High-Temperature Corrosion Performance of FeAl-Based Alloys Containing Carbon in Molten Salt
Abstract
:1. Introduction
2. Materials and Methods
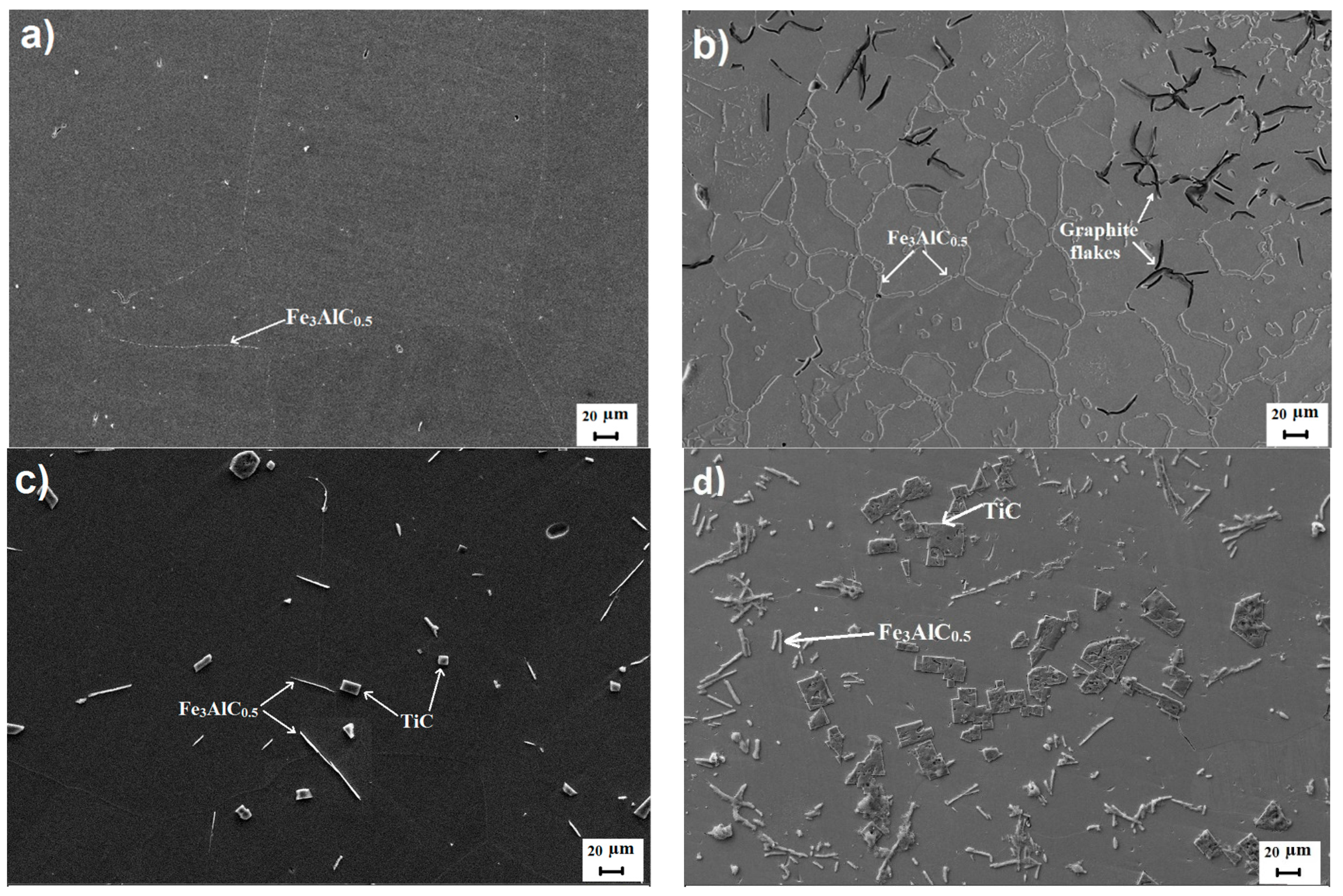
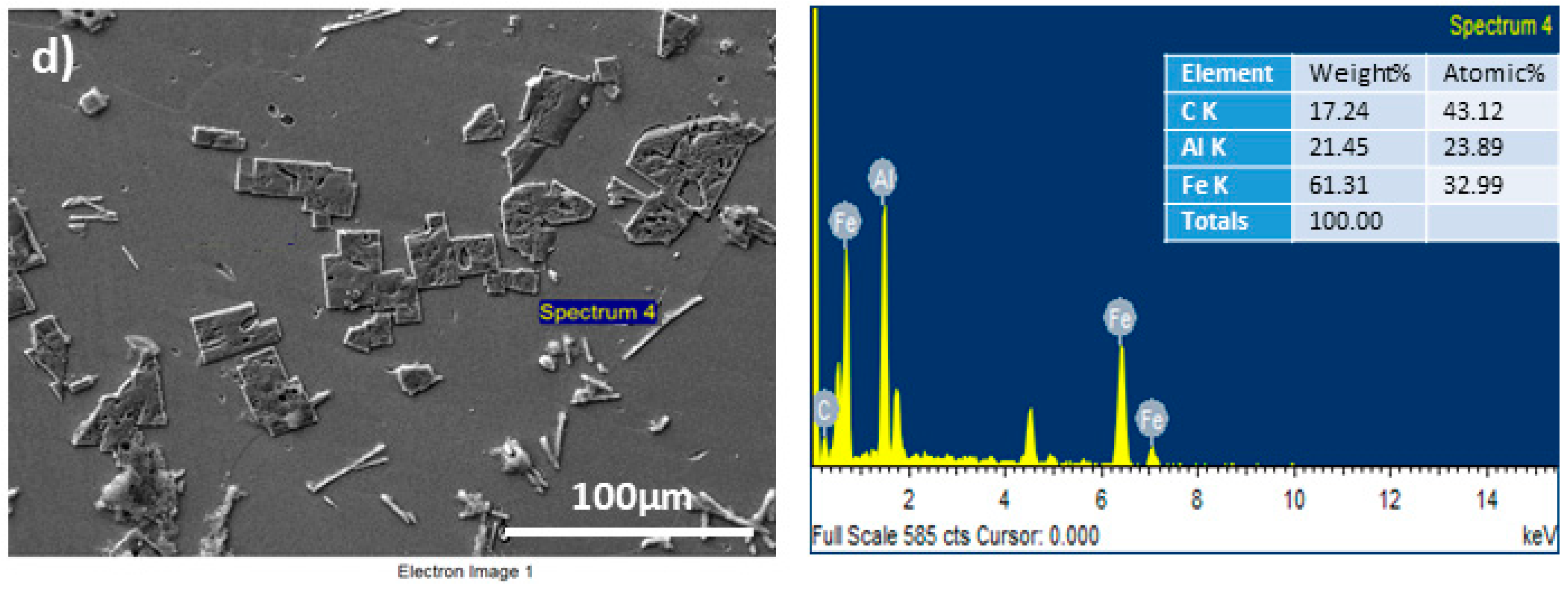
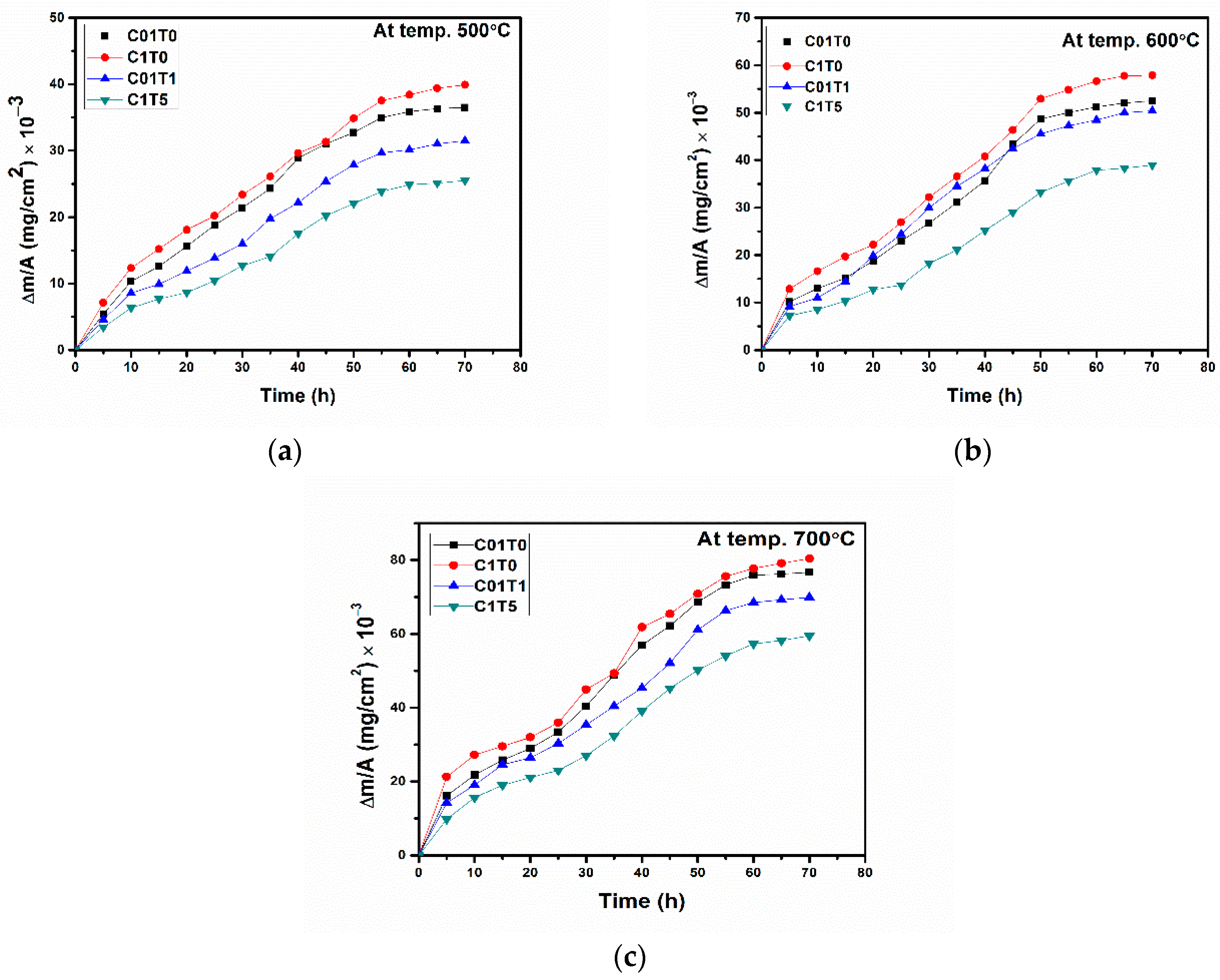
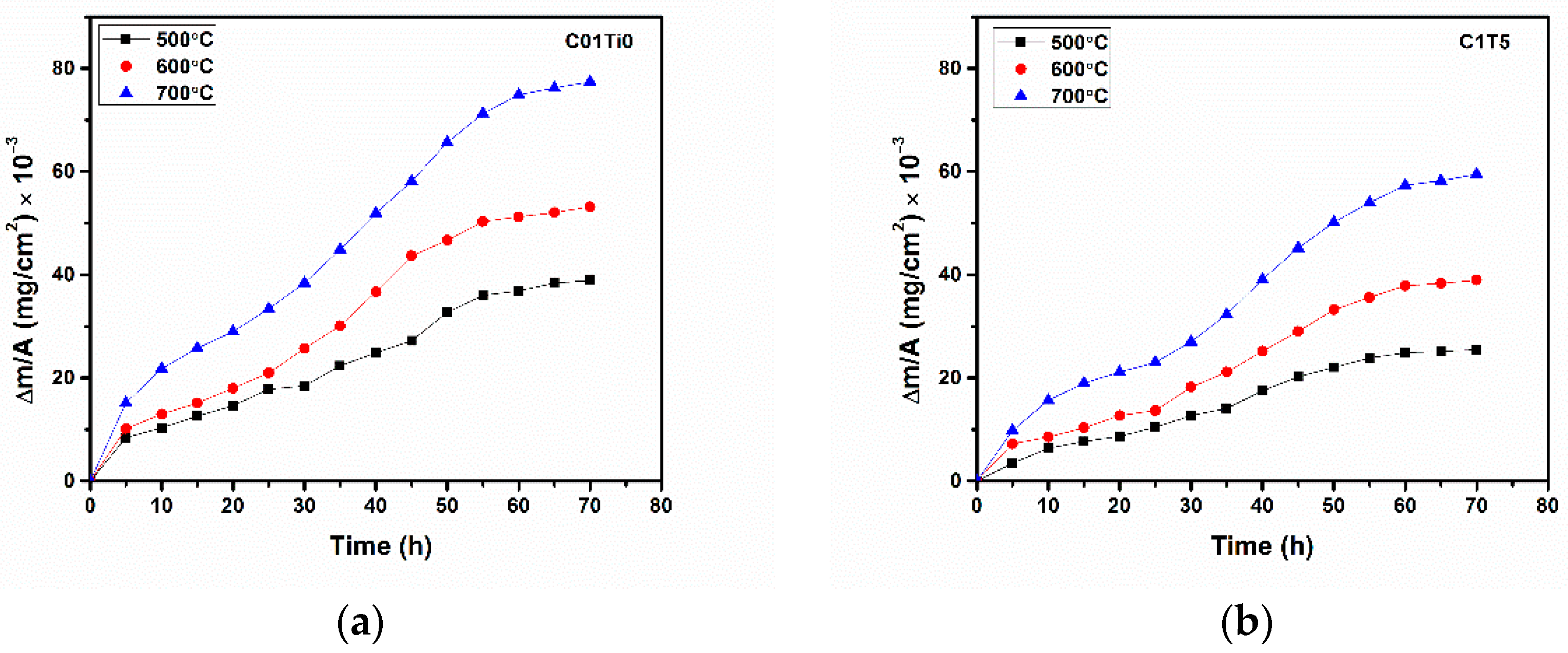
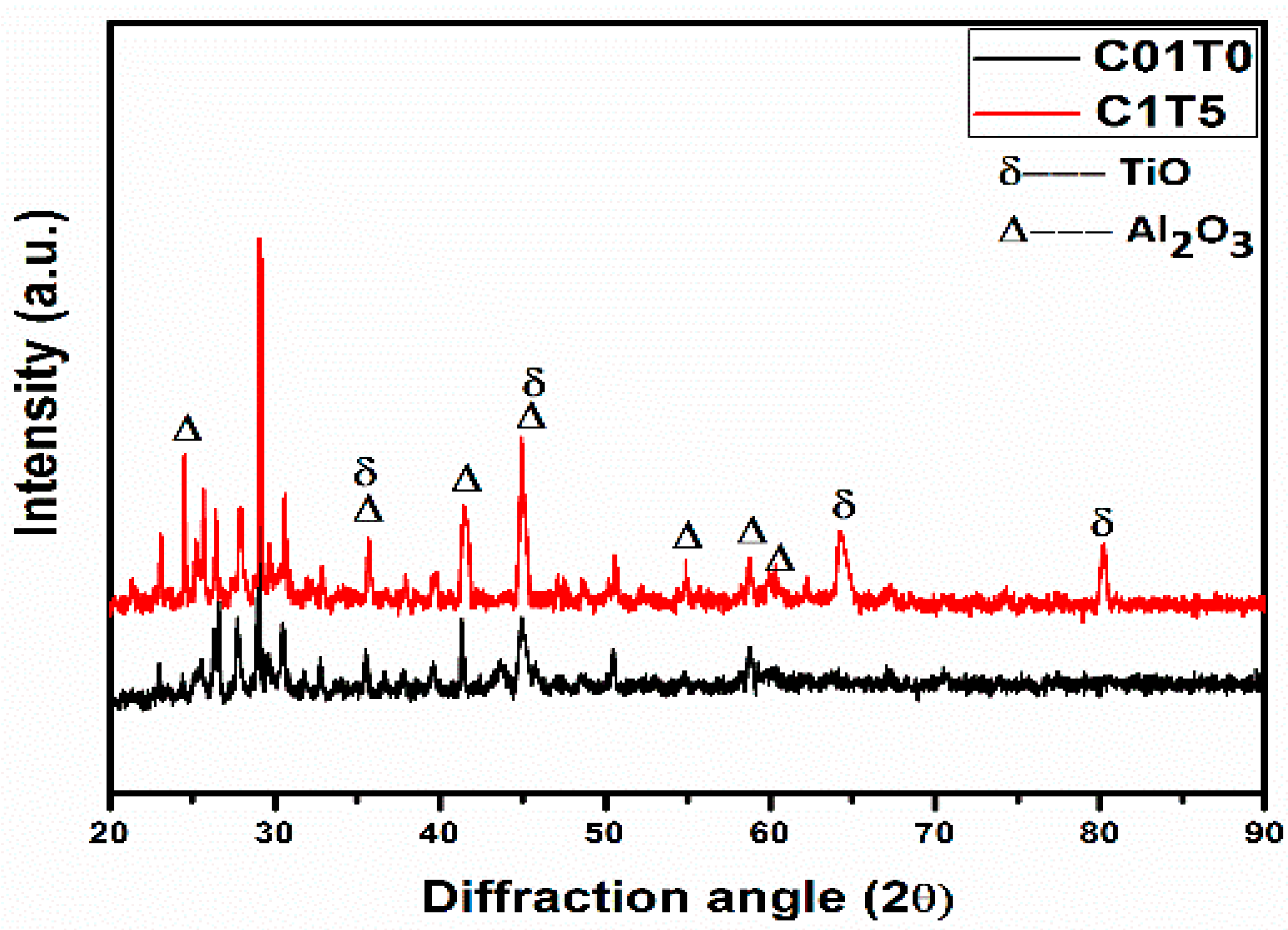
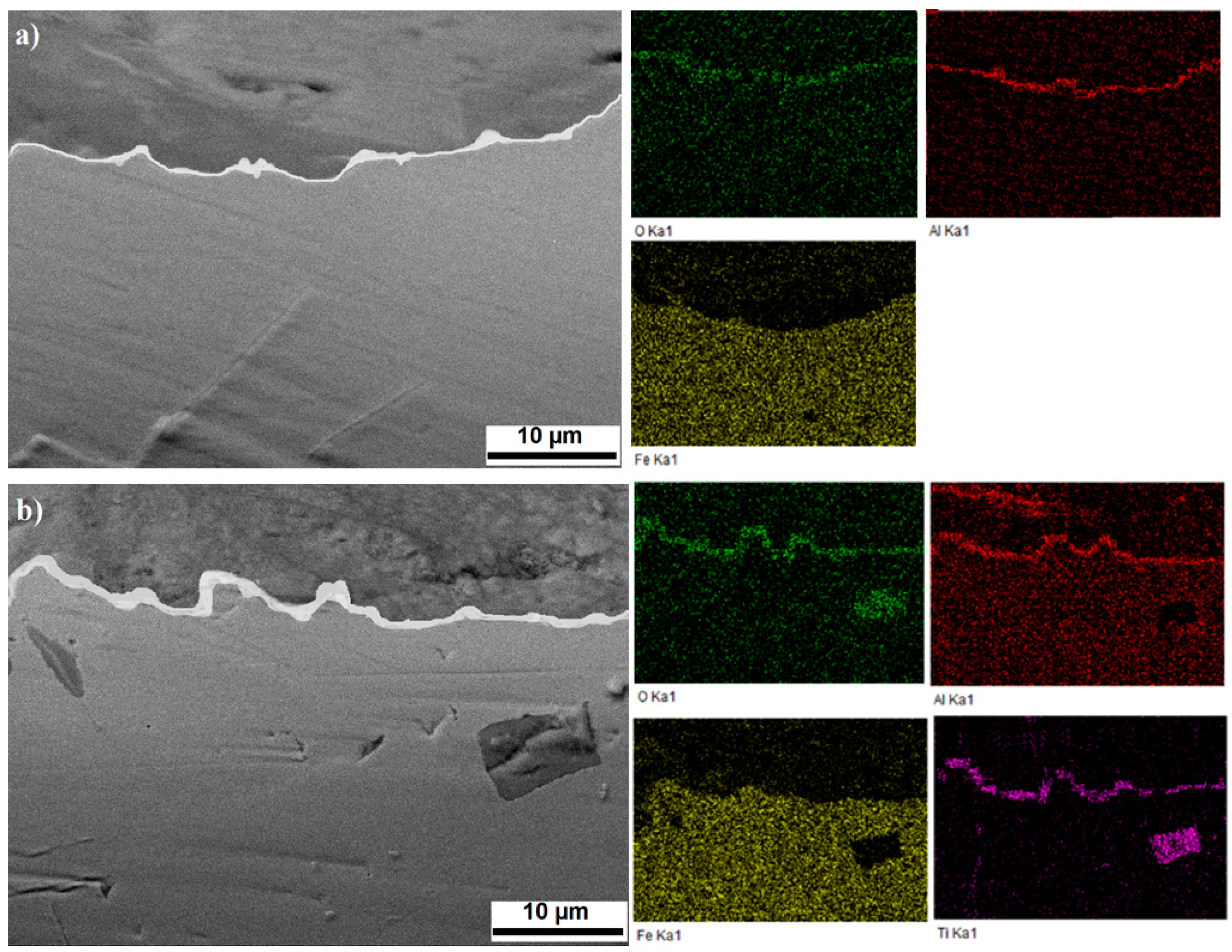
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Lee, D.B. Corrosion of Fe3Al-4Cr alloys at 1000 °C in N2-0.1%H2S gas. Key Eng. Mater. 2018, 765, 173–177. [Google Scholar] [CrossRef]
- Audigie, P.; Encinas-Sanchez, V.; Juez-Lorenzo, M.; Rodrıguezo, S.; Gutierrez, M.; Perez, F.J.; Aguero, A. High temperature molten salt corrosion behavior of aluminide and nickel aluminide coatings for heat storage in concentrated solar power plants. Surf. Coat. Technol. 2018, 349, 1148–1157. