Investigation of the Sn-0.7 wt.% Cu Solder Reacting with C194, Alloy 25, and C1990 HP Substrates
Abstract
:1. Introduction
2. Materials and Methods
3. Results
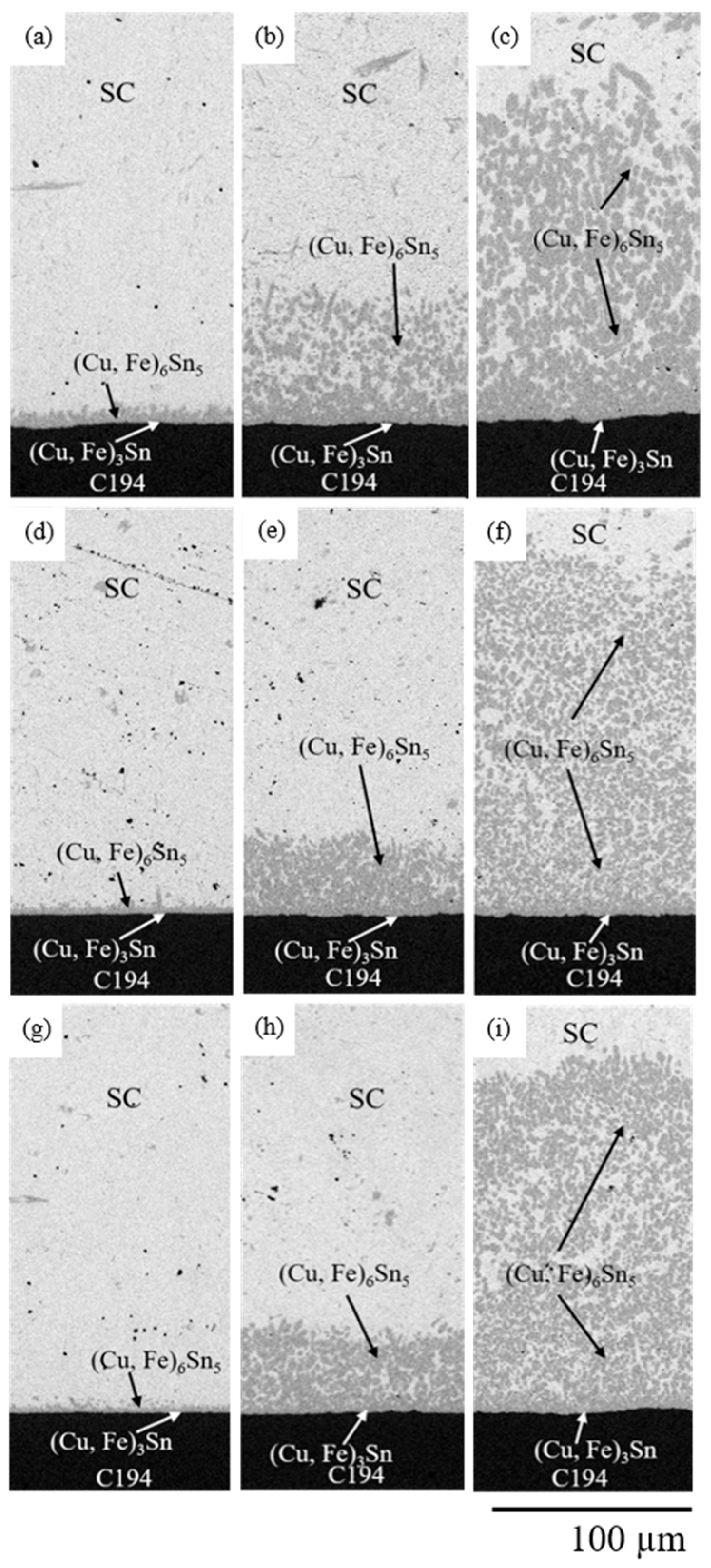
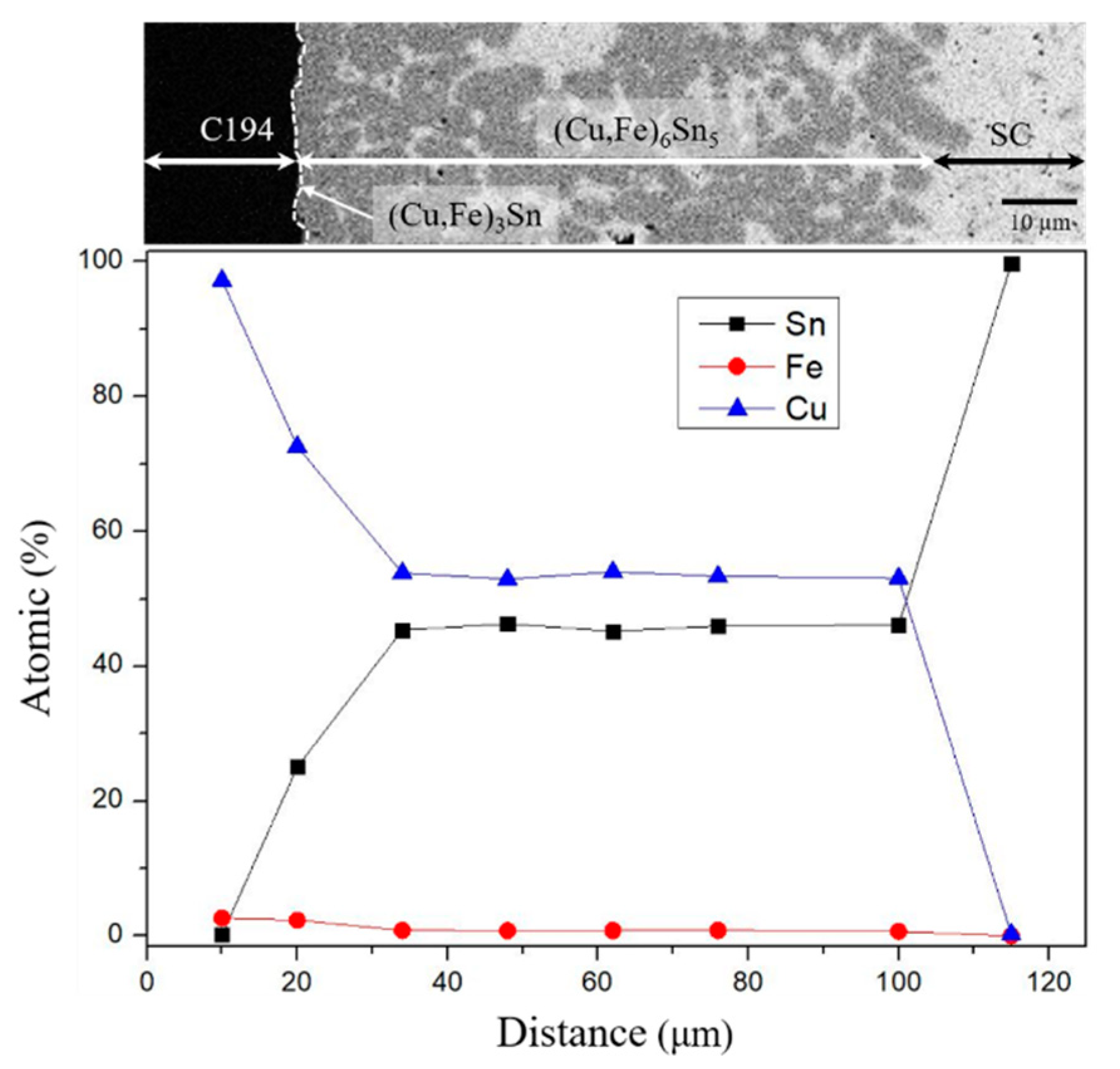
3.1. SC/C194 Reaction Couples
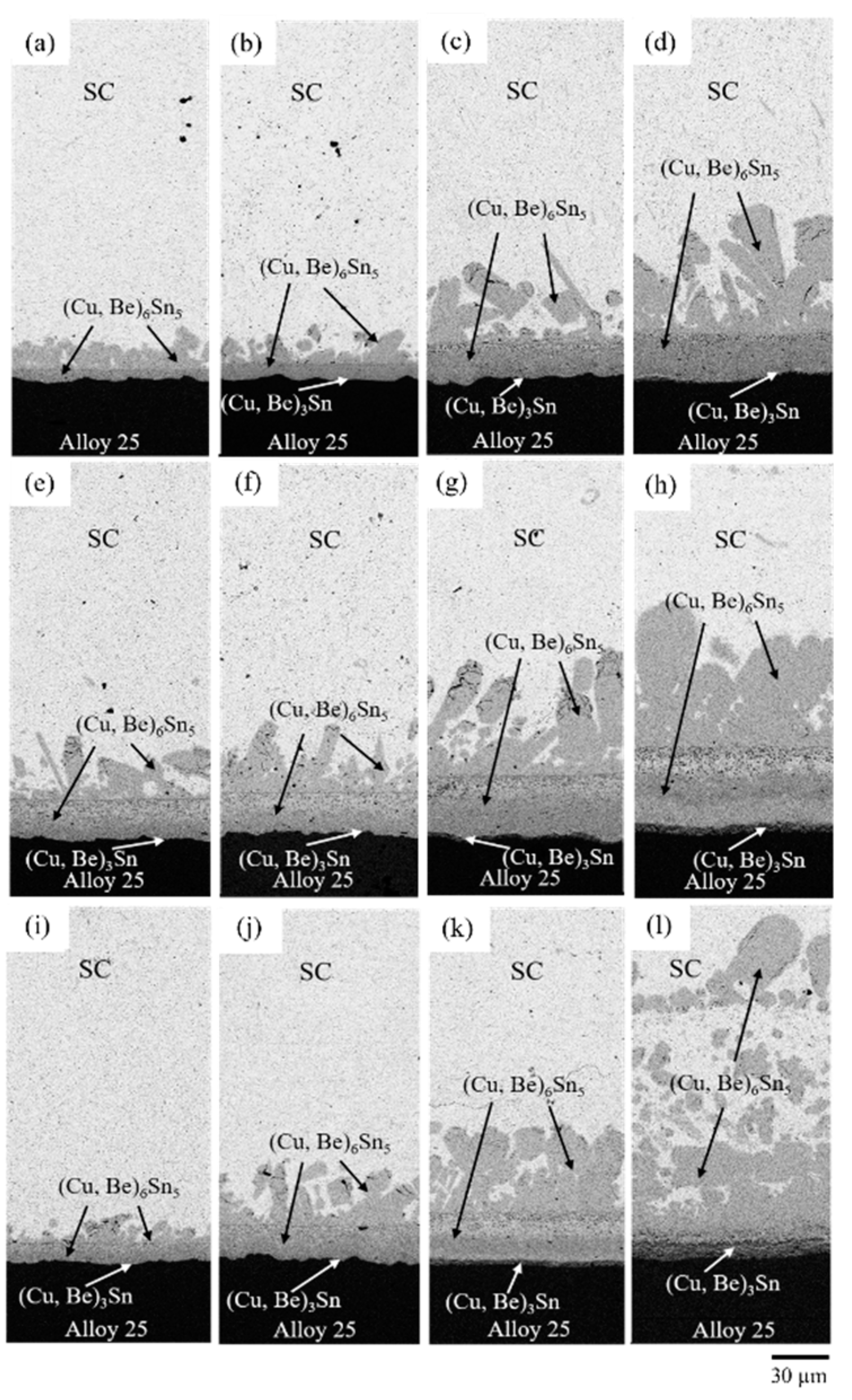
3.2. SC/Alloy 25 Reaction Couples
