1. Introduction
Mineral processing and metallurgical operations have taken a new turn according to the type of copper production being used. Innovation and new mineral processing alternatives are essential to maintaining copper production [
1,
2,
3]. Production by hydrometallurgical processes has started to decline, and new techniques are required to overcome various processing challenges [
4,
5,
6,
7], one of which is the increasing arsenic content in copper deposits. According to government regulations, when arsenic cannot be recovered from a process and is eliminated as waste, it must be confined in a stable environment [
8,
9,
10].
Arsenic is one of the most toxic and carcinogenic elements. In solution, it can be absorbed by vegetation and pass through the food chain to human beings [
11,
12,
13,
14]. Arsenic can be found in a natural state, as well as in sulphides such as realgar, enargite and orpiment, as arsenide in oxides and as arsenate. Arsenopyrite (FeSAs) is the most common arsenic mineral [
15]. In copper ores, arsenic is often contained in tennantite (Cu
12As
4S
13) and enargite (Cu
3AsS
4) [
16], which are respectively 20.2% and 19.0% arsenic [
17]. This is why the final disposition of arsenic must be controlled, and to do this it is necessary to establish the form of arsenic that is present in order to stabilize and confine it over the long term.
Numerous studies have considered the most stable arsenic form for final disposal. It has been concluded that As
5+ is more stable than As
3+ [
18]. Studies by [
19] have indicated that arsenate (As
5+) can precipitate in the presence of (Mn
3+) in oxidizing environments. Therefore, the presence of a high concentration of manganese can positively influence arsenic precipitation. Precipitation with lime is a widespread practice despite consensus about the low arsenic concentration content and low long-term stability of the resulting precipitates. Precipitation of As
+5 with lime at room temperature results in the formation of various calcium arsenate compounds, including Ca
4(OH)
2(AsO
4)
2·4H
2O, Ca
5(AsO
4)
3OH and Ca
3(AsO
4)
2, as well as CaHAsO
4·
xH
2O and Ca
5H
2(AsO
4)
4. These compounds depend on the concentration and oxidation state of arsenic, calcium and SO
42− and/or HSO
4−. It should be noted that there is evidence that calcium arsenate compounds decompose upon contact with atmospheric CO
2 or carbonate ions to form calcium carbonate and a soluble arsenic acid [
18].
The best alternative for arsenic stabilization is the formation of scorodite (FeAsO
4·2H
2O) [
20,
21,
22], which is capable of containing between 20–25% of As [
23] and is stable under oxidizing conditions in the pH range of 2.0–6.0 [
18,
24]. The formation of scorodite at atmospheric pressure requires strict rigor with respect to the pH. It has been determined that As
+5 precipitation at ambient pressure is directly affected by the pH level, with values over 4 resulting in the oversaturation of iron and arsenic in the solution, thus forming amorphous iron-arsenic compounds that are not stable for depositing [
25]. Scorodite formation is reflected in the kinetics of its transformation, pH 1 is the established value, while at pH 2 it is moderate and at pH 4.5 the kinetics are slow [
26]. Another factor to consider in the coprecipitation of As is the oxidation of As
3+ to As
5+. Oxidation is important because the successful removal of arsenic requires that it be in the form of arsenate, especially when scorodite is formed [
21,
27].
This research analyzes the feasibility of incorporating the effluent solution from a metallurgical flue dust treatment plant (PLS-P) from a copper smelter to a copper hydrometallurgical plant. In this way, it is expected that the copper, acid and water, that are currently lost due to the high arsenic content, can be recovered and that the arsenic can be fixed in a stable form in the heap leaching residue, allowing for its disposal in a confined and environmentally safe area.
2. Materials and Methods
To determine the behavior of arsenic and iron in the effluent flue dust solution (PLS-P) of a copper smelter when it is incorporated into an industrial copper leaching process, a set of six column-leaching tests were performed.
Table 1 shows the details of the pH and solution potential (Eh) for the leaching columns (C-1, C2, C3, C4, C5 and C6).