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, M.; Sharma, S.K.; Kim, D.Y.; Kumar, S.; Chavan, N.M.; Joshi, S.V.; Singh, N.; Singh, H. Study of mechanical properties and high temperature oxidation behavior of a novel cold-spray Ni-20Cr coating on boiler steels. Appl. Surf. Sci. 2015, 328, 13–25. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikka, V.K. Nickel and iron aluminides: An overview on properties, processing, and applications. Intermetallics 1996, 4, 357–375. [Google Scholar] [CrossRef]
- Gonzalez-Rodrıguez, J.G.; Luna-Ramirez, A.; Salazar, M.; Porcayo-Calderon, J.; Rosas, G.; Martinez-Villafane, A. Molten salt corrosion resistance of FeAl alloy with additions of Li, Ce and Ni. Mater. Sci. Eng. A 2005, 399, 344–350. [Google Scholar] [CrossRef]
- Amaya, M.; Espinosa-Medina, M.A.; Porcayo-Calderon, J.; Martinez, L.; Gonzalez-Rodriguez, J.G. High temperature corrosion performance of FeAl intermetallic alloys in molten salts. Mater. Sci. Eng. A 2003, 349, 12–19. [Google Scholar] [CrossRef]
- MEspinosa-Medina, A.; Carbajal-De la Torre, G.; Liu, H.B.; Martınez-Villafane, A.; Gonzalez-Rodriguez, J.G. Hot corrosion behaviour of Fe-Al based intermetallic in molten NaVO3 salt. Corros. Sci. 2009, 51, 1420–1427. [Google Scholar] [CrossRef]
- Senderowski, C.; Cinca, N.; Dosta, S.; Cano, I.G.; Guilemany, J.M. The effect of hot treatment on composition and microstructure of HVOF iron aluminide coatings in Na2SO4 molten salts. J. Therm. Spray Technol. 2019, 28, 1492–1510. [Google Scholar] [CrossRef] [Green Version]
- Troysi, F.D.; Brito, P.P. Development and characterization of an iron aluminide coating on mild steel substrate obtained by friction surfacing and heat treatment. Int. J. Adv. Manuf. Technol. 2020, 111, 2569–2576. [Google Scholar] [CrossRef]