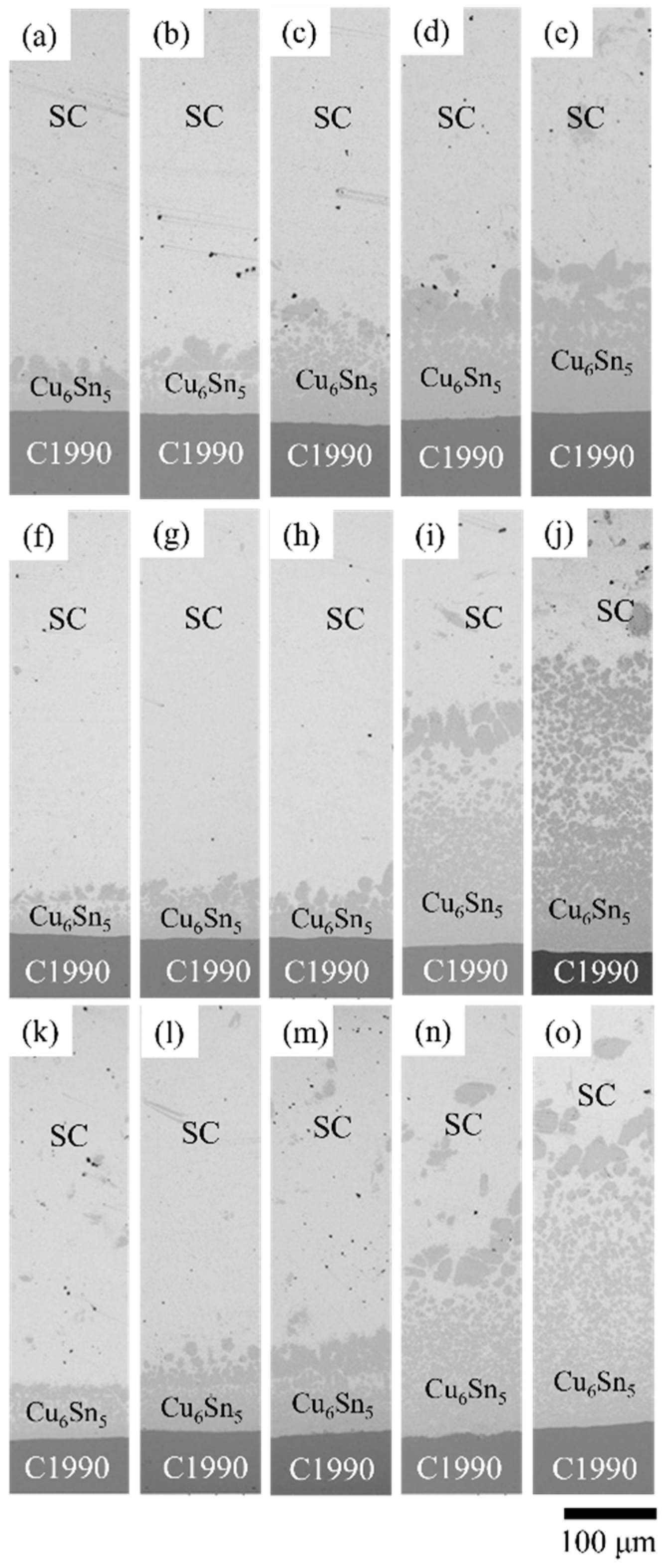
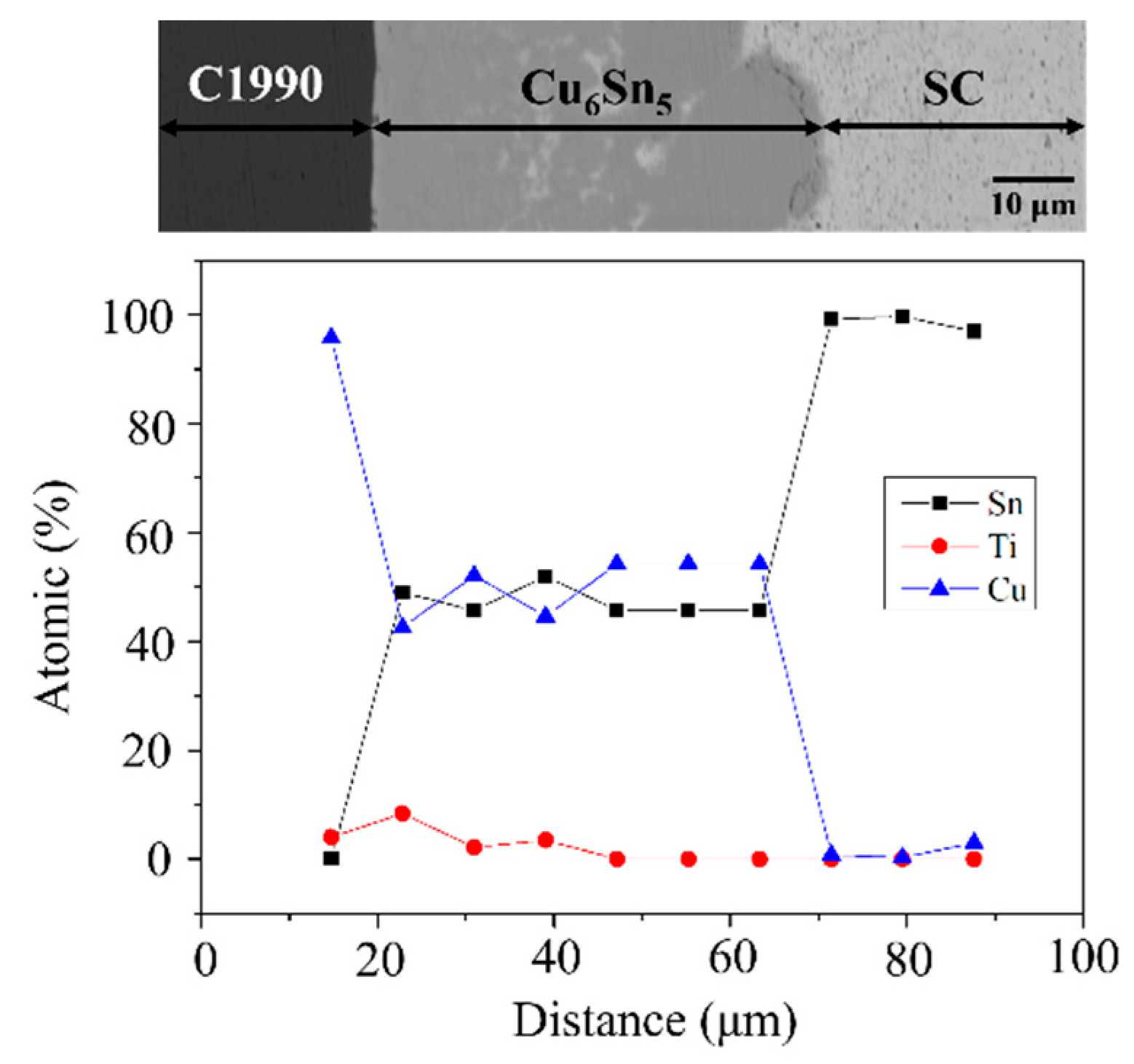
3.3. SC/C1990 HP Reaction Couples
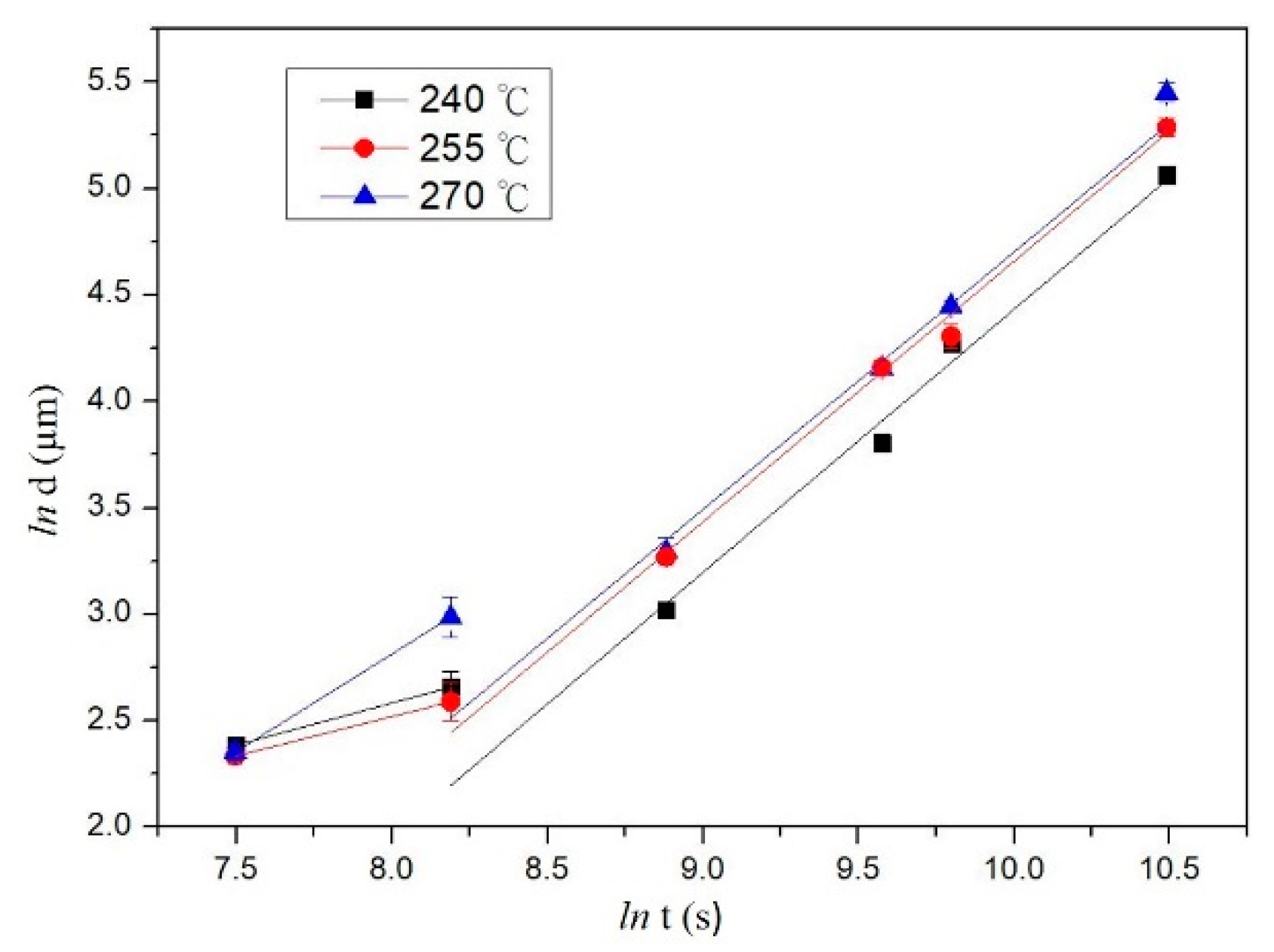
