for high-temperature power device packaging. Mater. Design 2016, 108, 590–596. [Google Scholar] [CrossRef]
- Lin, C.; Jao, C.; Lee, C.; Yen, Y. The effect of non-reactive alloying elements on the growth kinetics of the intermetallic compound between liquid Sn-based eutectic solders and Ni substrates. J. Alloys Compd. 2007, 440, 333–340. [Google Scholar] [CrossRef]
- He, H.; Huang, S.; Xiao, Y.; Goodall, R. Diffusion reaction-induced microstructure and strength evolution of Cu joints bonded with Sn-based solder containing Ni-foam. Mater. Lett. 2020, 281, 128642. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, K.; Liu, J.; He, Y.; Xiao, Y. Effect of Thermal aging on the interfacial reaction behavior and failure mechanism of Ni-xCu/Sn soldering joints under shear loading. Materials 2023, 16, 5253. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Huang, J.; Wang, W.; Ye, Z.; Chen, S.; Zhao, Y. First-principles calculations on physical properties of Ni3Snx binary system intermetallic compounds and Ni/Ni3Sn interfaces in Nickel-Tin TLPS bonding layer. Intermetallics 2018, 101, 27–38. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, P. First-Principles Study of Substitution of Au for Ni in Ni3Sn4. J. Electron. Mater. 2018, 47, 2600–2608. [Google Scholar] [CrossRef]
