Structure and Heat Transfer Characteristic Evolution of CaO-SiO2-CaF2-Based Solid Mold Flux Film upon Solidification
Abstract
:1. Introduction
2. Experiments
2.1. Mold Flux Selection and Slag Film Solidification
2.2. Measurements and Analysis
3. Result and Discussions
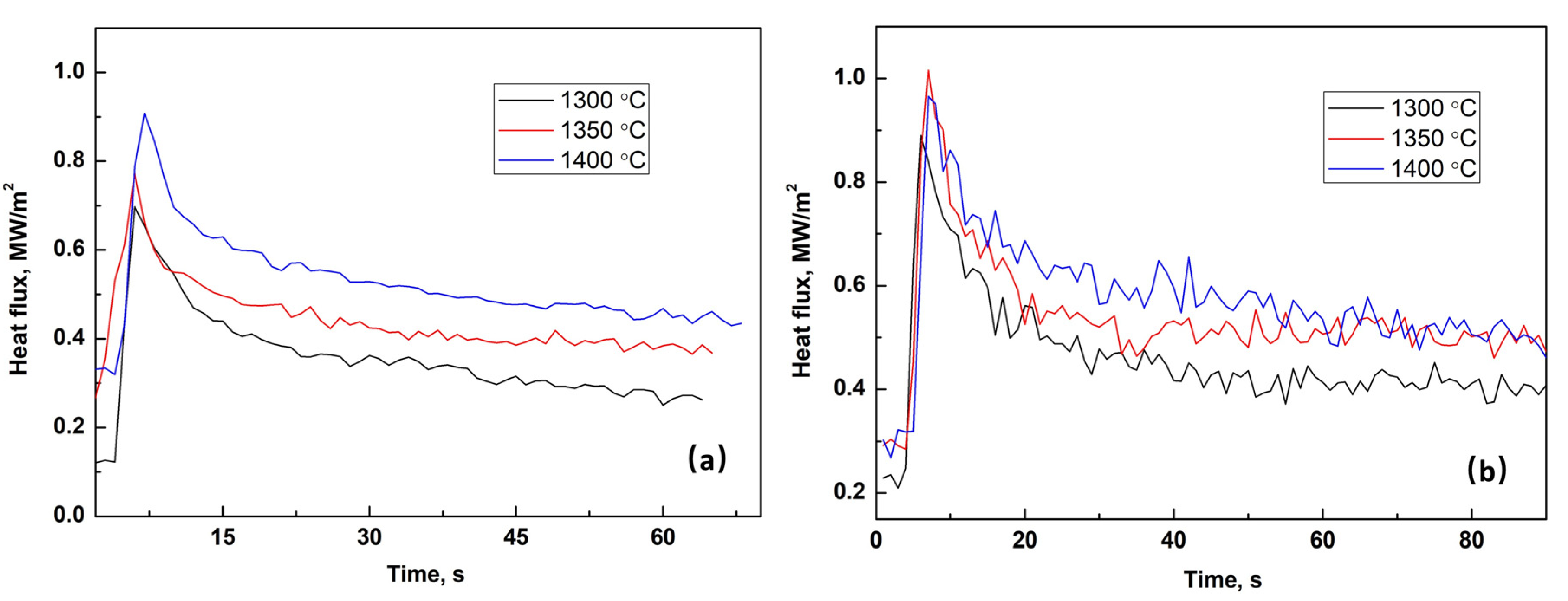
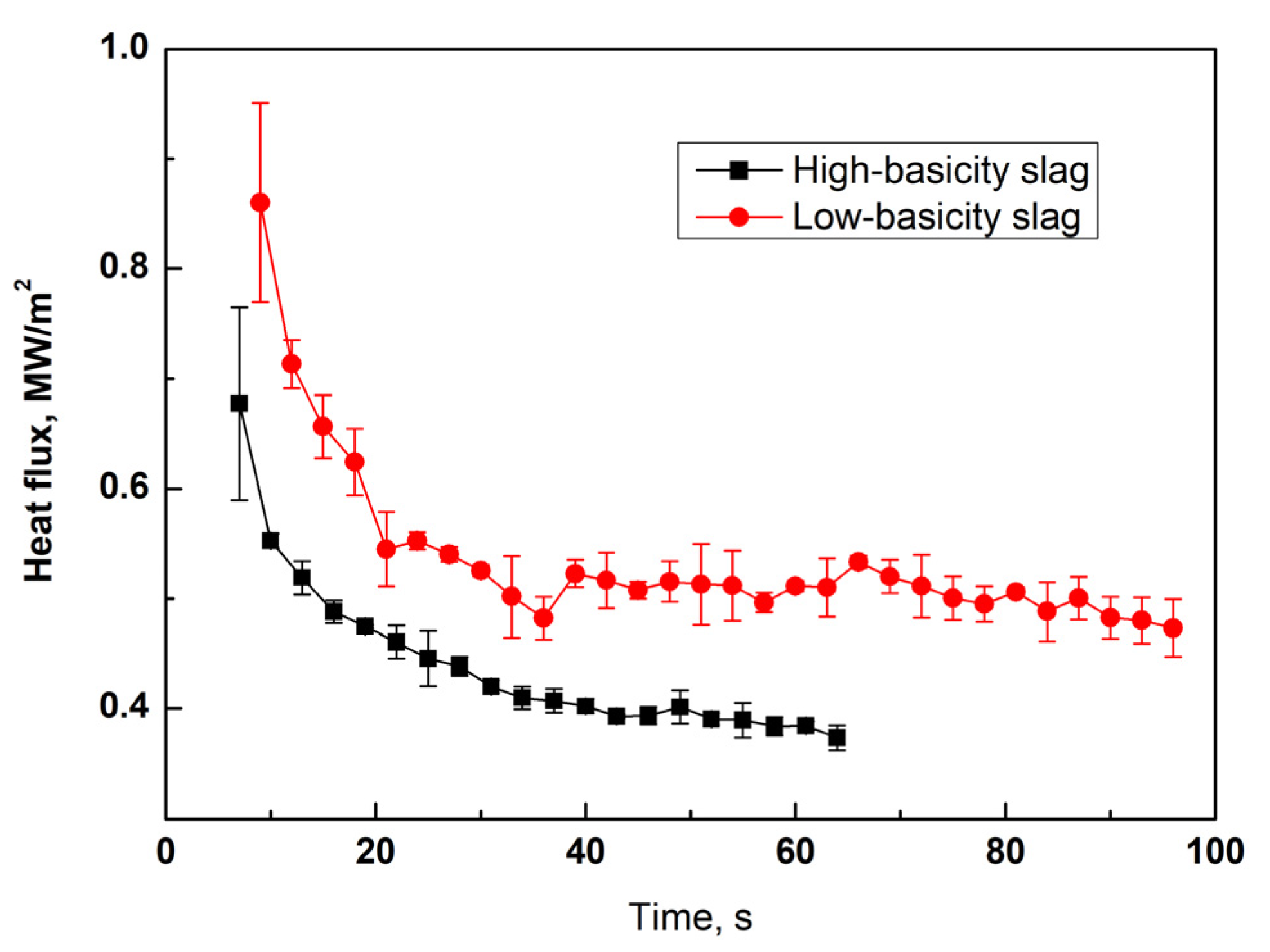
3.1. Heat Flux through Slag Film upon Solidification
3.2. Structure Evolution of Solid Films
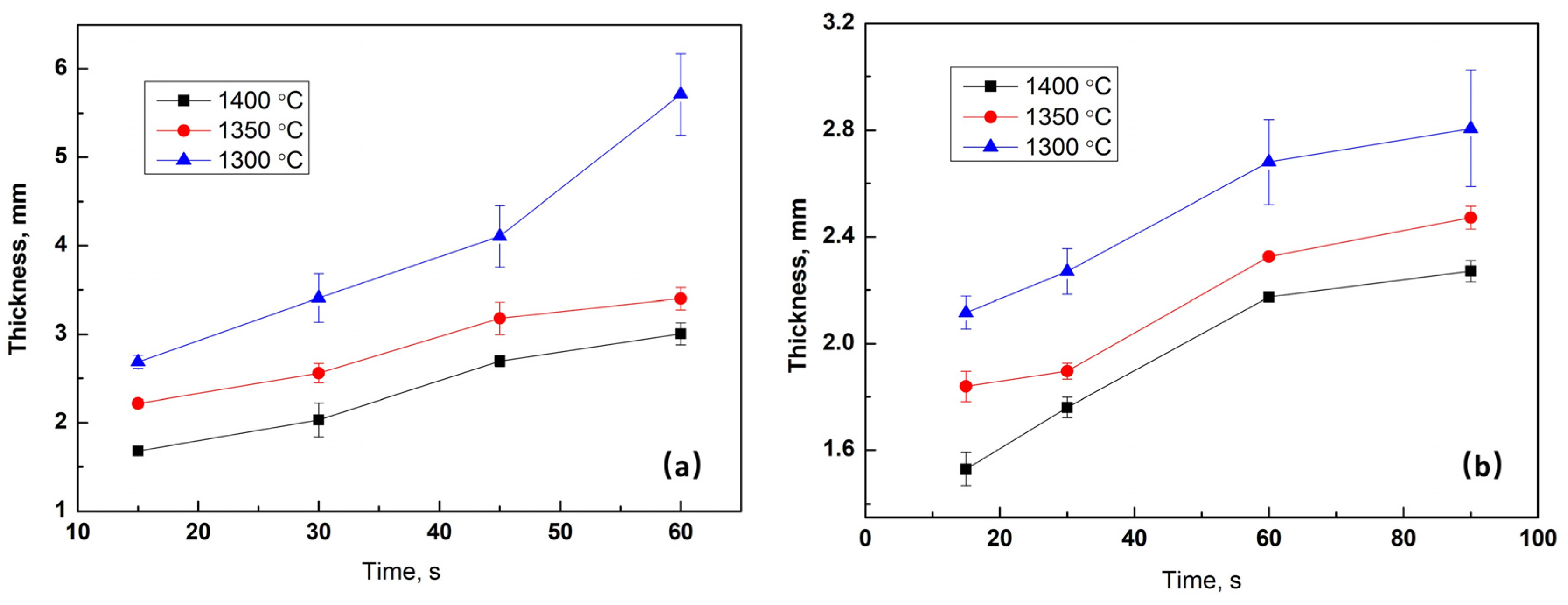
3.2.1. Total Thickness of Films
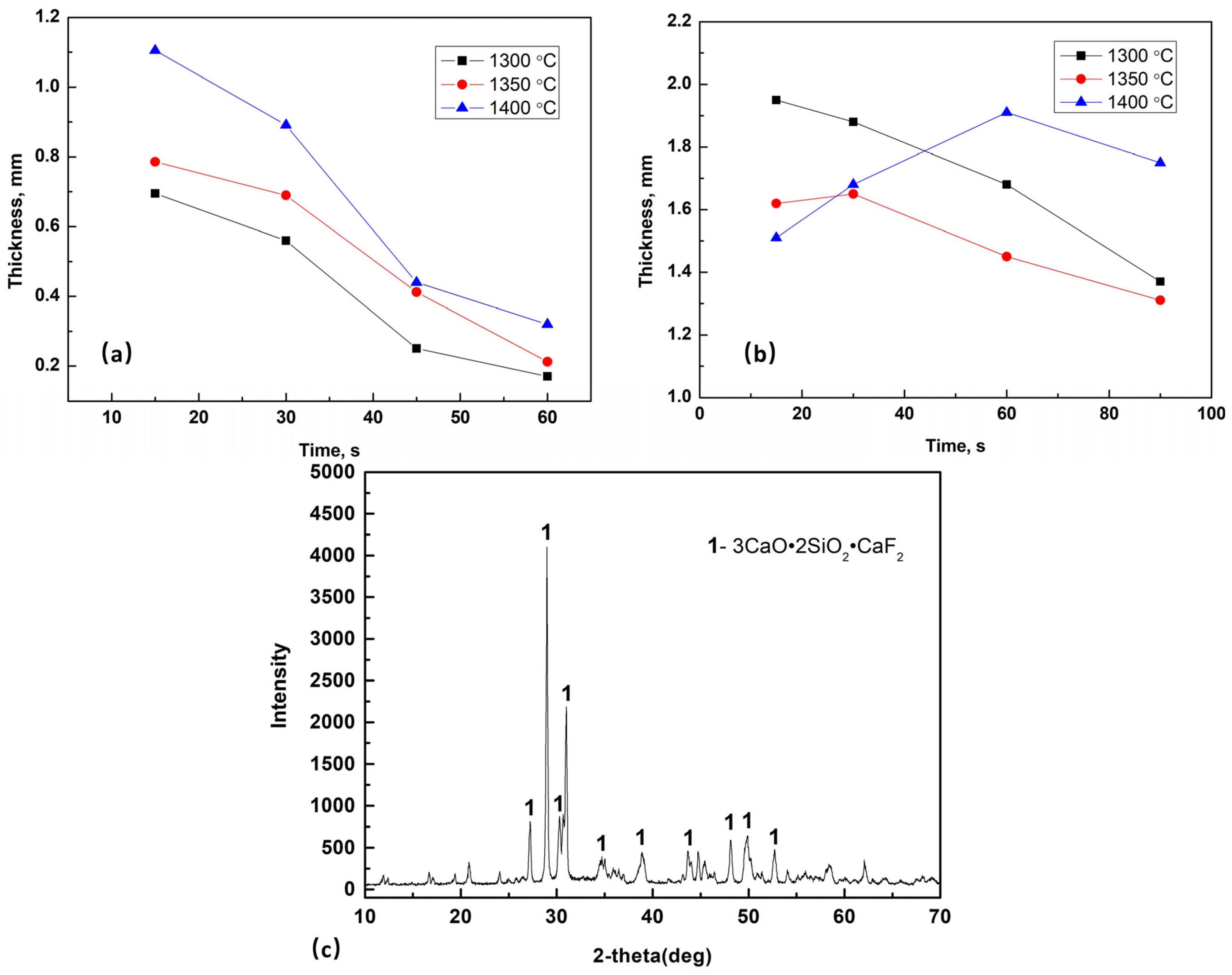
3.2.2. Thickness of Glassy Layer of Films
3.2.3. Fusion Cracks within Glassy Layers
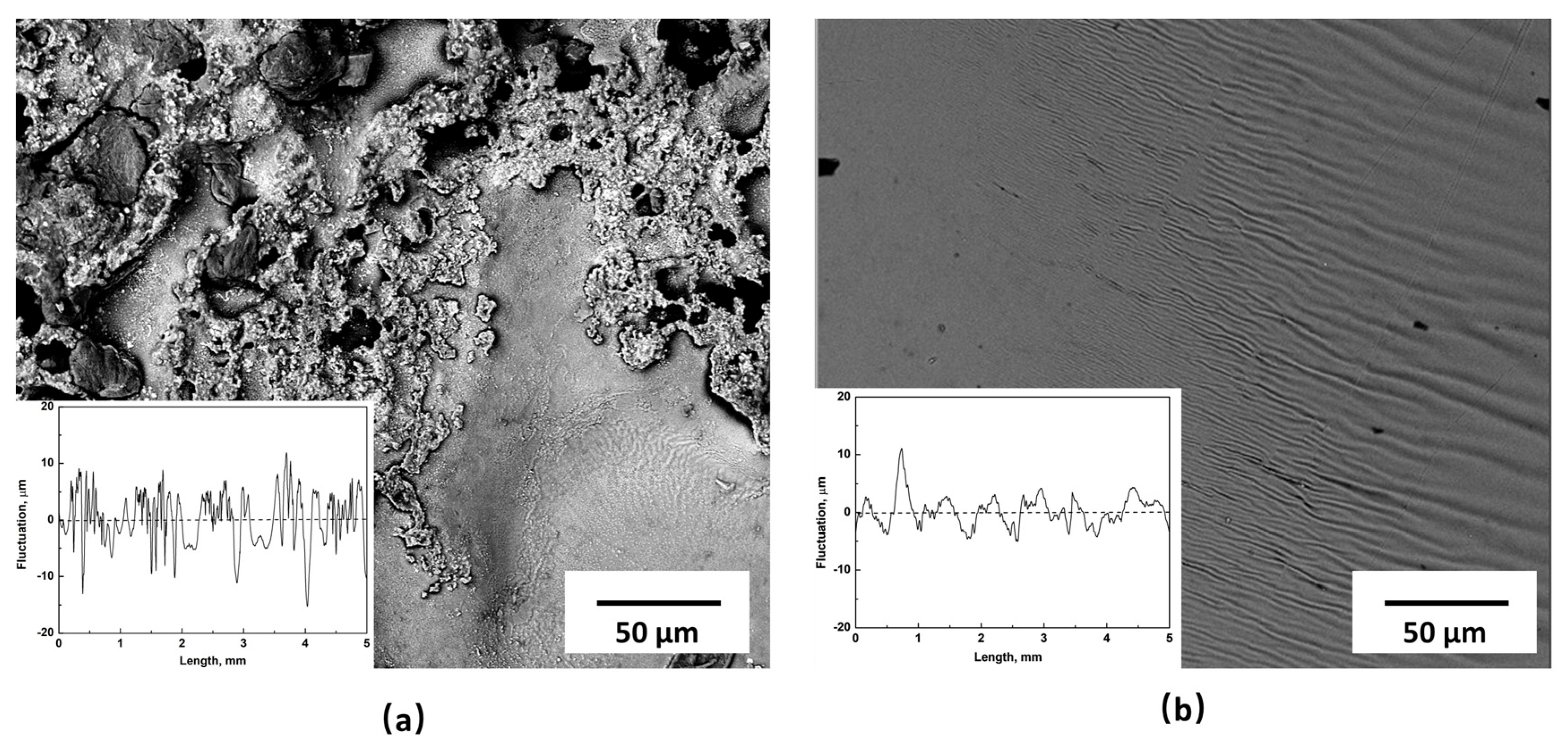
3.2.4. Roughness of Film Surfaces Contacted with Copper Wall
3.2.5. Closed Porosity of Films
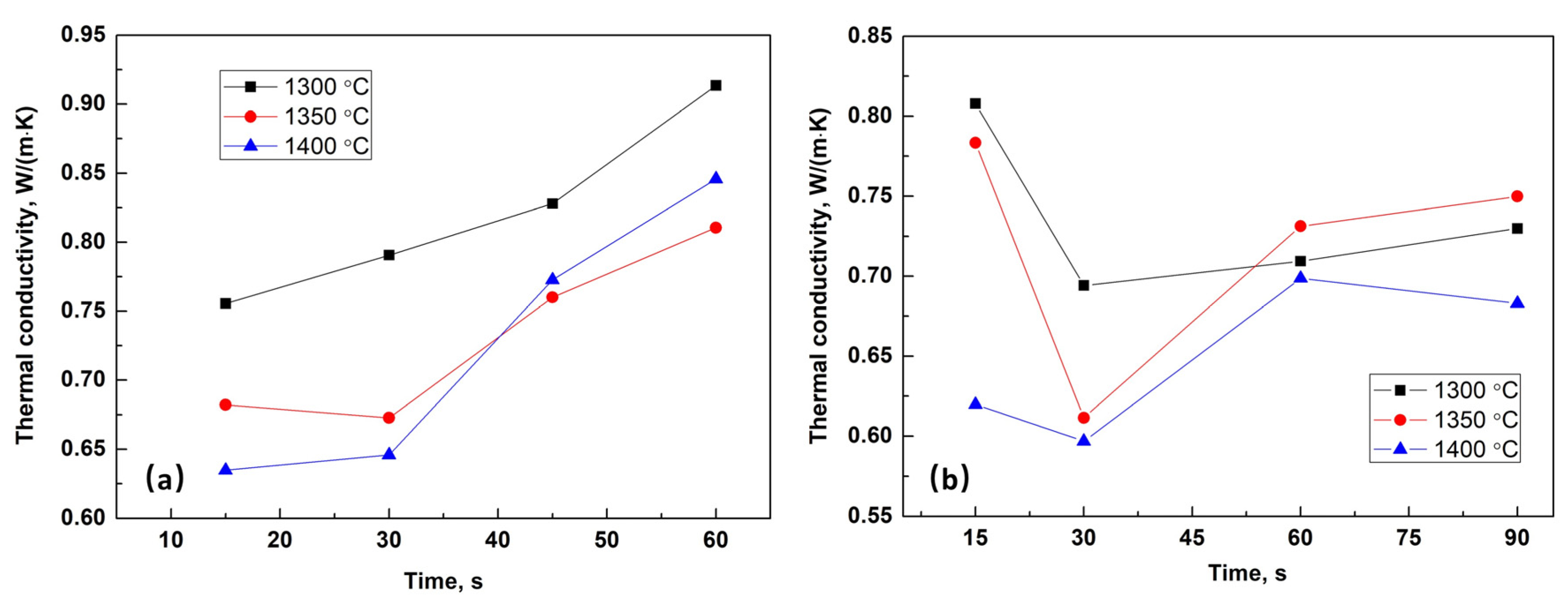
3.3. Evolution of Heat Transfer Characteristics of Slag Films upon Solidification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mills, K.C.; Fox, A.B. The role of mould fluxes in continuous casting-so simple and yet so complex. ISIJ Int. 2003, 43, 1479–1486. [Google Scholar] [CrossRef]