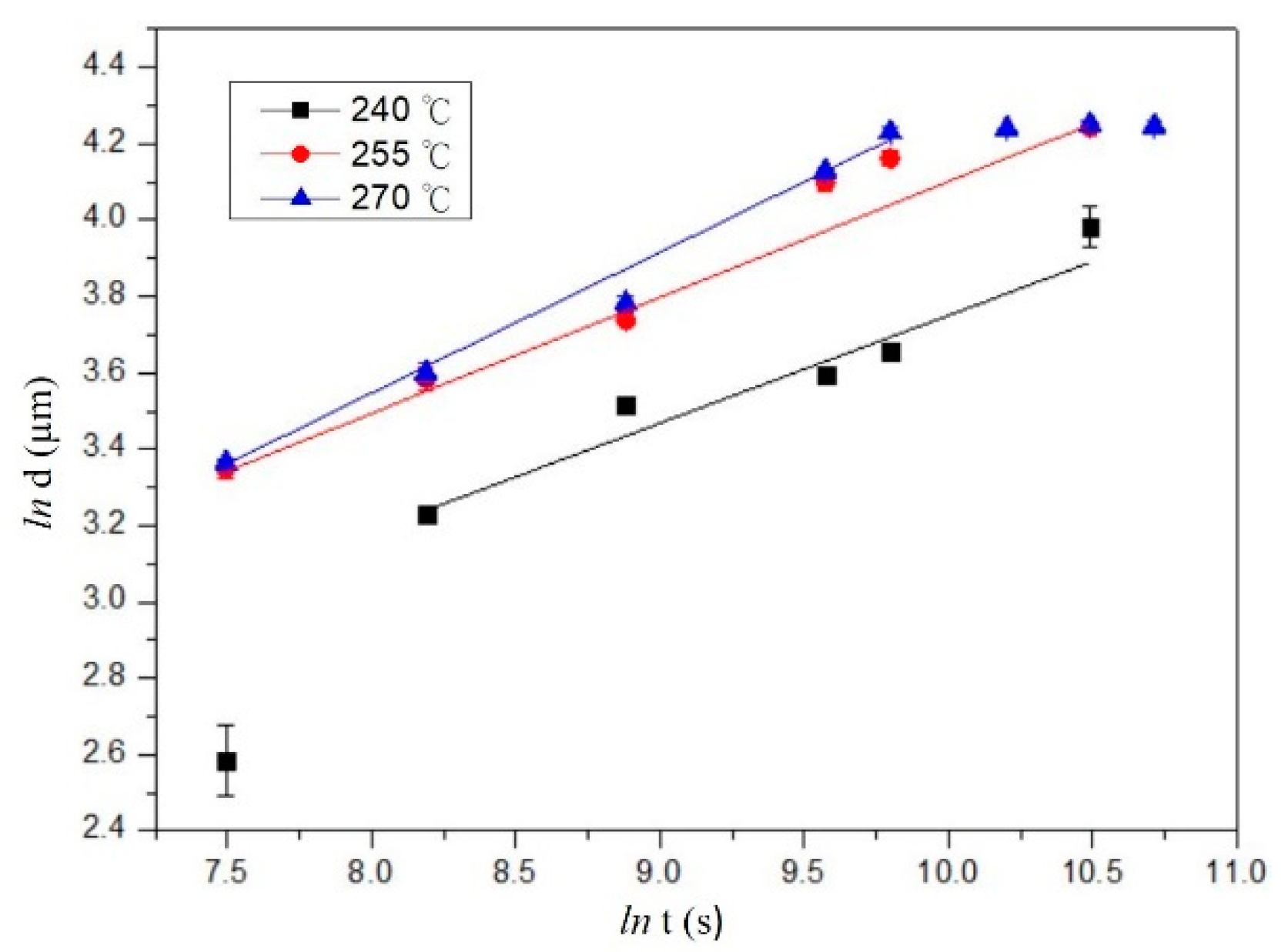
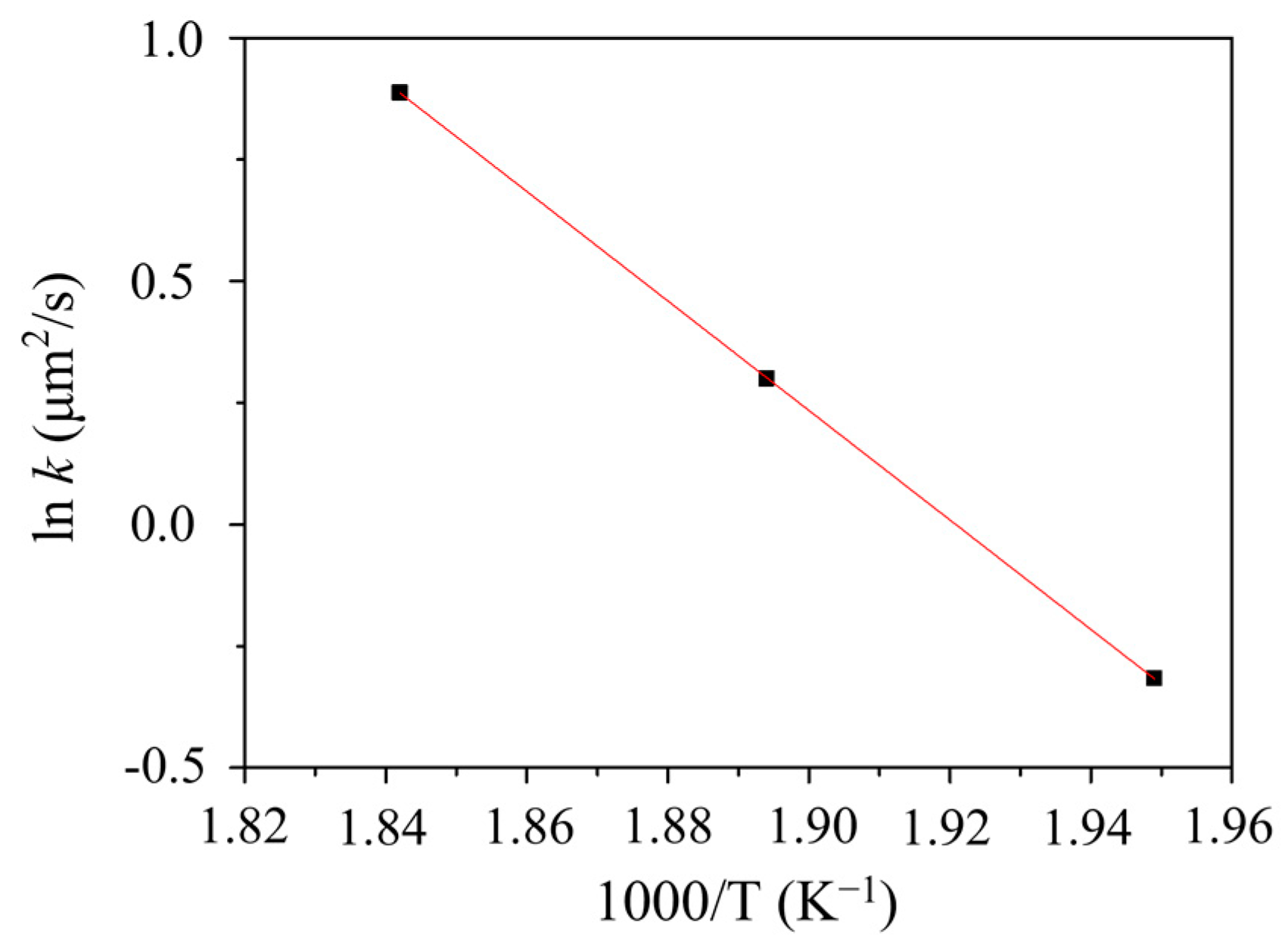
3.4. Kinetics in the Interfacial Reaction Couples
4. Conclusions
- The Fe atoms from the C194 substrate provide good nucleation sites for the IMC reaction that results in the formation of the (Cu, Fe)6Sn5 phase, which cannot be dense and thick.