- Jeitschko, W.; Jaberg, B. Structure refinement of Ni3Sn4. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1982, 38, 598–600. [Google Scholar] [CrossRef]
- Han, Y.; Chen, J.; Lin, M.; Zhang, K.; Lu, H. Synergistic effects of alloy elements on the structural stability, mechanical properties and electronic structure of Ni3Sn4: Using first principles. Vacuum 2023, 214, 112239. [Google Scholar] [CrossRef]
- Zhang, W.W.; Ma, Y.; Zhou, W.; Wu, P. The Structural, Elastic and Electronic Properties of Ni3−xCuxSn4 (x = 0, 0.5, 1 and 1.5) Intermetallic Compounds via Ab Initio Calculations. J. Electron. Mater. 2019, 48, 4533–4543. [Google Scholar] [CrossRef]
- Yao, P.; Liu, P.; Liu, J. Effects of multiple reflows on intermetallic morphology and shear strength of SnAgCu–xNi composite solder joints on electrolytic Ni/Au metallized substrate. J. Alloys Compd. 2008, 462, 73–79. [Google Scholar] [CrossRef]
- Yao, P.; Liu, P.; Liu, J. Interfacial reaction and shear strength of SnAgCu–xNi/Ni solder joints during aging at 150 °C. Microelectron. Eng. 2009, 86, 1969–1974. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.; Probert, M.A.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Hu, X.; Jiang, X.; Li, Q. Effect of Cu additions on mechanical properties of Ni3Sn4-based intermetallic compounds: First-principles calculations and nano-indentation measurements. Vacuum 2019, 164, 7–14. [Google Scholar] [CrossRef]
