Influence of Basicity and Calcium-Containing Substances on the Consolidation Mechanism of Fluxed Iron Ore Pellets
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental Method
2.2.1. Pelletizing
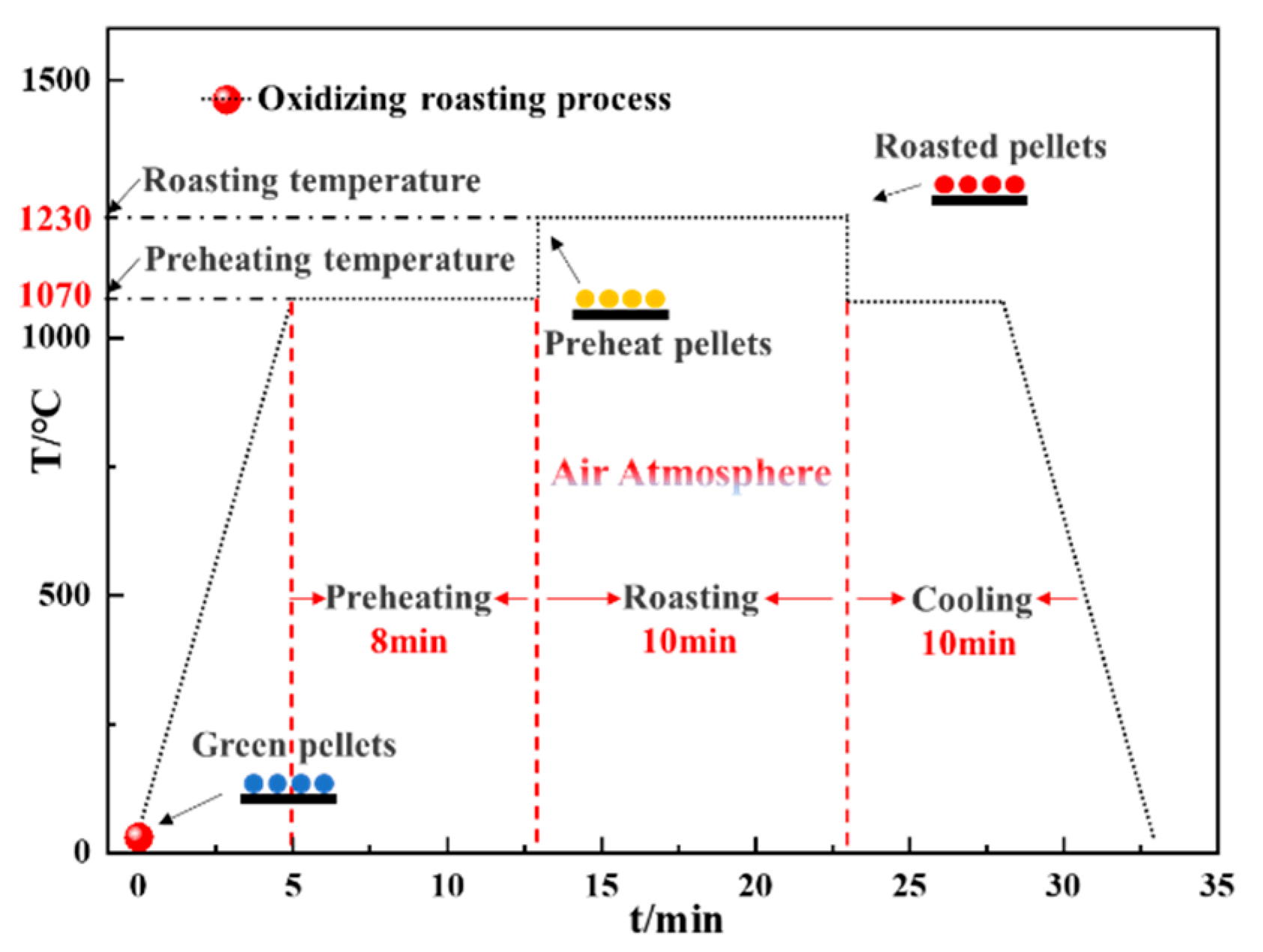
2.2.2. Preparation of Oxidized Roasting Pellets
2.2.3. Analysis and Characterization
3. Results and Discussion
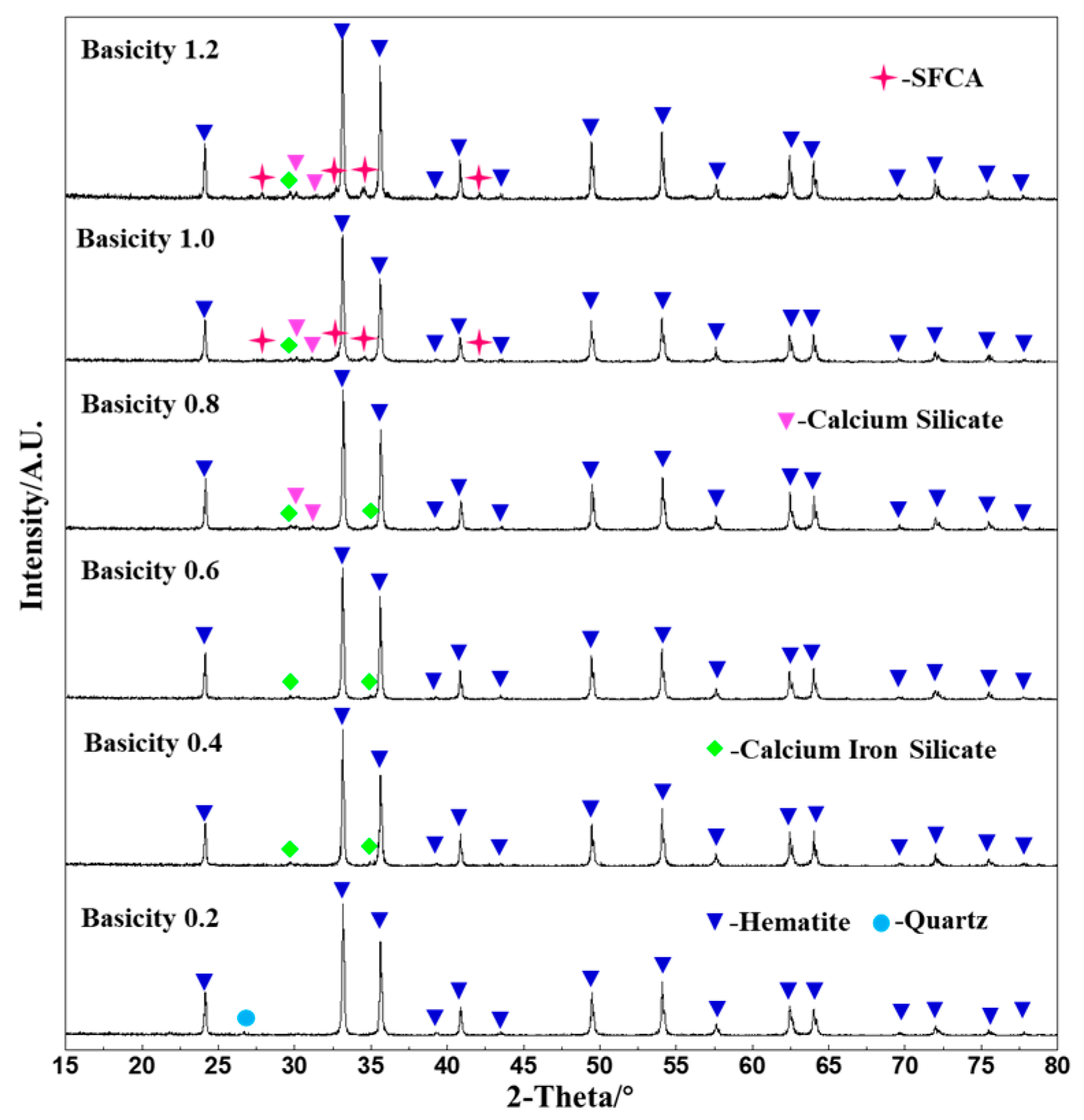
3.1. Phase Composition of Fluxed Pellets with Different Basicity
3.2. Microstructures Analysis of Different Basicity Fluxed Pellets
3.2.1. Electron Microscope Microstructures of Different Basicity Pellets
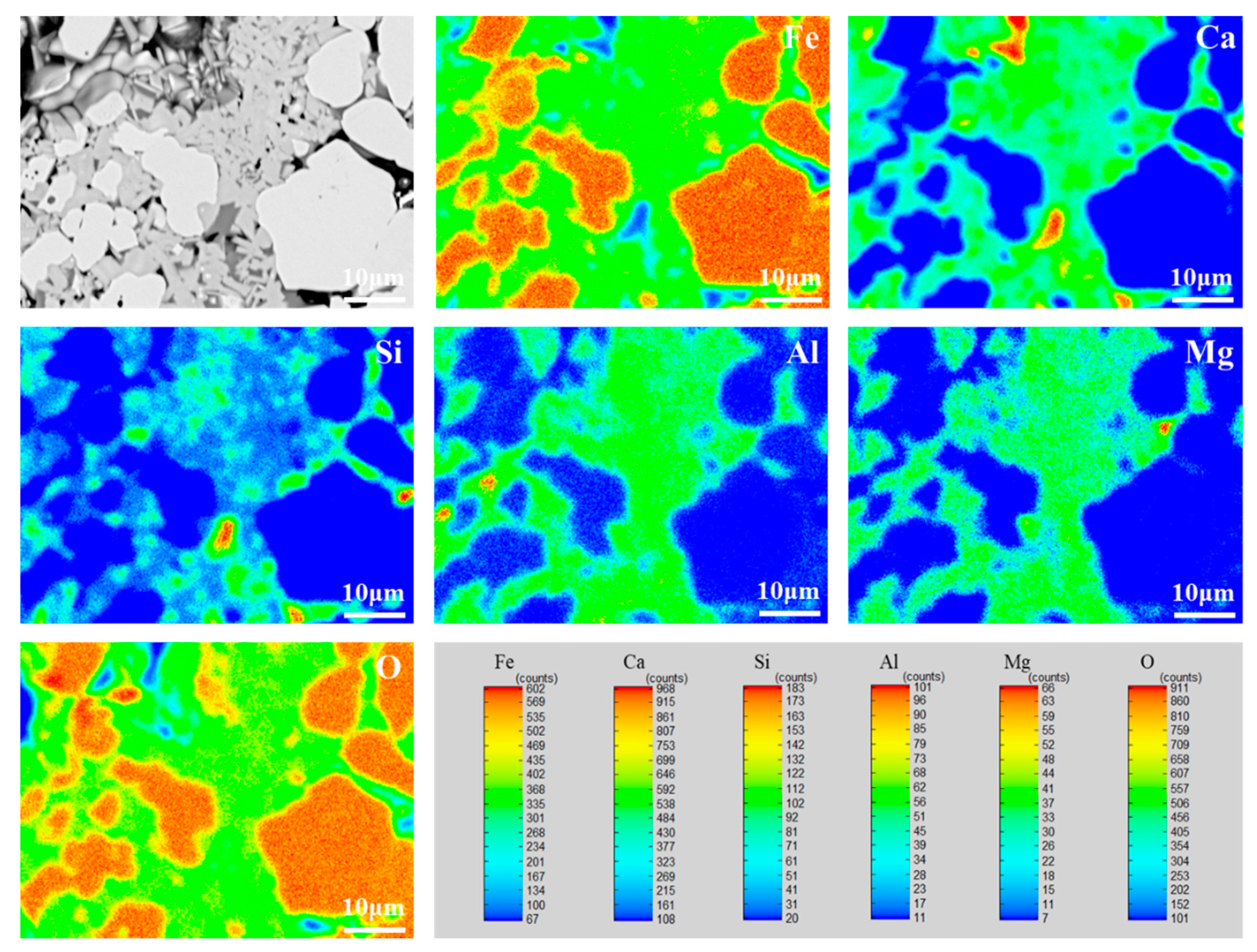
3.2.2. Element Distribution Analysis of High-Basicity Pellets
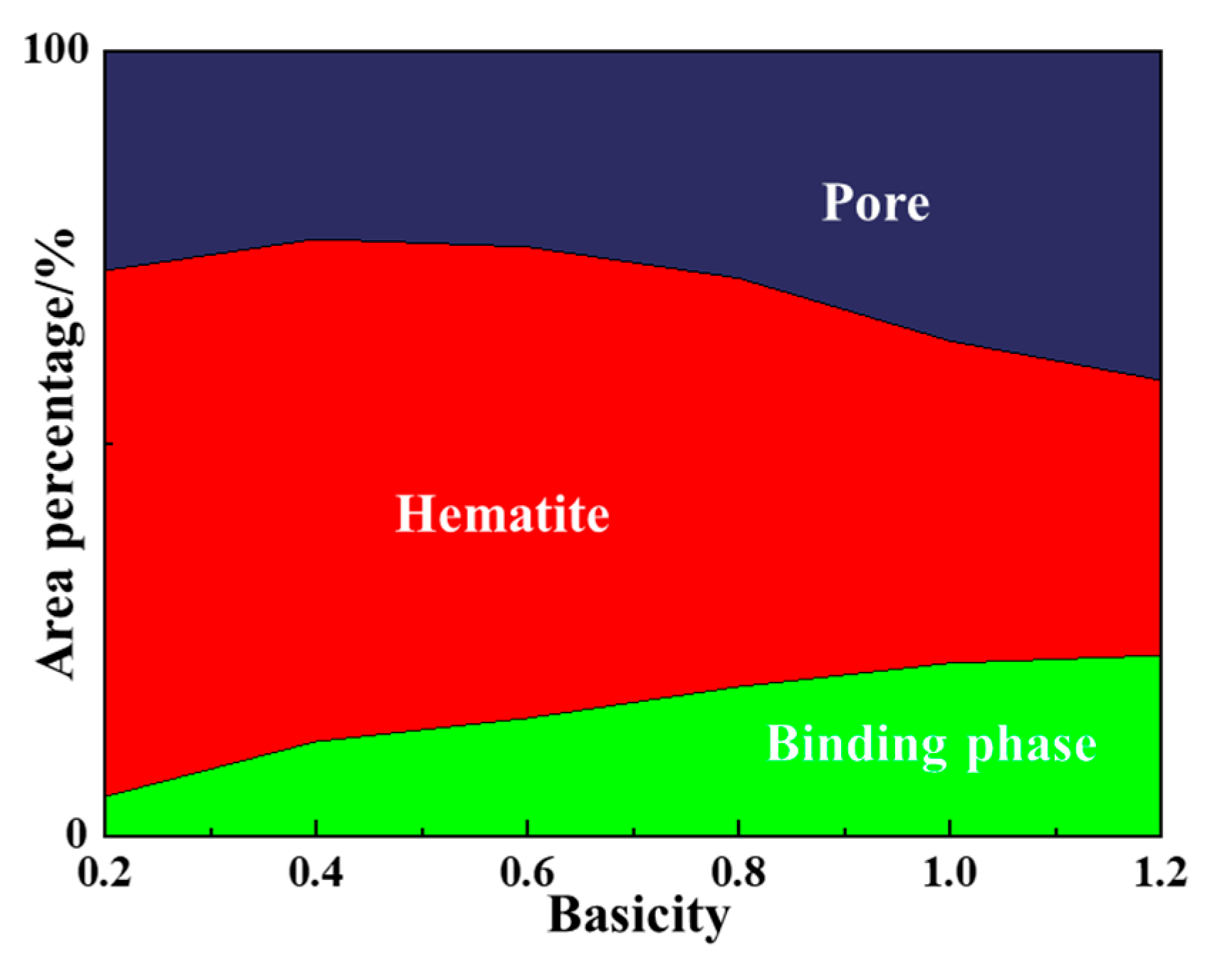
3.2.3. Proportion Variation of Different Microstructures with Basicity
3.3. Consolidation Mechanism of Fluxed Pellets
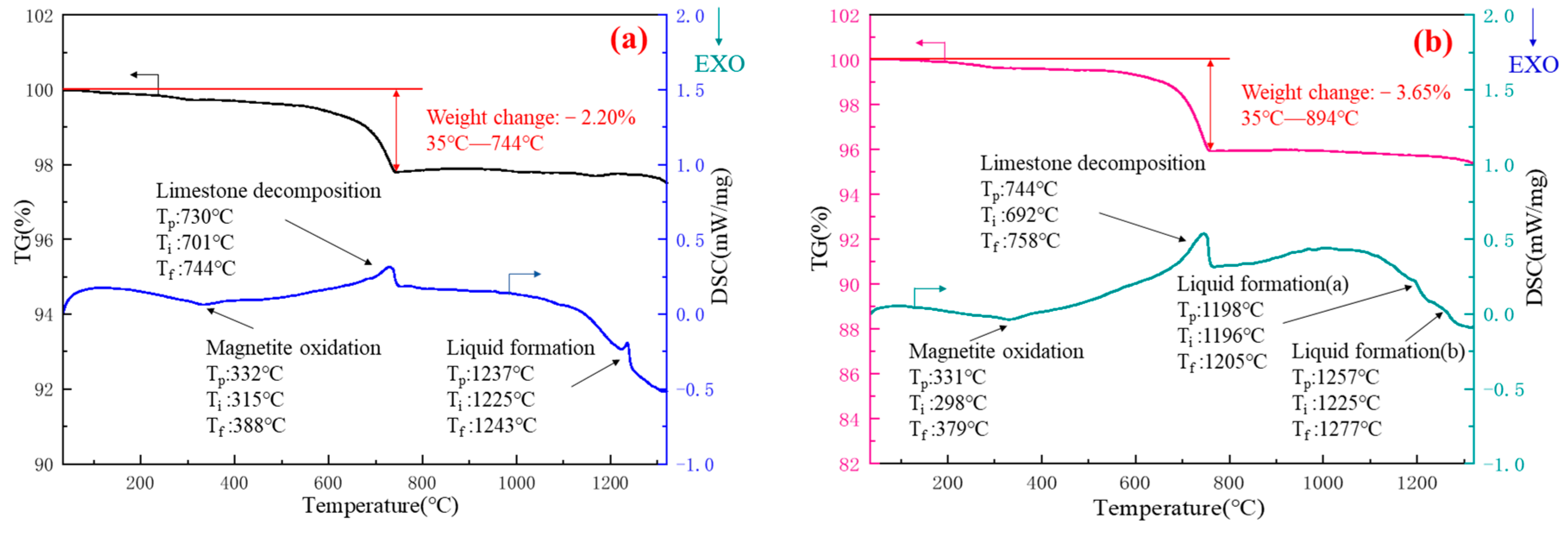
3.4. Thermal Analysis of Fluxed Pellet Preparation Process
3.5. Influence of Basicity on the Consolidation Strength of Fluxed Pellets