- Mills, K.C. Structure and Properties of slags used in the continuous casting of steel: Part 1 conventional mould powders. ISIJ Int. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Wang, W.; Lu, B.; Xiao, D. A review of mold flux development for the casting of high-Al steels. Metall. Mater. Trans. B 2016, 47, 384–389. [Google Scholar] [CrossRef]
- Cho, J.W.; Emi, T.; Shibata, H.; Suzuki, M. Heat transfer across mold flux film in mold during initial solidification in continuous casting of steel. ISIJ Int. 1998, 38, 834–842. [Google Scholar] [CrossRef]
- Yoon, D.W.; Cho, J.W.; Kim, S.H. Controlling radiative heat transfer across the mold flux layer by the scattering effect of the borosilicate mold flux system with metallic iron. Metall. Mater. Trans. B 2017, 48, 1951–1961. [Google Scholar] [CrossRef]
- Bhagurkar, A.G.; Qin, R. The microstructure formation in slag solidification at continuous casting mold. Metals 2022, 12, 617. [Google Scholar] [CrossRef]
- Mills, K.C. Treatise on Process Metallurgy; Elsevier: Oxford, UK, 2014; Volume 3, pp. 435–475. [Google Scholar]
- Long, X.; He, S.-P.; Xu, J.-F.; Huo, X.-L.; Wang, Q. Properties of high basicity mold fluxes for peritectic steel slab casting. J. Iron Steel Res. Int. 2012, 19, 39–45. [Google Scholar] [CrossRef]
- Nakai, K.; Sakashita, T.; Hashio, M.; Kawasaki, M.; Nakajima, K.; Sugitani, Y. Effect of mild cooling in mould upon solidified shell formation of continuous cast slab. Tetsu-Hagane 1987, 73, 498–504. [Google Scholar] [CrossRef]
- Hunt, A.; Stewart, B. Techniques for controlling heat transfer in the mould-strand gap in order to use fluoride free mould powder for continuous casting of peritectic steel grades. In Advances in Molten Slags, Fluxes, and Salts, Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts, Seattle, WA, USA, 22–25 May 2016; Springer: Cham, Switzerland, 2016; pp. 349–356. [Google Scholar]
- Ji, J.; Mao, Y.; Zhang, X.; Chen, W.; Zhang, L.; Wang, Q. Initial solidification and heat transfer at different locations of slab continuous casting mold through 3D coupled model. Steel Res. Int. 2021, 92, 2000714. [Google Scholar] [CrossRef]
- Yan, X.; Pan, W.; Wang, X.; Zhang, X.; He, S.; Wang, Q. Electrical conductivity, viscosity and structure of CaO–Al2O3-based mold slags for continuous casting of high-Al steels. Metall. Mater. Trans. B 2021, 52, 2526–2535. [Google Scholar] [CrossRef]