- Bi, X.; Hu, X.; Li, Q. Effect of Co addition into Ni film on shear strength of solder/Ni/Cu system: Experimental and theoretical investigations. Mater. Sci. Eng. A 2020, 788, 139589. [Google Scholar] [CrossRef]
- Beckstein, O.; Klepeis, J.E.; Hart, G.; Pankratov, O. First-principles elastic constants and electronic structure of α−Pt2Si and PtSi. Phys. Rev. B 2001, 63, 134112. [Google Scholar] [CrossRef]
- Nye, J.F. Physical Properties of Crystals: Their Representation by Tensors and Matrices; Oxford University Press: Oxford, UK, 1985; pp. 206–210. [Google Scholar]
- Heciri, D.; Belkhir, H.; Belghit, R.; Bouhafs, B.; Khenata, R.; Ahmed, R.; Bouhemadou, A.; Ouahrani, T.; Wang, X.; Omran, S.B. Insight into the structural, elastic and electronic properties of tetragonal inter-alkali metal chalcogenides CsNaX (X = S, Se, and Te) from first-principles calculations. Mater. Chem. Phys. 2019, 221, 125–137. [Google Scholar] [CrossRef]
- Cheng, H.; Yu, C.; Chen, W. First-principles density functional calculation of mechanical, thermodynamic and electronic properties of CuIn and Cu2In crystals. J. Alloys Compd. 2013, 546, 286–295. [Google Scholar] [CrossRef]