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dwarapudi, S.; Banerjee, P.; Chaudhary, P.; Sinha, S.; Chakraborty, U.; Sekhar, C.; Venugopalan, T.; Venugopal, R. Effect of fluxing agents on the swelling behavior of hematite pellets. Int. J. Miner. Process. 2014, 126, 76–89. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, M.; Liu, Z.; Cheng, M.; Chen, J. Modeling NOx emission of coke combustion in iron ore sintering process and its experimental validation. Fuel 2016, 179, 322–331. [Google Scholar] [CrossRef]
- Mohanty, M.K.; Mishra, S.; Mishra, B.; Sarkar, S. Effect of Basicity on the Reduction Behavior of Iron Ore Pellets. Arab. J. Sci. Eng. 2018, 43, 5989–5998. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Liu, Z.; Li, Y. Effect of lime addition on the mineral structure and compressive strength of magnesium containing pellets. Powder Technol. 2020, 376, 222–228. [Google Scholar] [CrossRef]
- Pal, J.; Arunkumar, C.; Rajshekhar, Y.; Das, G.; Goswami, M.C.; Venugopalan, T. Development on Iron Ore Pelletization Using Calcined Lime and MgO Combined Flux Replacing Limestone and Bentonite. ISIJ Int. 2014, 54, 2169–2178. [Google Scholar] [CrossRef] [Green Version]