- Ji, J.; Cui, Y.; Wang, S.; He, S.; Wang, Q.; Zhang, X. Effect of TiO2 substituting SiO2 on the rheological and crystallization behavior of mold slags for casting Ti-containing steel. Ceram. Int. 2022, 48, 256–265. [Google Scholar] [CrossRef]
- Nakada, H.; Susa, M.; Seko, Y.; Hayashi, M.; Nagata, K. Mechanism of heat transfer reduction by crystallization of mold flux for continuous casting. ISIJ Int. 2008, 48, 446–453. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, W.; Zhou, L.; Lu, B.; Kang, Y.B. Effects of MnO on crystallization, melting, and heat transfer of CaO-Al2O3-based mold flux used for high Al-TRIP steel casting. Metall. Mater. Trans. B 2014, 45, 1510–1519. [Google Scholar] [CrossRef]
- Susa, M.; Kushimoto, A.; Toyota, H.; Hayashi, M.; Endo, R.; Kobayashi, Y. Effects of both crystallisation and iron oxides on the radiative heat transfer in mould fluxes. ISIJ Int. 2009, 49, 1722–1729. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Lv, M.; Fang, M.; Liu, K. Numerical simulation of the fluid flow, heat transfer, and solidification in ultrahigh speed continuous casting billet mold. Steel Res. Int. 2022, 93, 2100673. [Google Scholar] [CrossRef]