- The Be atoms from Alloy 25 are the solids dissolved into the (Cu, Be)3Sn and (Cu, Be)6Sn5 phases, resulting in lattice distortion with unevenness.
- The Ti element in the C1990 HP substrate facilitates heterogeneous nucleation, resulting in no formation of other IMCs, which is responsible for the longer diffusion distance in the case of Cu6Sn5.
- The IMC growth mechanism of each system has different types, which are reaction control, grain boundary diffusion control, and diffusion control for the SC/C194, SC/Alloy 25, and SC/C1990 HP systems, respectively.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Freitas, E.S.; Osório, W.R.; Spinelli, J.E.; Garcia, A. Mechanical and corrosion resistances of a Sn–0.7 wt.%Cu lead-free solder alloy. Microelectron. Reliab. 2014, 54, 1392–1400. [Google Scholar] [CrossRef]
- Tao, Y.; Ding, D.; Li, T.; Guo, J.; Yu, Y. Influence of protective atmosphere on the solderability and reliability of OSP-based solder joints. J. Mater. Sci. Mater. Electron. 2016, 27, 4898–4907. [Google Scholar] [CrossRef]
- Kumar, P.M.; Gergely, G.; Horváth, D.K.; Gácsi, Z. Investigating the microstructural and mechanical properties of pure lead-free soldering materials (SAC305 & SAC405). Powder Metall. Prog. 2018, 18, 49–57. [Google Scholar]
- Morando, C.; Fornaro, O.; Garbellini, O.; Palacio, H. Thermal properties of Sn-based solder alloys. J. Mater. Sci. Mater. Electron. 2014, 25, 3440–3447. [Google Scholar] [CrossRef]
- Li, R.; Cong, S.; Mei, J.; Zhang, L.; Chen, Z.; Li, T.; Yuan, X. Interface evolution and mechanical properties of Sn–36Pb–2Ag solder joints under different aging conditions. J. Mater. Res. Technol. 2021, 10, 868–881. [Google Scholar] [CrossRef]
- Li, G.; Shi, Y.; Hao, H.; Xia, Z.; Lei, Y.; Guo, F. Effect of phosphorus element on the comprehensive properties of Sn–Cu lead-free solder. J. Alloys Compd. 2010, 491, 382–385. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Hammad, A.E. Enhancement of creep resistance and thermal behavior of eutectic Sn–Cu lead-free solder alloy by Ag and In-additions. Mater. Design 2012, 40, 292–298. [Google Scholar] [CrossRef]
- Zeng, G.; Xue, S.; Zhang, L.; Gao, L. Recent advances on Sn–Cu solders with alloying elements: Review. J. Mater. Sci. Mater. Electron. 2011, 22, 565–578. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, L.; Liu, Z.Q.; Xiong, M.Y.; Sun, L. Structure and properties of Sn-Cu lead-free solders in electronics packaging. Sci. Technol. Adv. Mater. 2019, 20, 421–444. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Xu, H.; Chen, J.; Liang, Q.; Wang, J.; Zhang, J. Aging properties and precipitates analysis of Cu–2.3Fe–0.03P alloy by thermomechanical treatments. Mater. Res. Express 2017, 4, 116511. [Google Scholar] [CrossRef]
- Chang, J.; Yen, Y. Investigation of the interfacial reactions and mechanical strength in the Sn-Ag-Cu (SAC)/Cu-Be alloy (Alloy 25) couple. In Proceedings of the 2018 International Conference on Electronics Packaging and iMAPS All Asia Conference (ICEP-IAAC), Kuwana, Japan, 17–21 April 2018. [Google Scholar]
- Soffa, W.A.; Laughlin, D.E. High-strength age hardening copper–titanium alloys: Redivivus. Prog. Mater. Sci. 2004, 49, 347–366. [Google Scholar] [CrossRef]
- Ong, C.G.; Lau, K.T.; Zaimi, M.; Afiq, M.; Queck, K.P. Effect of electroless Ni-P thickness on EFTECH 64-Ni, EFTECH 64-Cu and C194-Ni bump. In Proceedings of the 11th International Microsystems, Packaging, Assembly and Circuits Technology Conference (IMPACT), Taipei, Taiwan, 26–28 October 2016. [Google Scholar]
- Liu, T.; Ding, D.; Hu, Y.; Gong, Y. Effect of interfacial reaction on tin whisker formation of Sn/Ni films deposited on copper lead-frame. In Proceedings of the 15th International Conference on Electronic Packaging Technology, Chengdu, China, 12–15 August 2014. [Google Scholar]
- Li, Y.C.; Chang, C.H.; Pasana, A.S.; Hsiao, H.M.; Yen, Y.W. Interfacial reactions in lead-free solder/Cu-2.0Be (Alloy 25) couples. J. Electron. Mater. 2021, 50, 903–913. [Google Scholar] [CrossRef]