- Dwarapudi, S.; Ghosh, T.K.; Shankar, A.; Tathavadkar, V.; Bhattacharjee, D.; Venugopal, R. Effect of pellet basicity and MgO content on the quality and microstructure of hematite pellets. Int. J. Miner. Process. 2011, 99, 43–53. [Google Scholar] [CrossRef]
- Prakash, S.; Goswami, M.; Mahapatra, A.; Ghosh, K.; Das, S.; Sinha, A.; Mishra, K. Morphology and reduction kinetics of fluxed iron ore pellets. Ironmak. Steelmak. 2000, 27, 194–201. [Google Scholar] [CrossRef]
- Li, W.; Wang, N.; Fu, G.; Chu, M.; Zhu, M. Influence of roasting characteristics on gas-based direct reduction behavior of Hongge vanadium titanomagnetite pellet with simulated shaft furnace gases. Powder Technol. 2017, 310, 343–350. [Google Scholar] [CrossRef]
- Li, J.; An, H.F.; Liu, W.X.; Yang, A.M.; Chu, M.S. Effect of basicity on metallurgical properties of magnesium fluxed pellets. J. Iron Steel Res. Int. 2019, 27, 239–247. [Google Scholar] [CrossRef]
- Sharma, T.; Gupta, R.; Prakash, B. Effect of gangue content on the swelling behaviour of iron ore pellets. Miner. Eng. 1990, 3, 509–516. [Google Scholar] [CrossRef]