A representative sample of mineral used in the Lomas Bayas mining process was characterized by chemical analysis using inductively coupled plasma atomic emission spectroscopy (ICP-AES) via the model Optima 2000 DV (PerkinElmer, Überlinge, Germany), with a detection limit of 0.01 ppm for Cu and Fe and 0.5 ppm for As. A pulverized mineral sample was analyzed by X-ray analysis (Siemens model D5600, Bruker, Billerica, MA, USA), with an analysis time of one hour. The ICDD (International Center for Diffraction Data) database was used to identify the species that were present.
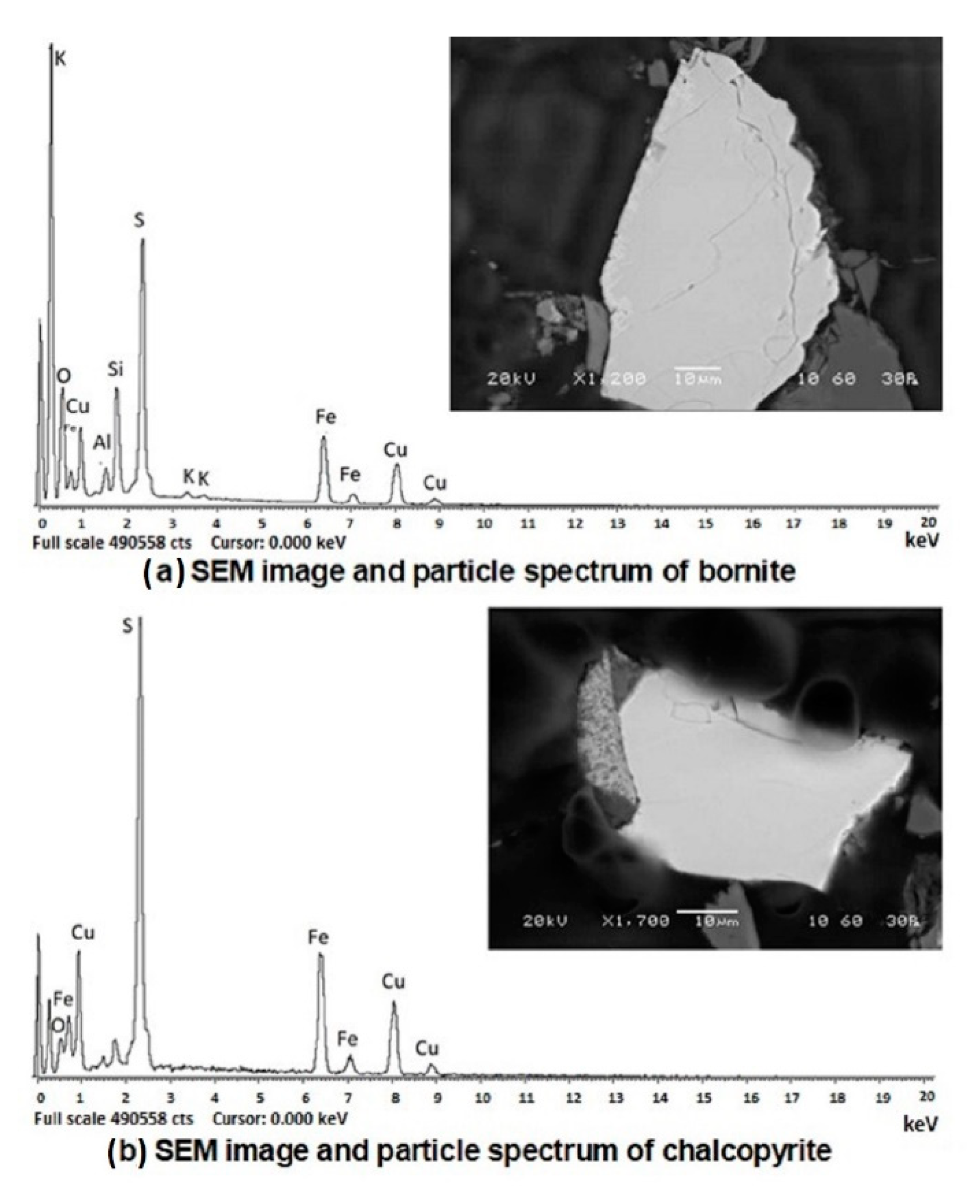
To perform the microscopy analysis, a polished briquette (Buehler Simplimet 2 briquetting machine) was made with transoptic powders and was characterized by scanning electron microscopy (SEM) using JEOL 6360-LV equipment (JEOL USA Inc., Peabody, MA, USA) and by an energy-dispersive X-ray spectroscopy (EDX) microanalysis system (Zeiss Ultra Plus, Zeiss, Jena, Germany), operated at 30 kV under high vacuum conditions. Finally, the samples were also studied under a BX-51 reflected-light microscope (Olympus, Tokyo, Japan).