- Yen, Y.W.; Laksono, A.D.; Yang, C.Y. Investigation of the interfacial reactions between Sn-3.0 wt%Ag-0.5 wt%Cu solder and CuTi alloy (C1990HP). Microelectron. Reliab. 2019, 96, 29–36. [Google Scholar] [CrossRef]
- Laksono, A.D.; Chang, J.S.; Yan, J.; Yen, Y.W. Interfacial Reaction between Sn and Cu-Ti Alloy (C1990HP). Mater. Sci. Forum 2019, 964, 263–269. [Google Scholar] [CrossRef]
- Laksono, A.D.; Chou, J.T.; Chiang, T.Y.; Yen, Y.W. Study on Interfacial Reactions and Tensile Properties in the Sn/C1990 HP Systems. In Proceedings of the 17th International Microsystems, Packaging, Assembly and Circuits Technology Conference (IMPACT), Taipei, Taiwan, 26–28 October 2022. [Google Scholar]
- Ma, H.R.; Wang, Y.P.; Chen, J.; Ma, H.T.; Zhao, N. The effect of reflow temperature on IMC growth in Sn/Cu and Sn0.7Cu/Cu solder bumps during multiple reflows. In Proceedings of the 2017 18th International Conference on Electronic Packaging Technology (ICEPT), Harbin, China, 16–19 August 2017. [Google Scholar]
- Lee, L.M.; Mohamad, A.A. Interfacial reaction of Sn-Ag-Cu lead-free solder alloy on Cu: A Review. Adv. Mater. Sci. Eng. 2013, 2013, 123697. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, H. Phase Diagrams for Binary Alloys, 2nd ed.; Okamoto, H., Ed.; ASM International: Materials Park, OH, USA, 2000; Volume 44, pp. 307–356. [Google Scholar]
- Xie, X.C.; Zhao, X.C.; Liu, Y.; Cheng, J.W.; Zheng, B.; Gu, Y. Effect of Ag addition on growth of the interfacial intermetallic compounds between Sn-0.7 Cu solder and Cu substrate. Mater. Sci. Forum 2015, 815, 129–134. [Google Scholar] [CrossRef]
- Lai, Y.; Hu, X.; Li, Y.; Jiang, X. Interfacial microstructure evolution and shear strength of Sn0.7Cu–xNi/Cu solder joints. J. Mater. Sci. Mater. Electron. 2018, 29, 11314–11324. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, L. Properties and microstructures of Sn-Ag-Cu-X lead-free solder joints in electronic packaging. Adv. Mater. Sci. Eng. 2015, 2015, 639028. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.H.; Yu, C.Y.; Ho, C.Y.; Duh, J.G. Retardation of (Cu, Ni)6Sn5 spalling in Sn–Ag–Cu/Ni solder joints via controlling the grain structure of Ni metallization layer. Mater. Lett. 2013, 105, 40–42. [Google Scholar] [CrossRef]
- Lin, S.K.; Chen, K.D.; Chen, H.; Liou, W.K.; Yen, Y.W. Abnormal spalling phenomena in the Sn-0.7Cu/Au/Ni/SUS304 interfacial reactions. J. Mater. Res. 2010, 25, 2278–2286. [Google Scholar] [CrossRef]
- Arenas, M.F.; Acoff, V.L. Contact angle measurements of Sn-Ag and Sn-Cu lead-free solders on copper substrates. J. Electron. Mater. 2004, 33, 1452–1458. [Google Scholar] [CrossRef]
- Mookam, N.; Tunthawiroon, P.; Kanlayasiri, K. Effects of copper content in Sn-based solder on the intermetallic phase formation and growth during soldering. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Phuket, Thailand, 27–29 January 2018. [Google Scholar]
- Saunders, N.; Miodownik, A.P. The Cu-Sn (Copper-Tin) system. Bull. Alloy Phase Diagr. 1990, 11, 278–287. [Google Scholar] [CrossRef]
- Yen, Y.W.; Chou, W.T.; Tseng, Y.; Lee, C.; Hsu, C.L. Investigation of dissolution behavior of metallic substrates and intermetallic compound in molten lead-free solders. J. Electron. Mater. 2008, 37, 73–83. [Google Scholar] [CrossRef]
- Yen, Y.W.; Lee, C.Y.; Kuo, M.H.; Chao, K.S.; Chen, K.D. Interfacial reactions between lead-free solders and the multilayer Au/Ni/SUS304 substrate. Int. J. Mater. Res. 2009, 100, 672–676. [Google Scholar] [CrossRef]
- Kim, H.; Liou, H.; Tu, K.N. Three-dimensional morphology of a very rough interface formed in the soldering reaction between eutectic SnPb and Cu. Appl. Phys. Lett. 1995, 66, 2337–2339. [Google Scholar] [CrossRef]
- Liu, A.A.; Kim, H.; Tu, K.N.; Totta, P.A. Spalling of Cu6Sn5 spheroids in the soldering reaction of eutectic SnPb on Cr/Cu/Au thin films. J. Appl. Phys. 1996, 80, 2774–2780. [Google Scholar] [CrossRef]
- Tu, K.N.; Zeng, K. Tin–lead (SnPb) solder reaction in flip chip technology. Mater. Sci. Eng. R Rep. 2001, 34, 1–58. [Google Scholar] [CrossRef]