- Iljana, M.; Kemppainen, A.; Paananen, T.; Mattila, O.; Pisilä, E.; Kondrakov, M.; Fabritius, T. Effect of adding limestone on the metallurgical properties of iron ore pellets. Int. J. Miner. Process. 2015, 141, 34–43. [Google Scholar] [CrossRef]
- Panigrahy, S.C.; Jena, B.C.; Rigaud, M. Characterization of bonding and crystalline phases in fluxed pellets using peat moss and bentonite as binders. Met. Mater. Trans. A 1990, 21, 463–474. [Google Scholar] [CrossRef]
- Yang, C.C.; Zhu, D.Q.; Pan, J.; Zhou, B.Z.; Xun, H. Oxidation and Induration Characteristics of Pellets Made from Western Australian Ultrafine Magnetite Concentrates and Its Utilization Strategy. J. Iron Steel Res. Int. 2016, 23, 924–932. [Google Scholar] [CrossRef]
- Gao, Q.J.; Jiang, X.; Wei, G.; Shen, F.M. Effects of MgO on densification and consolidation of oxidized pellets. J. Cent. South Univ. 2014, 21, 877–883. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.S.; Feng, C.; Li, F.; Liu, Z.G. Phases Transition and Consolidation Mechanism of High Chromium Vanadium-Titanium Magnetite Pellet by Oxidation Process. High Temp. Mater. Process. 2015, 35, 729–738. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, D.Q.; Pan, J.; Guo, Z.Q.; Xu, M.J. Improving roasting performance and consolidation of pellets made of ultrafine and super-high-grade magnetite concentrates by modifying basicity. J. Iron Steel Res. Int. 2020, 27, 770–781. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, K.; Chen, F.; Wang, S.; Zheng, F.; Yang, L.; Liu, Y. Effect of basicity on the reduction swelling behavior and mechanism of limestone fluxed iron ore pellets. Powder Technol. 2021, 393, 291–300. [Google Scholar] [CrossRef]
- Fan, X.H.; Gan, M.; Jiang, T.; Yuan, L.S.; Chen, X.L. Influence of flux additives on iron ore oxidized pellets. J. Cent. South Univ. Technol. 2010, 17, 732–737. [Google Scholar] [CrossRef]
- Umadevi, T.; Kumar, P.; Lobo, N.F.; Prabhu, M.; Mahapatra, P.; Ranjan, M. Influence of Pellet Basicity (CaO/SiO2) on Iron Ore Pellet Properties and Microstructure. ISIJ Int. 2011, 51, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Nicol, S.; Chen, J.; Pownceby, M.I.; Webster, N.A.S. A Review of the Chemistry, Structure and Formation Conditions of Silico-Ferrite of Calcium and Aluminum (‘SFCA’) Phases. ISIJ Int. 2018, 58, 2157–2172. [Google Scholar] [CrossRef] [Green Version]