The size distribution was characterized in two stages, the first using mesh sizes of 1″, , , , 6#, 10# and −10# mesh (Tyler). In the second stage, a 500 g (−10#) mineral sample was classified using 20, 30, 50, 70, 100, 140, 200, 270, 325 and 400# meshes (Tyler). Moisture was determined by the weight difference of a mineral sample before and after being dried at 95 °C in an oven for 14 h.
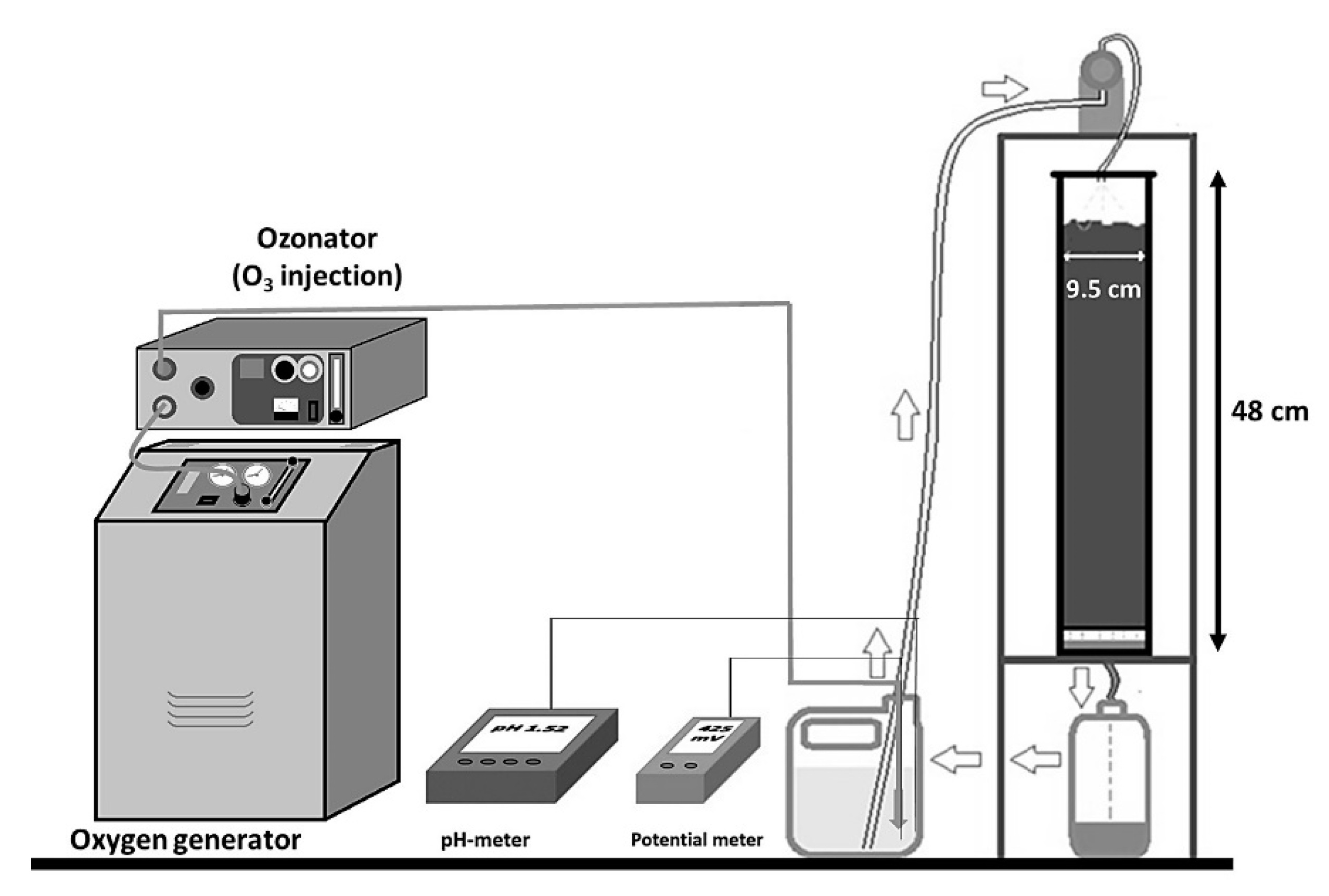
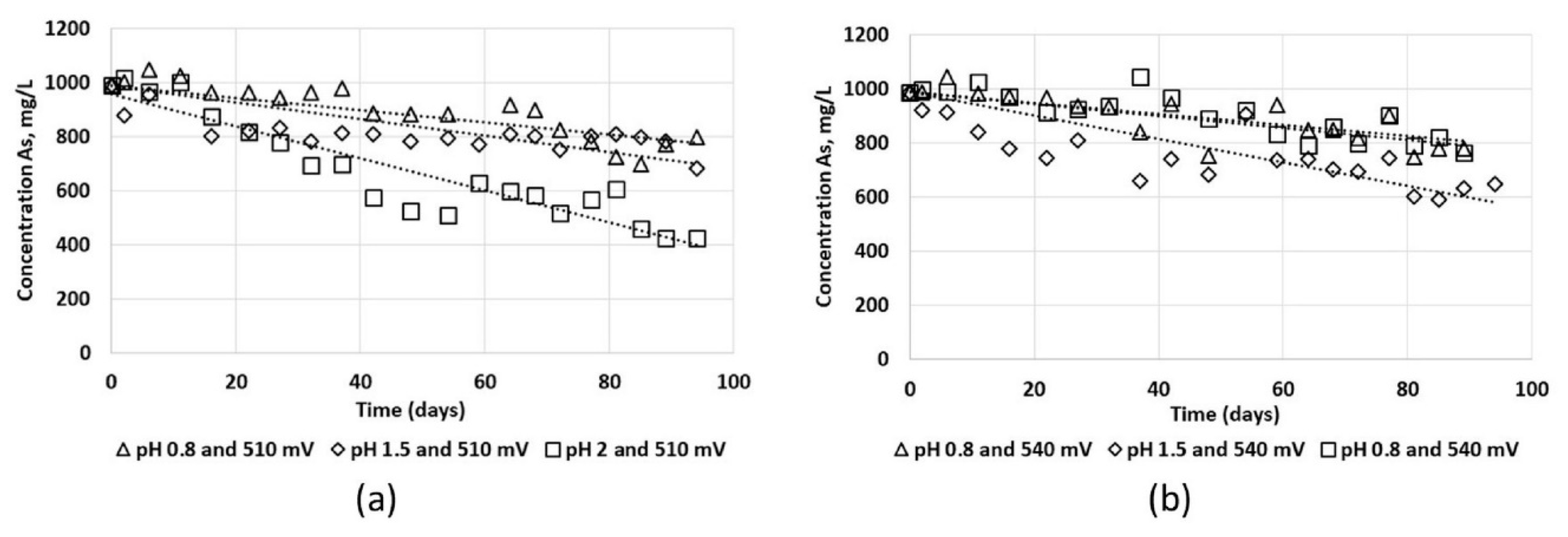
The ore from the heap leaching was loaded into columns 48 cm high and 9.5 cm in diameter. The columns were irrigated independently in duplicate with a mixed solution of PLS-P and Lomas Bayas heap leaching solution in a 1/10.74 ratio, with 1 g/L arsenic. The initial pH (0.8) and the solution potential (510 mV) with respect to the standard hydrogen electrode (SHE), were the natural values of the blend. Both the pH and the solution potential were conditioned according to
Table 1, and NaOH and H
2SO
4 (Merk, analytical grade) were used to control the pH of the solution. To regulate the solution potential up to 540 mV, ozone was injected into the solution for 5 min, using an ozonator model L21 (Pacific Ozone, Benicia, CA, USA), fed with oxygen through an oxygen generator system. The conditioned solution was irrigated in the columns, and the pregnant leach solution (PLS) was recirculated in the columns until completion of a 94-day leaching cycle.