- Baligidad, R.G.; Prakash, U.; Radhakrishna, A.; Rao, V.R.; Rao, P.K.; Ballal, N.B. Effect of carbon content on high temperature tensile properties of Fe3Al based intermetallic alloys. Scipta Mater. 1997, 36, 667. [Google Scholar] [CrossRef]
- Radhakrishna, A.; Baligidad, R.G.; Sarma, D.S. Effect of carbon on structure and properties of FeAl based intermetallic alloy. Scr. Mater. 2001, 45, 1077. [Google Scholar] [CrossRef]
- Kant, R.; Prakash, U.; Agarwala, V.; Prasad, V.S. Effect of carbon and titanium additions on mechanical properties of B2 FeAl. Trans. Indian Inst. Met. 2016, 68, 1155. [Google Scholar] [CrossRef]
- Kant, R.; Prakash, U.; Agarwala, V.; Prasad, V.S. Microstructure and wear behaviour of FeAl-based composites containing in-situ carbides. Bull. Mater. Sci. 2016, 39, 1827–1834. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.X.; Lin, D. Oxidation behaviour of FeAl alloy with and without titanium. J. Mater. Sci. 2001, 36, 979. [Google Scholar] [CrossRef]
- Novak, P.; Bartak, Z.; Nova, K.; Prusa, F. Effect of nickel and titanium on properties of Fe-Al-Si alloy prepared by mechanical alloying and spark plasma sintering. Materials 2020, 13, 800. [Google Scholar] [CrossRef] [Green Version]
- Rommerskirchen, I.; Eltester, I.B.; Grabke, H.J. Oxidation of β-FeAl and Fe-Al alloys. Mater. Corros. 1996, 47, 646. [Google Scholar] [CrossRef]
- Radoslaw, L. High-temperature oxidation of Fe3Al intermetallic alloy prepared by additive manufacturing LENS. Materials 2015, 8, 1499. [Google Scholar]
- Ko, S.H.; Hanada, S. In-situ production and microstructures of iron aluminide/TiC composites. Intermetallics 1999, 7, 947. [Google Scholar] [CrossRef]
- Aaronson, H.I.; Spanos, G.; Masamura, R.A.; Vardiman, R.G.; Moon, D.W.; Menon, E.S.; Hall, K.M.J. Sympathetic nucleation: An overview. Mater. Sci. Eng. B 1995, 32, 107. [Google Scholar] [CrossRef]