- Cai, B.; Watanabe, T.; Kamijo, C.; Susa, M.; Hayashi, M. Comparison between Reducibilities of Columnar Silico-ferrite of Calcium and Aluminum (SFCA) Covered with Slag and Acicular SFCA with Fine Pores. ISIJ Int. 2018, 58, 642–651. [Google Scholar] [CrossRef] [Green Version]
- Koryttseva, A.; Webster, N.A.S.; Pownceby, M.; Navrotsky, A. Thermodynamic stability of SFCA (silico-ferrite of calcium and aluminum) and SFCA-I phases. J. Am. Ceram. Soc. 2017, 100, 3646–3651. [Google Scholar] [CrossRef]
- Zhang, F.; An, S.L.; Luo, G.P.; Wang, Y.C. Effect of Basicity and Alumina-Silica Ratio on Formation of Silico-Ferrite of Calcium and Aluminum. J. Iron Steel Res. Int. 2012, 19, 1–5. [Google Scholar] [CrossRef]
- Firth, A.R.; Garden, J.F. Interactions between Magnetite Oxidation and Flux Calcination during Iron Ore Pellet Induration. Met. Mater. Trans. A 2008, 39, 524–533. [Google Scholar] [CrossRef]
- Firth, A.R.; Garden, J.F.; Douglas, J.D. Phase Equilibria and Slag Formation in the Magnetite Core of Fluxed Iron Ore Pellets. ISIJ Int. 2008, 48, 1485–1492. [Google Scholar] [CrossRef] [Green Version]