- Hill, R. The elastic behaviour of a crystalline aggregate. Proc. Phys. Society. Sect. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Hossain, K.M.; Mitro, S.K.; Rahman, M.M.; Khatun, A.A.; Parvin, F. Analyzing the physical properties of perovskite oxides BaMO3 (M = Ru, Os) for predicting potential applications. Comput. Condens. Matter 2023, 34, e00782. [Google Scholar] [CrossRef]
- Zeng, G.; Xue, S.; Zhang, L.; Gao, L.; Dai, W.; Luo, J. A review on the interfacial intermetallic compounds between Sn–Ag–Cu based solders and substrates. J. Mater. Sci. Mater. Electron. 2010, 21, 421–440. [Google Scholar] [CrossRef]
- Eklund, C.; Fennie, C.J.; Rabe, K.M. Strain-induced ferroelectricity in orthorhombic CaTiO3 from first principles. Phys. Rev. B 2009, 79, 220101. [Google Scholar] [CrossRef]
- Gschneidner, K.A., Jr.; Ji, M.; Wang, C.Z.; Ho, K.M.; Russell, A.M.; Mudryk, Y.; Becker, A.T.; Larson, J.L. Influence of the electronic structure on the ductile behavior of B2 CsCl-type AB intermetallics. Acta Mater. 2009, 57, 5876–5881. [Google Scholar] [CrossRef]
- Fu, C.L.; Wang, X.; Ye, Y.Y.; Ho, K.M. Phase stability, bonding mechanism, and elastic constants of Mo5Si3 by first-principles calculation. Intermetallics 1999, 7, 179–184. [Google Scholar] [CrossRef]
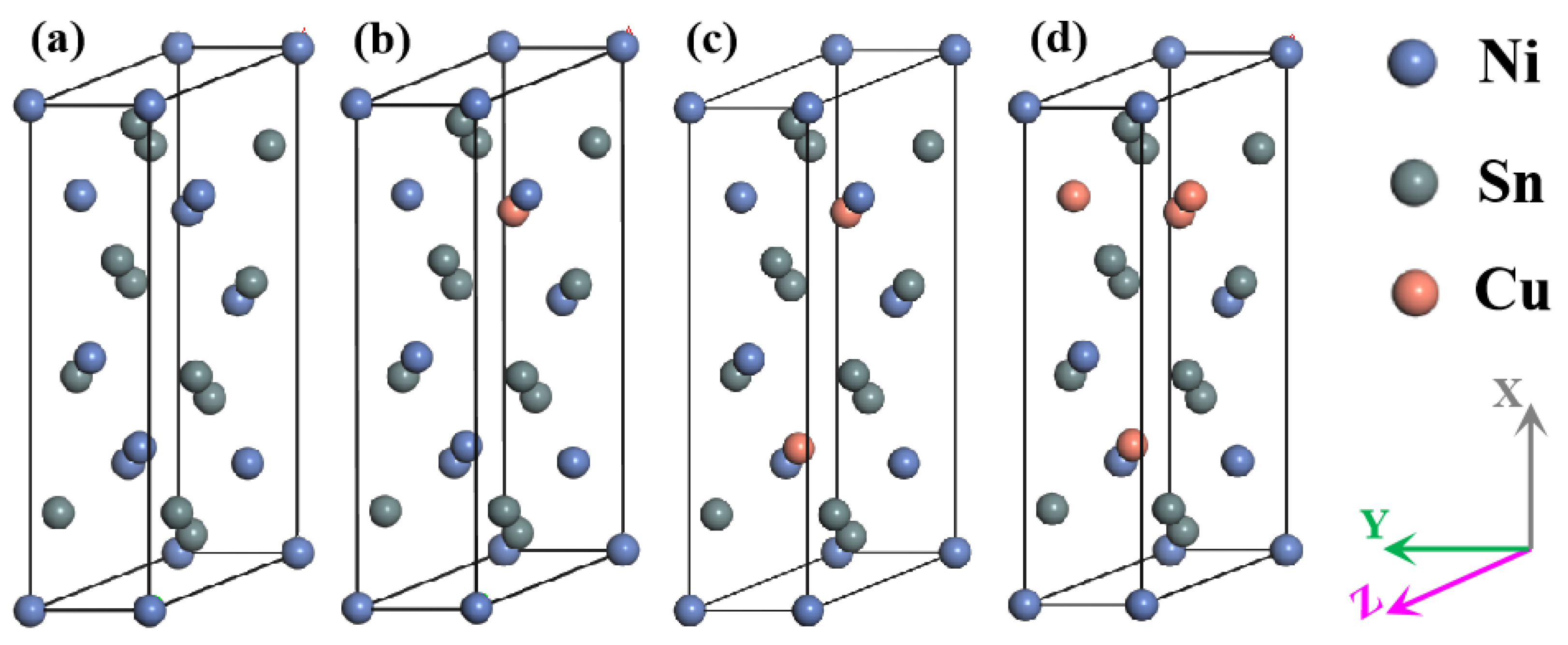
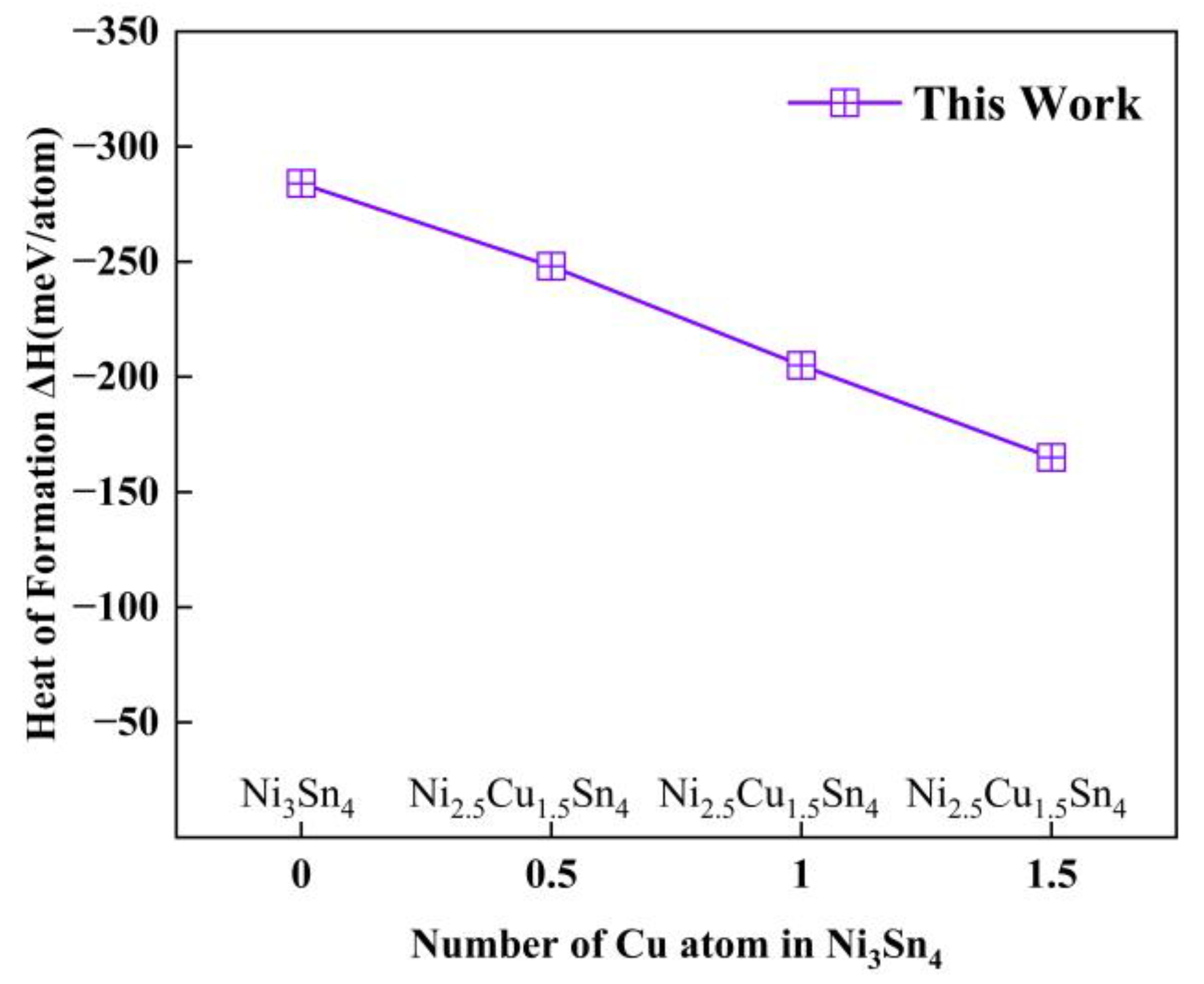
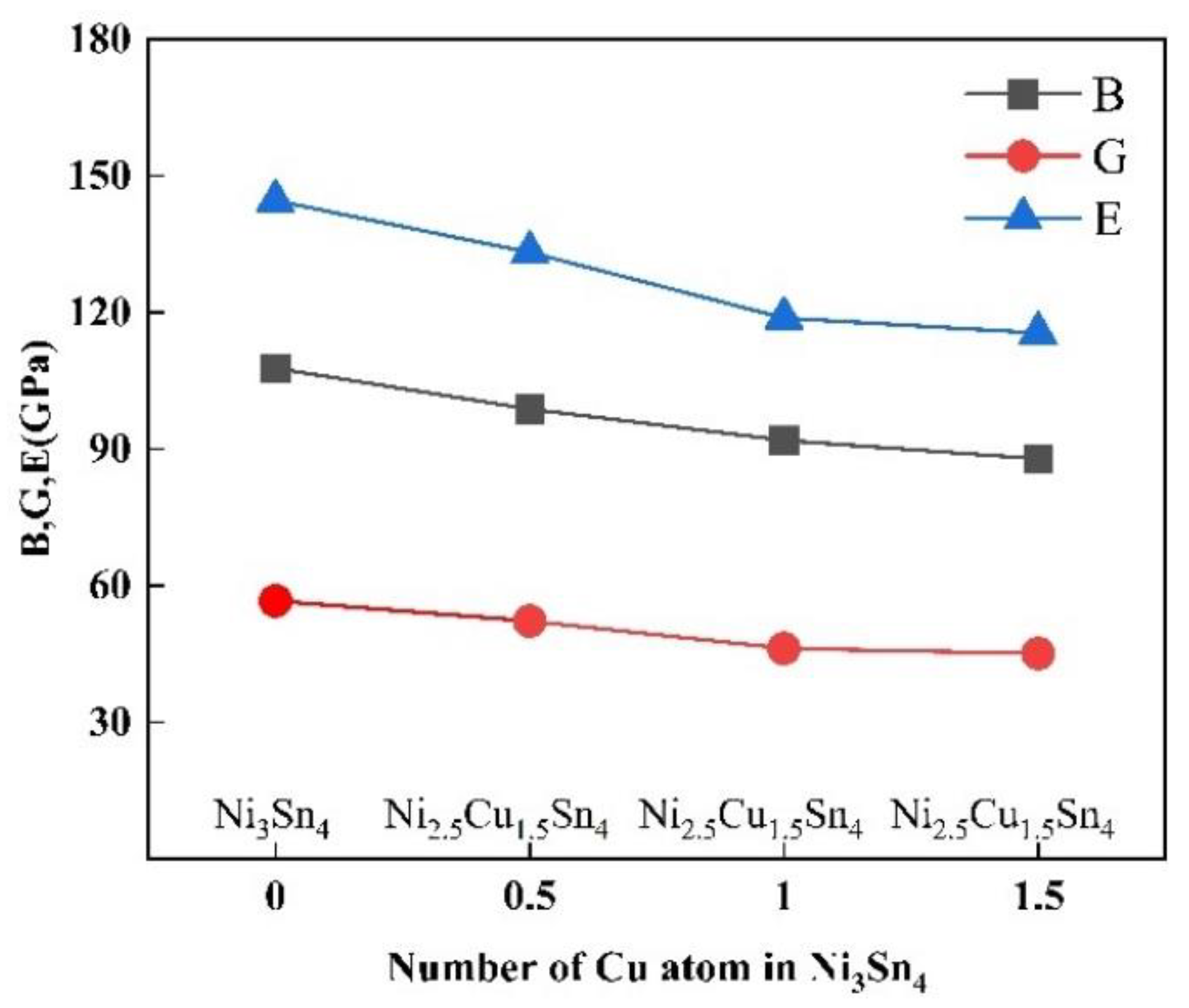



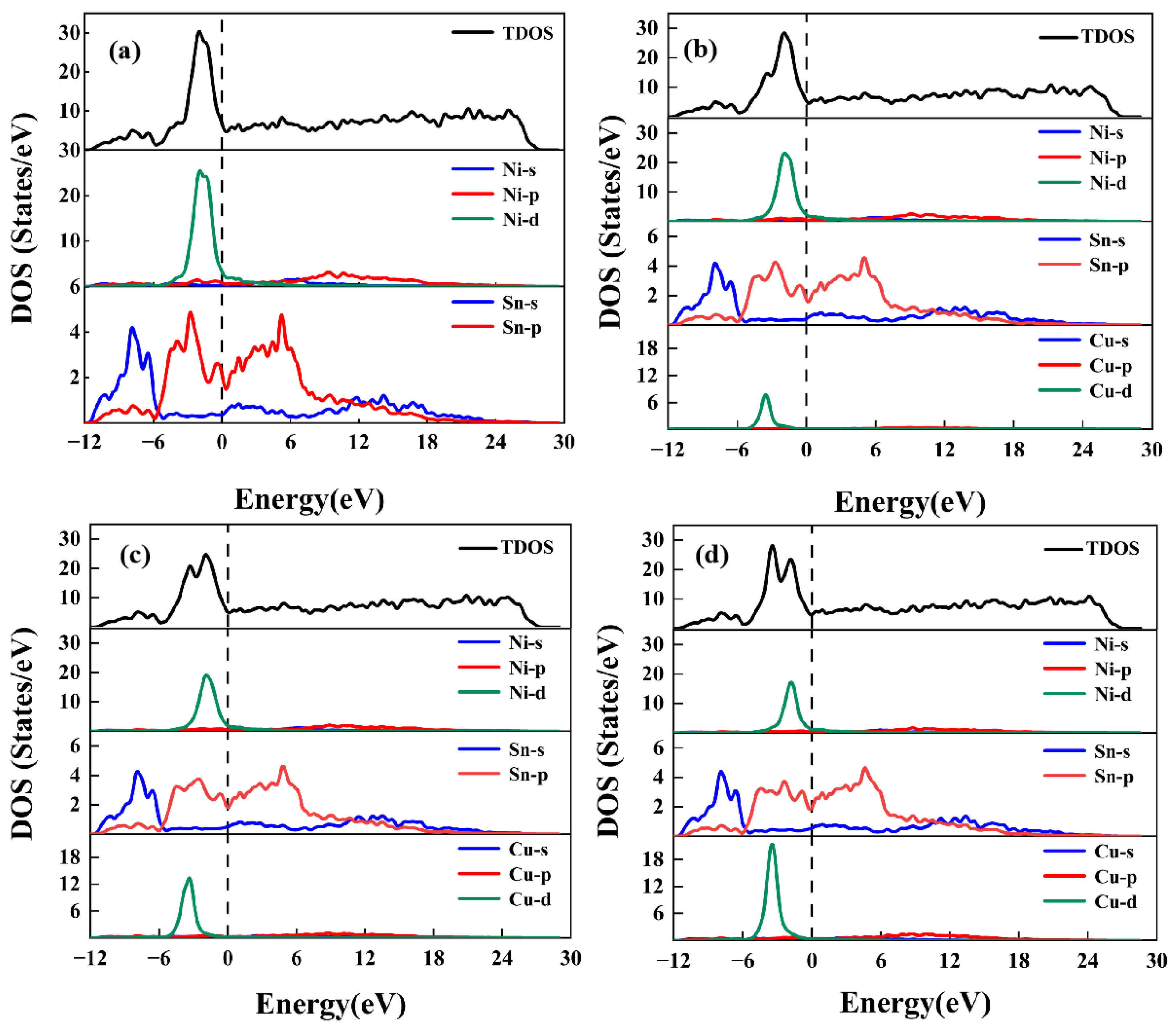
| Phases | Reference | a | b | c | β | ΔH |