The columns were irrigated using a multi-head MasterFlex peristaltic pump at a rate of 10 (L/h)/m
2. The drained solutions from the columns were collected separately, sampled every five days and analyzed with an Absorption Spectrometer, (SpectrAA-50/55, Varian, Santa Clara, CA, USA) for As(total), As
3+, As
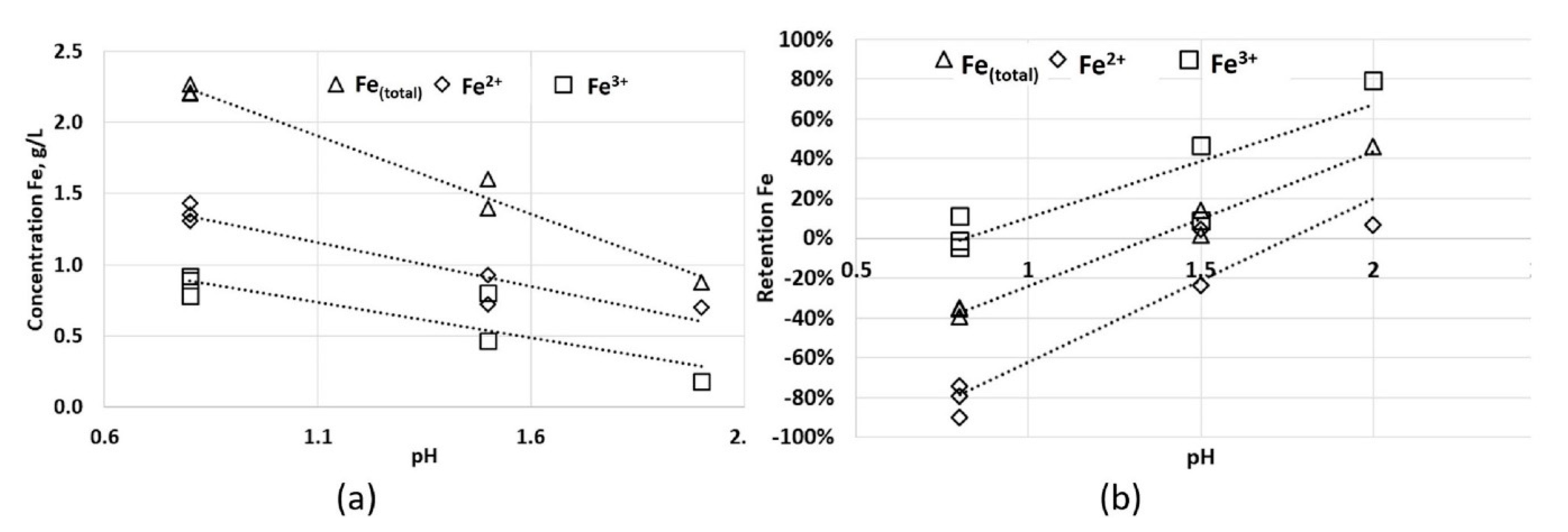
5+ using HG-FAAS (hydride generation with pH speciation), and using FAAS (flame atomic adsorption spectrometry with pH speciation) in the case of Fe
(total), Fe
2+, Fe
3+ and Cu
2+.
Figure 1 shows a schematic of the system used. At the end of the cycle (94 days), the irrigation was discontinued and the columns were drained for three days and discharged. A sample was taken from the leaching residue that was obtained in each column for physical, chemical, X-ray and SEM analysis.