- Yang, J.; Chen, D.; Long, M.; Duan, H. An approach for modelling slag infiltration and heat transfer in continuous casting mold for high Mn–high Al steel. Metals 2020, 10, 51. [Google Scholar] [CrossRef]
- Wen, G.H.; Zhu, X.B.; Tang, P.; Yang, B.; Yu, X. Influence of raw material type on heat transfer and structure of mould slag. ISIJ Int. 2011, 51, 1028–1032. [Google Scholar] [CrossRef]
- Xu, C.; Wang, W.; Zhou, L.; Xie, S.; Zhang, C. The effects of Cr2O3 on the melting, viscosity, heat transfer, and crystallization behaviors of mold flux used for the casting of Cr-bearing alloy steels. Metall. Mater. Trans. B 2015, 46, 882–892. [Google Scholar] [CrossRef]
- Anisimov, K.N.; Longinov, A.M.; Gusev, M.P.; Zarubin, S.V. Influence of mold flux on the thermal processes in the mold. Steel Transl. 2016, 46, 589–594. [Google Scholar] [CrossRef]
- Assis, K.L.S. Heat Transfer through Mold Fluxes: A New Approach to Measure Thermal Properties of Slags. Ph. D. Thesis, Carnegie Mellon University, Pittsburgh, PA, USA, 2016. [Google Scholar]