- Yin, Z.; Lin, M.; Li, Q.; Wu, Z. Effect of doping Ni nanoparticles on microstructure evolution and shear behavior of Sn–3.0Ag–0.5Cu (SAC305)/Cu–2.0Be solder joints during reflowing. J. Mater. Sci. Mater. Electron. 2020, 31, 4905–4914. [Google Scholar] [CrossRef]
- Shen, J.; Chan, Y.C.; Liu, S.Y. Growth mechanism of Ni3Sn4 in a Sn/Ni liquid/solid interfacial reaction. Acta Mater. 2009, 57, 5196–5206. [Google Scholar] [CrossRef]
- Yen, Y.W.; Tsai, P.H.; Fang, Y.K.; Lo, S.C.; Hsieh, Y.P.; Lee, C. Interfacial reactions on Pb-free solders with Au/Pd/Ni/Cu multilayer substrates. J. Alloys Compd. 2010, 503, 25–30. [Google Scholar] [CrossRef]
- Shen, J.; Pu, Y.; Wu, D.; Tang, Q.; Zhao, M. Effects of minor Bi, Ni on the wetting properties, microstructures, and shear properties of Sn–0.7Cu lead-free solder joints. J. Mater. Sci. Mater. Electron. 2015, 26, 1572–1580. [Google Scholar] [CrossRef]
- Tian, S.; Zhou, J.; Xue, F.; Cao, R.; Wang, F. Microstructure, interfacial reactions and mechanical properties of Co/Sn/Co and Cu/Sn/Cu joints produced by transient liquid phase bonding. J. Mater. Sci. Mater. Electron. 2018, 29, 16388–16400. [Google Scholar] [CrossRef]
- Hu, X.B.; Wen, J.; Yen, Y.W. Interfacial reactions in the Sn-3.0Ag-0.5Cu/C194 couples. In Proceedings of the International Conference on Electronics Packaging (ICEP), Tokyo, Japan, 11–14 May 2022. [Google Scholar]
- Madeni, J.; Liu, S.; Siewert, T. Intermetallics formation and growth at the interface of tin-based solder alloys and copper substrates. In Proceedings of the 2nd International Brazing and Soldering Conference (ISBC), San Diego, CA, USA, 17–19 February 2003. [Google Scholar]




| Components | C194 | Alloy 25 | C1990 HP |
|---|---|---|---|
| Cu | 97.8 | 97.8 | 96.7 |
| Fe | 2.2 | - | - |
| Be | - | 2.2 | - |
| Ti | - | - | 3.3 |
| SC/C194 Couples | ||||||
| Temp (°C) | Reaction Time (h) | |||||
| 0.5 | 1 | 2 | 4 | 5 | 10 | |
| 240 | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn |
| 255 | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn |
| 270 | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn | (Cu, Fe)6Sn5(Cu, Fe)3Sn |
| SC/Alloy 25 Couples | ||||||
| Temp (°C) | Reaction Time (h) | |||||
| 0.5 | 1 | 2 | 4 | 5 | 10 | |
| 240 | (Cu, Be)6Sn5 | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn |
| 255 | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn |
| 270 | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn | (Cu, Be)6Sn5(Cu, Be)3Sn |
| SC/C1990HP Couples | ||||||
| Temp (°C) | Reaction Time (h) | |||||
| 0.5 | 1 | 2 | 4 | 5 | ||
| 240 | Cu6Sn5 | Cu6Sn5 | Cu6Sn5 | Cu6Sn5 | Cu6Sn5 | |
| 255 | Cu6Sn5 | Cu6Sn5 | Cu6Sn5 | Cu6Sn5 | Cu6Sn5 | |
| 270 | Cu6Sn5 | Cu6Sn5 | Cu6Sn5 | Cu6Sn5 | Cu6Sn5 | |
| Temperature | Time | n |
|---|---|---|
| 240 °C | 0.5~1 h | 0.46 |
| 240 °C | 1~10 h | 1.05 |
| 255 °C | 0.5~1 h | 0.47 |
| 255 °C | 1~10 h | 1.04 |
| 270 °C | 0.5~10 h | 0.96 |
| Temperature | Time | n |
|---|---|---|
| 240 °C | 1~10 h | 0.28 |
| 255 °C | 0.5~10 h | 0.30 |
| 270 °C | 0.5~5 h | 0.34 |
| System | Growth Rate Constant k (×1014 m2/s) | Q (kJ/mole) | Reference | ||
|---|---|---|---|---|---|
| 240 °C | 255 °C | 270 °C | |||
| SC/C1990 HP | 53.0 | 180.0 | 590.0 | 186.0 | This Study |
| Sn/Alloy 25 | 273.8 | 531 | 286.8 | 27.3 | [15] |
| SAC/Alloy 25 | 162.8 | 351.3 | 342.9 | 58.0 | [15] |
| SAC/C1990 HP | 73.1 | 80.8 | 123.9 | 40.6 | [16] |
| SAC/C194 | - | - | - | 45.2 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laksono, A.D.; Tsai, T.-Y.; Chung, T.-H.; Chang, Y.-C.; Yen, Y.-W. Investigation of the Sn-0.7 wt.% Cu Solder Reacting with C194, Alloy 25, and C1990 HP Substrates. Metals 2023, 13, 12. https://doi.org/10.3390/met13010012
Laksono AD, Tsai T-Y, Chung T-H, Chang Y-C, Yen Y-W. Investigation of the Sn-0.7 wt.% Cu Solder Reacting with C194, Alloy 25, and C1990 HP Substrates. Metals. 2023; 13(1):12. https://doi.org/10.3390/met13010012
Chicago/Turabian StyleLaksono, Andromeda Dwi, Tzu-Yang Tsai, Tai-Hsuan Chung, Yong-Chi Chang, and Yee-Wen Yen. 2023. "Investigation of the Sn-0.7 wt.% Cu Solder Reacting with C194, Alloy 25, and C1990 HP Substrates" Metals 13, no. 1: 12. https://doi.org/10.3390/met13010012






