Toxicity of Selected Acaricides to Honey Bees (Apis mellifera) and Varroa (Varroa destructor Anderson and Trueman) and Their Use in Controlling Varroa within Honey Bee Colonies
Abstract
:1. Introduction
2. Materials and Methods
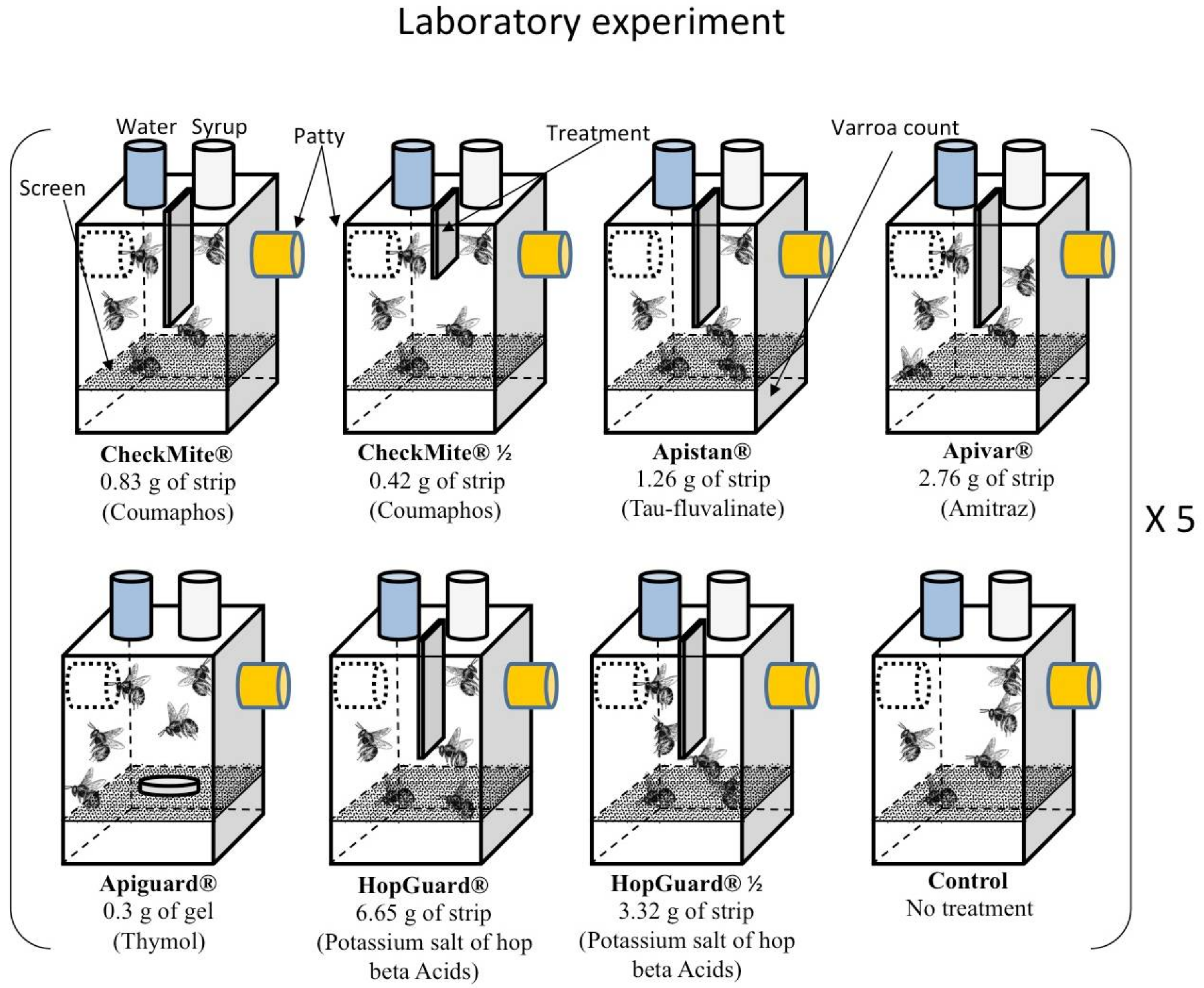
2.1. Laboratory Toxicity Experiment