- Assis, K.L.S.; Pistorius, P.C. Improved cold-finger measurement of heat flux through solidified mould flux. Ironmak. Steelmak. 2018, 45, 502–508. [Google Scholar] [CrossRef]
- Long, X.; He, S.; Wang, Q.; Pistorius, P.C. Structure of solidified films of mold flux for peritectic steel. Metall. Mater. Trans. B 2017, 48, 1652–1658. [Google Scholar] [CrossRef]
- Masahito, H.; Masayuki, K.; Tadao, W. Influence of Na2O on phase relation between mold flux composition and cuspidine. ISIJ Int. 2004, 44, 827–835. [Google Scholar]
- Nakada, H.; Fukuyama, H.; Nagata, K. Effect of NaF addition to mold flux on cuspidine primary field. ISIJ Int. 2006, 46, 1660–1667. [Google Scholar] [CrossRef]
- Watanabe, T.; Fukuyama, H.; Nagata, K. Stability of cuspidine (3CaO·2SiO2·CaF2) and phase relations in the CaO-SiO2-CaF2 system. ISIJ Int. 2002, 42, 489–497. [Google Scholar] [CrossRef]
- Zeng, J.; Long, X.; You, X.; Li, M.; Wang, Q.; He, S. Structure of solidified films of CaO-SiO2-Na2O based low-fluorine mold flux. Metals 2019, 9, 93. [Google Scholar] [CrossRef]