Author Contributions
Conceptualization, O.B., M.C.H. and Y.Z.; methodology, O.B., M.C.H. and Y.S.; validation, O.B.; formal analysis, O.B., E.M. and V.Q.; investigation, O.B., and M.C.H.; resources, O.B., M.C.H., E.M. and Y.Z.; data curation, O.B. and Y.S.; writing—original draft preparation and writing—review and editing, O.B., E.M. and V.Q.; visualization, V.Q. and O.B.; supervision, O.B., M.C.H. and E.M.; project administration, O.B. and Y.Z.; funding acquisition, O.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank the Universidad Católica del Norte and Compañia Minera Lomas Bayas for the opportunity and funding provided to develop this research. We also appreciate the contribution of the Scientific Equipment Unit-MAINI, Universidad Católica del Norte, for support in the preparation of samples, analysis and data generation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cánovas, M.; Valenzuela, J.; Romero, L.; González, P. Characterization of electroosmotic drainage: Application to mine tailings and solid residues from leaching. J. Mater. Res. Technol. 2020, 9, 2960–2968. [Google Scholar] [CrossRef]
- Valenzuela-Elgueta, J.; Cánovas, M.; García, A.; Zárate, R. Electrocoalescence of emulsions in raffinate from the solvent extraction phase under AC electrical fields. J. Mater. Res. Technol. 2020, 9, 490–497. [Google Scholar] [CrossRef]
- Beiza, L.; Quezada, V.; Melo, E.; Valenzuela, G. Electrochemical Behaviour of Chalcopyrite in Chloride Solutions. Metals 2019, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Cochilco. Proyección de la Producción de Cobre en Chile 2019–2030. Santiago de Chile. 2019. Available online: https://www.cochilco.cl/Listado%20Temtico/Proyecci%C3%B3n%20de%20la%20producci%C3%B3n%20esperada%20de%20cobre%202019%20-%202030%20Vfinal.pdf (accessed on 24 July 2020).
- Velásquez-Yévenes, L.; Quezada-Reyes, V. Influence of seawater and discard brine on the dissolution of copper ore and copper concentrate. Hydrometallurgy 2018, 180, 88–95. [Google Scholar] [CrossRef]
- Benavente, O.; Hernández, M.; Melo, E.; Núñez, D.; Quezada, V.; Zepeda, Y. Copper Dissolution from Black Copper Ore under Oxidizing and Reducing Conditions. Metals 2019, 9, 799. [Google Scholar] [CrossRef] [Green Version]
- Quezada, V.; Roca, A.; Benavente, O.; Cruells, M.; Keith, B.; Melo, E. Effect of pretreatment prior to leaching on a chalcopyrite mineral in acid media using NaCl and KNO3. J. Mater. Res. Technol. 2020, 9, 10316–10324. [Google Scholar] [CrossRef]
- Pantuzzo, F.L.; Ciminelli, V.S.T. Arsenic association and stability in long-term disposed arsenic residues. Water Res. 2010, 44, 5631–5640. [Google Scholar] [CrossRef]
- Raghav, M.; Shan, J.; Sáez, A.E.; Ela, W.P. Scoping candidate minerals for stabilization of arsenic-bearing solid residuals. J. Hazard. Mater. 2013, 263, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Xiu, W.; Guo, H.; Shen, J.; Liu, S.; Ding, S.; Hou, W.; Ma, J.; Dong, H. Stimulation of Fe(II) Oxidation, Biogenic Lepidocrocite Formation, and Arsenic Immobilization by Pseudogulbenkiania Sp. Strain 2002. Environ. Sci. Technol. 2016, 50, 6449–6458. [Google Scholar] [CrossRef]
- Lihareva, N. Arsenic solubility, mobility and speciation in the deposits from a copper production waste storage. Microchem. J. 2005, 81, 177–183. [Google Scholar] [CrossRef]
- Phenrat, T.; Marhaba, T.F.; Rachakornkij, M. Leaching behaviors of arsenic from arsenic-iron hydroxide sludge during TCLP. J. Environ. Eng. 2008, 134, 671–682. [Google Scholar] [CrossRef]
- Otgon, N.; Zhang, G.; Zhang, K.; Yang, C. Removal and fixation of arsenic by forming a complex precipitate containing scorodite and ferrihydrite. Hydrometallurgy 2019, 186, 58–65. [Google Scholar] [CrossRef]