| Raw Material | TFe | FeO | CaO | SiO2 | MgO | Al2O3 | K2O | Na2O | S | P | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Iron concentrates | 66.23 | 10.52 | 0.36 | 3.42 | 0.48 | 0.73 | 0.053 | 0.077 | 0.048 | 0.026 | 1.02 |
| bentonite | 3.06 | - | 4.55 | 60.14 | 2.88 | 9.10 | 0.93 | 2.08 | 0.031 | 0.049 | 10.25 |
| limestone | 0.36 | - | 54.53 | 1.06 | 0.53 | 0.44 | 0.14 | 0.037 | 0.018 | 0.0084 | 42.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Chen, F.; Guo, Y.; Liu, Y.; Wang, S.; Yang, L. Influence of Basicity and Calcium-Containing Substances on the Consolidation Mechanism of Fluxed Iron Ore Pellets. Metals 2022, 12, 1057. https://doi.org/10.3390/met12061057
Liu K, Chen F, Guo Y, Liu Y, Wang S, Yang L. Influence of Basicity and Calcium-Containing Substances on the Consolidation Mechanism of Fluxed Iron Ore Pellets. Metals. 2022; 12(6):1057. https://doi.org/10.3390/met12061057
Chicago/Turabian StyleLiu, Kuo, Feng Chen, Yufeng Guo, Yajing Liu, Shuai Wang, and Lingzhi Yang. 2022. "Influence of Basicity and Calcium-Containing Substances on the Consolidation Mechanism of Fluxed Iron Ore Pellets" Metals 12, no. 6: 1057. https://doi.org/10.3390/met12061057