- Andersson, S.P.; Eggertson, C. Thermal conductivity of powders used in continuous casting of steel, part 1—glassy and crystalline slags. Ironmak. Steelmak. 2015, 42, 456–464. [Google Scholar] [CrossRef]




| No. | CaO | SiO2 | F | Na2O | Al2O3 | Li2O | MgO | Basicity |
|---|---|---|---|---|---|---|---|---|
| 1 | 41 | 32 | 10 | 11 | 3 | - | 1 | 1.28 |
| 2 | 34 | 40 | 8 | 13 | 2 | 1.5 | 1 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, X.; Luo, W.; Li, X.; Long, S.; Ma, H.; Luo, D.; Zheng, C. Structure and Heat Transfer Characteristic Evolution of CaO-SiO2-CaF2-Based Solid Mold Flux Film upon Solidification. Metals 2024, 14, 1. https://doi.org/10.3390/met14010001
Long X, Luo W, Li X, Long S, Ma H, Luo D, Zheng C. Structure and Heat Transfer Characteristic Evolution of CaO-SiO2-CaF2-Based Solid Mold Flux Film upon Solidification. Metals. 2024; 14(1):1. https://doi.org/10.3390/met14010001
Chicago/Turabian StyleLong, Xiao, Wenbo Luo, Xiang Li, Shaolei Long, Honggang Ma, Dayang Luo, and Congxin Zheng. 2024. "Structure and Heat Transfer Characteristic Evolution of CaO-SiO2-CaF2-Based Solid Mold Flux Film upon Solidification" Metals 14, no. 1: 1. https://doi.org/10.3390/met14010001