|---|---|---|---|---|---|---|
| Ni3Sn4 | This work | 12.323 | 4.119 | 5.303 | 105.416 | −284 |
| [26] | 12.299 | 4.084 | 5.288 | 105.190 | −260 | |
| [32] | 12.418 | 4.111 | 4.315 | 105.480 | −267 | |
| [33] | 12.334 | 4.100 | 5.325 | 105.010 | −269 | |
| Ni2.5Cu0.5Sn4 | This work | 12.419 | 4.146 | 5.291 | 105.555 | −248 |
| [26] | 12.357 | 4.102 | 5.307 | 105.150 | −219 | |
| [32] | 12.426 | 4.101 | 5.363 | 105.690 | −193 | |
| Ni2.0Cu1.0Sn4 | This work | 12.434 | 4.149 | 5.365 | 105.541 | −205 |
| [26] | 12.420 | 4.128 | 5.331 | 105.150 | −179 | |
| [27] | 12.422 | 4.133 | 5.331 | 105.160 | −188 | |
| Ni1.5Cu1.5Sn4 | This work | 12.538 | 4.173 | 5.382 | 105.958 | −165 |
| [26] | 12.495 | 4.145 | 5.381 | 105.16 | −135 | |
| [27] | 12.507 | 4.146 | 5.382 | 105.07 | −144 |
| Phases | Reference | C11 | C12 | C13 | C22 | C23 | C33 | C44 | C55 | C66 | C15 | C25 | C35 | C46 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni3Sn4 | This work | 165.96 | 74.58 | 72.13 | 183.59 | 68.85 | 191.67 | 62.35 | 64.28 | 55.35 | −17.26 | 12.83 | −5.70 | 9.67 |
| [26] | 176.60 | 64.30 | 57.40 | 146.00 | 76.10 | 169.70 | 60.80 | 49.50 | 46.70 | −22.90 | 7.00 | −9.60 | 6.90 | |
| [32] | 155.48 | 70.68 | 69.34 | 164.33 | 68.26 | 149.86 | 62.74 | 59.99 | 59.95 | −21.97 | 13.99 | −8.73 | 4.90 | |