- Quansah, R.; Armah, F.A.; Essumang, D.K.; Luginaah, I.; Clarke, E.; Marfoh, K.; Cobbina, S.J.; Edward, N.-A.; Namujju, P.B.; Obiri, S.; et al. Association of Arsenic with Adverse Pregnancy Outcomes/Infant Mortality: A Systematic Review and Meta-Analysis. Enviromental Heal. Perspect. 2015, 123, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Twidwell, L. Arsenic Hydrometallurgy; Fundamentals, Technology And Applications. In Arsenic Hydrometallurgy; Fundamentals, Technology and Applications: Vancouver, BC, Canada, 2014; p. 17. [Google Scholar]
- Viñals, J.; Roca, A.; Hernández, M.C.; Benavente, O. Topochemical transformation of enargite into copper oxide by hypochlorite leaching. Hydrometallurgy 2003, 68, 183–193. [Google Scholar] [CrossRef]
- Long, G.; Peng, Y.; Bradshaw, D. A review of copper-arsenic mineral removal from copper concentrates. Miner. Eng. 2012, 179–186. [Google Scholar] [CrossRef]
- Riveros, P.A.; Dutrizac, J.E.; Spencer, P. Arsenic disposal practices in the metallurgical industry. Can. Met. Q. 2001, 40, 395–420. [Google Scholar] [CrossRef]
- Nishimura, T.; Umetsu, Y. Oxidative precipitation of arsenic(III) with manganese(II) and iron(II) in dilute acidic solution by ozone. Hydrometallurgy 2001, 62, 83–92. [Google Scholar] [CrossRef]
- Filippou, D.; Demopoulos, G.P. Arsenic immobilization by controlled scorodite precipitation. JOM 1997, 49, 52–55. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Ito, M.; Hiroyoshi, N. Hematite-catalysed scorodite formation as a novel arsenic immobilisation strategy under ambient conditions. Chemosphere 2019, 233, 946–953. [Google Scholar] [CrossRef]
- Rong, Z.; Tang, X.; Wu, L.; Chen, X.; Dang, W.; Li, X.; Huang, L.; Wang, Y. The effect of precursor speciation on the growth of scorodite in an atmospheric scorodite synthesis. R. Soc. Open Sci. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Paktunc, D.; Bruggeman, K. Solubility of nanocrystalline scorodite and amorphous ferric arsenate: Implications for stabilization of arsenic in mine wastes. Appl. Geochem. 2010, 25, 674–683. [Google Scholar] [CrossRef]
- Ke, P.-C.; Liu, Z. Synthesis, in-situ coating and characterization of scorodite with high leaching stability. Trans. Nonferrous Met. Soc. China 2019, 29, 876–892. [Google Scholar] [CrossRef]
- Taboada, M.E.; Hernández, P.; Flores, E.; Graber, T. Crystallization of Arsenic Salts. Soc. Química Del Perú 2008, 74, 343–349. [Google Scholar]
- Paktunc, D.; Dutrizac, J.; Gertsman, V. Synthesis and phase transformations involving scorodite, ferric arsenate and arsenical ferrihydrite: Implications for arsenic mobility. Geochim. Cosmochim. Acta 2008, 72, 2649–2672. [Google Scholar] [CrossRef]
- Twidwell, L.G. Treatment of Arsenic-Bearing Minerals and Fixation of Recovered Arsenic Products: A Review. Sme Miner. Process. Extr. Met. Handb. 2018. [Google Scholar] [CrossRef]
- Nazari, A.M.; Radzinski, R.; Ghahreman, A. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 2017, 174, 258–281. [Google Scholar] [CrossRef]
- Gomez, M.A.; Becze, L.; Cutler, J.N.; Demopoulos, G.P. Hydrothermal reaction chemistry and characterization of ferric arsenate phases precipitated from Fe2(SO4)3-As 2O5-H2SO4 solutions. Hydrometallurgy 2011, 107, 74–90. [Google Scholar] [CrossRef]
- Monhemius, J. The Scorodite Process: A Technology for the disposal of arsenic in the 21st century. In Effluent Treatment in the Minning Industry; Castro, S., Vergara, F., Sánchez, M., Eds.; Universidad de Concepción: Concepción, Chile, 1998; pp. 119–161. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).