- Wood, G.C. The oxidation of iron-chromium alloys and stainless steels at high temperatures. Corros. Sci. 1962, 2, 173. [Google Scholar] [CrossRef]
- Pujilaksono, B.; Jonsson, T.; Halvarsson, M.; Panas, I.; Svenson, J.E.; Johansson, L.G. Paralinear oxidation of chromium in O2 + H2O environment at 600–700 °C. Oxid. Met. 2008, 70, 163. [Google Scholar] [CrossRef]
- Raja, V.S. High temperature oxidation behaviour of carbon containing two phase iron aluminides and Fe-Al alloys. Trans. Indian Inst. Met. 2004, 57, 525. [Google Scholar]
- Torterelli, P.F.; Natesan, K. Critical factors affecting the high-temperature oxidation performance of iron aluminides. Mater. Sci. Eng. A 1998, 258, 115. [Google Scholar] [CrossRef]
- Das, D.; Balasubramaniam, R.; Mungole, M.N. Hot oxidation of carbon-alloyed Fe3Al based iron aluminides. Mater. Sci. Eng. A 2002, 338, 24. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Kant, R.; Chand, S.; Prakash, U.; Sehgal, S.; Saxena, K.K.; Davim, J.P.; Prakash, C. High-Temperature Corrosion Performance of FeAl-Based Alloys Containing Carbon in Molten Salt. Metals 2021, 11, 2040. https://doi.org/10.3390/met11122040
Kumar M, Kant R, Chand S, Prakash U, Sehgal S, Saxena KK, Davim JP, Prakash C. High-Temperature Corrosion Performance of FeAl-Based Alloys Containing Carbon in Molten Salt. Metals. 2021; 11(12):2040. https://doi.org/10.3390/met11122040
Chicago/Turabian StyleKumar, Munish, Ravi Kant, Suresh Chand, Ujjwal Prakash, Shankar Sehgal, Kuldeep Kumar Saxena, Joao Paulo Davim, and Chander Prakash. 2021. "High-Temperature Corrosion Performance of FeAl-Based Alloys Containing Carbon in Molten Salt" Metals 11, no. 12: 2040. https://doi.org/10.3390/met11122040