| [33] | 153.09 | 68.22 | 63.25 | 144.31 | 75.37 | 176.53 | 57.42 | 57.35 | 53.84 | −22.70 | 12.94 | −11.11 | 9.09 | |
| Ni2.5Cu0.5Sn4 | This work | 153.70 | 67.24 | 63.10 | 165.94 | 63.53 | 186.08 | 48.84 | 59.64 | 57.98 | −21.22 | 12.00 | −4.30 | 9.61 |
| [32] | 146.95 | 71.79 | 64.07 | 176.30 | 73.29 | 154.47 | 57.86 | 55.11 | 55.41 | −21.79 | 11.93 | −6.18 | 8.22 | |
| Ni2.0Cu1.0Sn4 | This work | 138.64 | 63.51 | 62.15 | 156.72 | 60.21 | 166.06 | 45.23 | 47.76 | 56.05 | −20.92 | 10.26 | −4.43 | 8.55 |
| Ni1.5Cu1.5Sn4 | This work | 134.58 | 53.68 | 59.37 | 146.62 | 69.75 | 148.91 | 54.13 | 40.93 | 55.57 | −14.66 | 8.82 | −4.51 | 7.93 |
| Phases | Reference | B | G | E | ν | B/G |
|---|---|---|---|---|---|---|
| Ni3Sn4 | This work | 107.78 | 56.64 | 144.59 | 0.276 | 1.90 |
| [23] | 102.10 | 54.10 | 127.20 | 0.250 | 1.89 | |
| [26] | 97.90 | 49.08 | 126.11 | 0.285 | 2.00 | |
| [32] | 98.01 | 53.96 | 136.78 | 0.287 | 1.82 | |
| [33] | 98.30 | 49.27 | 126.65 | 0.290 | 2.00 | |
| Ni2.5Cu0.5Sn4 | This work | 98.79 | 52.23 | 133.21 | 0.275 | 1.89 |
| [27] | 94.25 | 49.07 | 125.43 | 0.278 | 1.92 | |
| [32] | 98.45 | 49.62 | 127.45 | 0.284 | 1.95 | |
| Ni2.0Cu1.0Sn4 | This work | 91.93 | 46.24 | 118.80 | 0.285 | 1.99 |
| Ni1.5Cu1.5Sn4 | This work | 87.84 | 45.08 | 115.48 | 0.281 | 1.95 |
| IMCs | Reference | GV | GR | BV | BR | GV/GR | BV/BR | AU |
|---|---|---|---|---|---|---|---|---|
| Ni3Sn4 | This work | 58.11 | 55.17 | 108.04 | 107.53 | 1.053 | 1.005 | 0.271 |
| [32] | 53.96 | 49.42 | 98.47 | 97.56 | 1.092 | 1.001 | 0.461 | |
| [33] | 51.79 | 46.74 | 99.07 | 97.53 | 1.108 | 1.016 | 0.556 | |
| Ni2.5Cu0.5Sn4 | This work | 54.08 | 50.38 | 99.27 | 98.31 | 1.077 | 1.010 | 0.378 |
| [32] | 51.58 | 47.66 | 99.56 | 97.33 | 1.082 | 1.013 | 0.423 | |
| Ni2.0Cu1.0Sn4 | This work | 48.18 | 44.30 | 92.57 | 91.28 | 1.088 | 1.014 | 0.454 |
| Ni1.5Cu1.5Sn4 | This work | 46.61 | 43.54 | 88.41 | 87.26 | 1.071 | 1.013 | 0.366 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Wang, L.; Guo, S.; Li, X.; Chen, X.; Shang, M.; Ma, H.; Ma, H. First-Principles Study of Cu Addition on Mechanical Properties of Ni3Sn4-Based Intermetallic Compounds. Metals 2024, 14, 64. https://doi.org/10.3390/met14010064
Yao J, Wang L, Guo S, Li X, Chen X, Shang M, Ma H, Ma H. First-Principles Study of Cu Addition on Mechanical Properties of Ni3Sn4-Based Intermetallic Compounds. Metals. 2024; 14(1):64. https://doi.org/10.3390/met14010064
Chicago/Turabian StyleYao, Jinye, Li Wang, Shihao Guo, Xiaofu Li, Xiangxu Chen, Min Shang, Haoran Ma, and Haitao Ma. 2024. "First-Principles Study of Cu Addition on Mechanical Properties of Ni3Sn4-Based Intermetallic Compounds" Metals 14, no. 1: 64. https://doi.org/10.3390/met14010064