2.2. Field Experiment
2.3. Data and Statistical Analyses
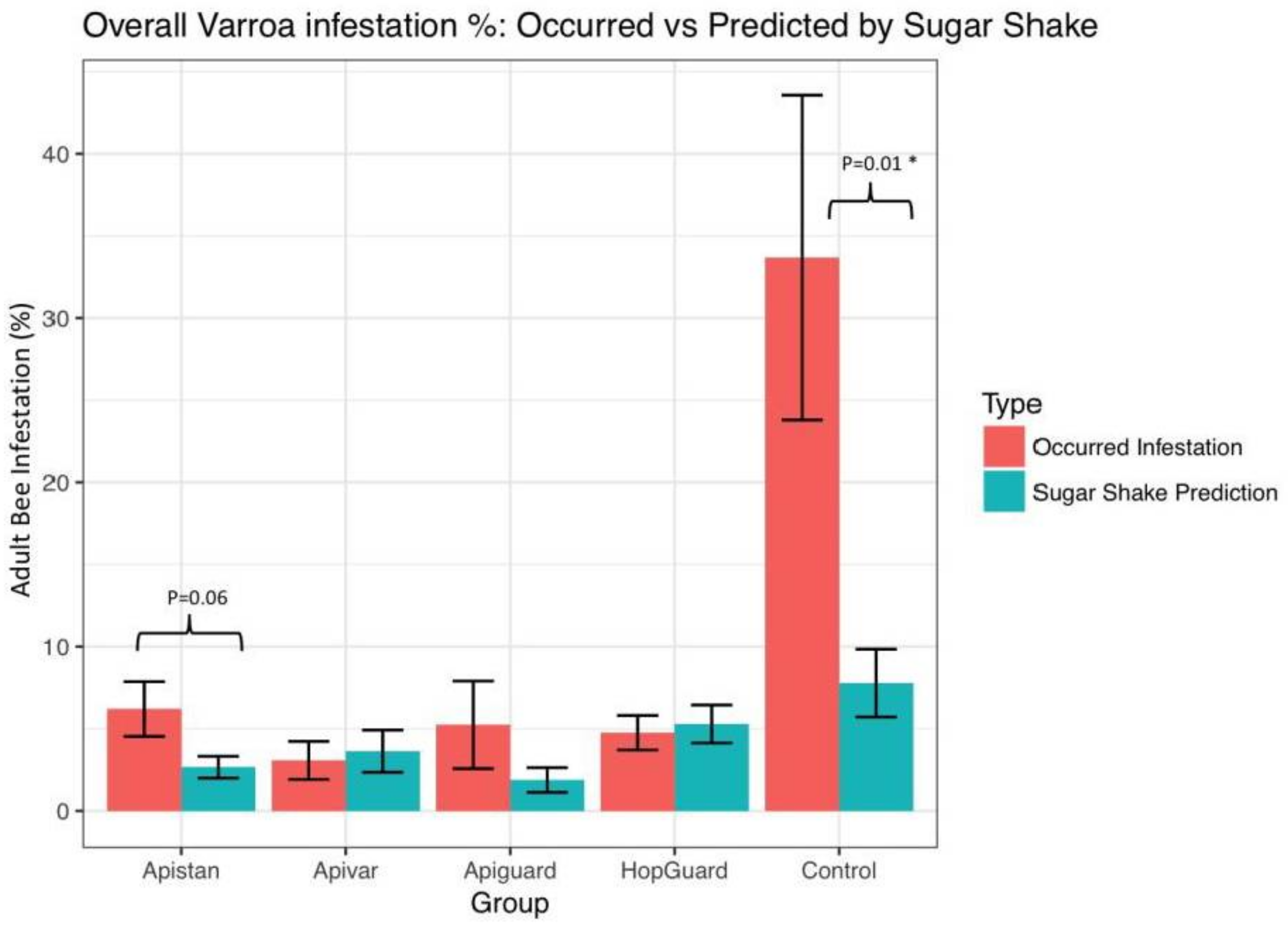
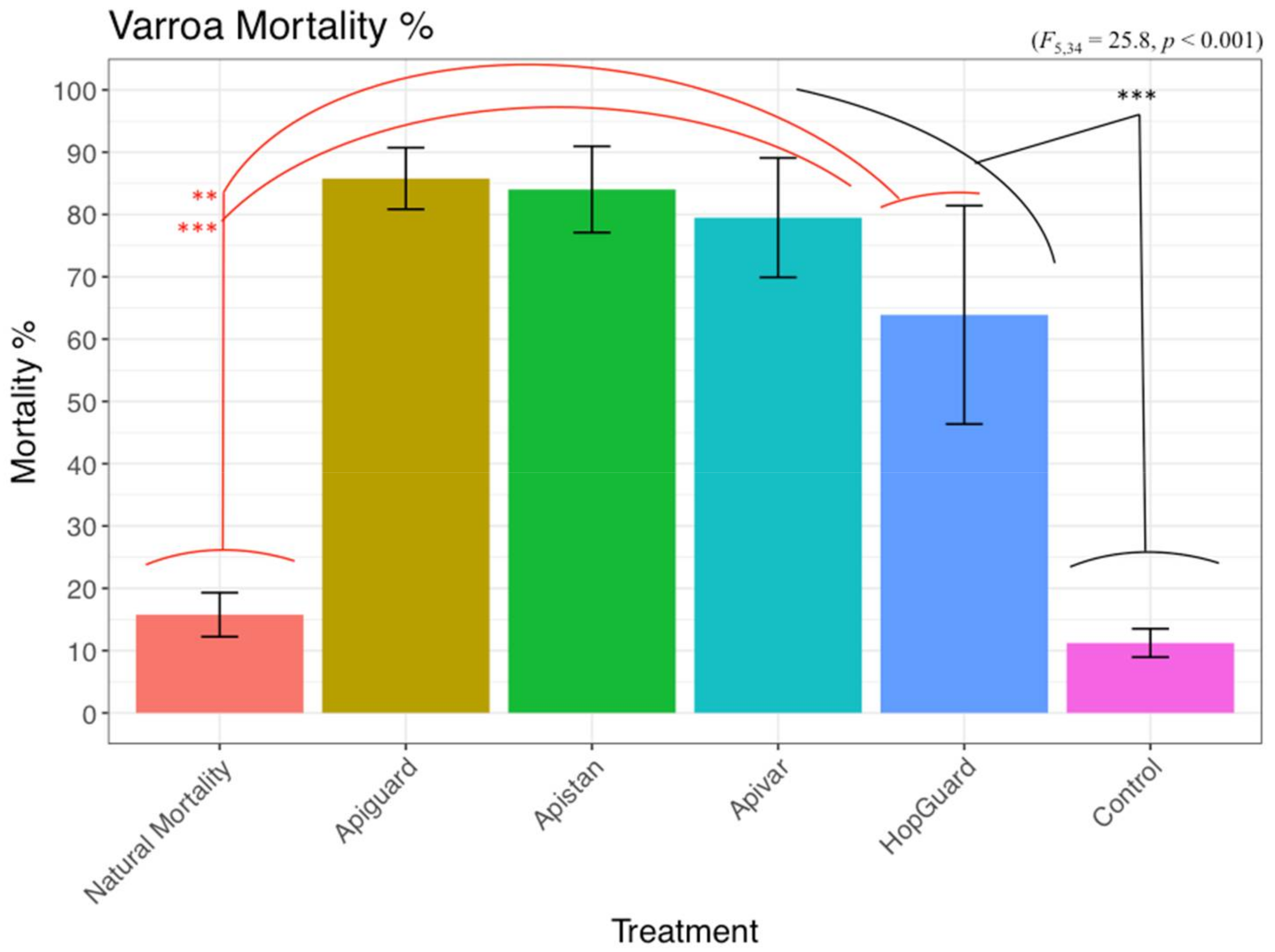
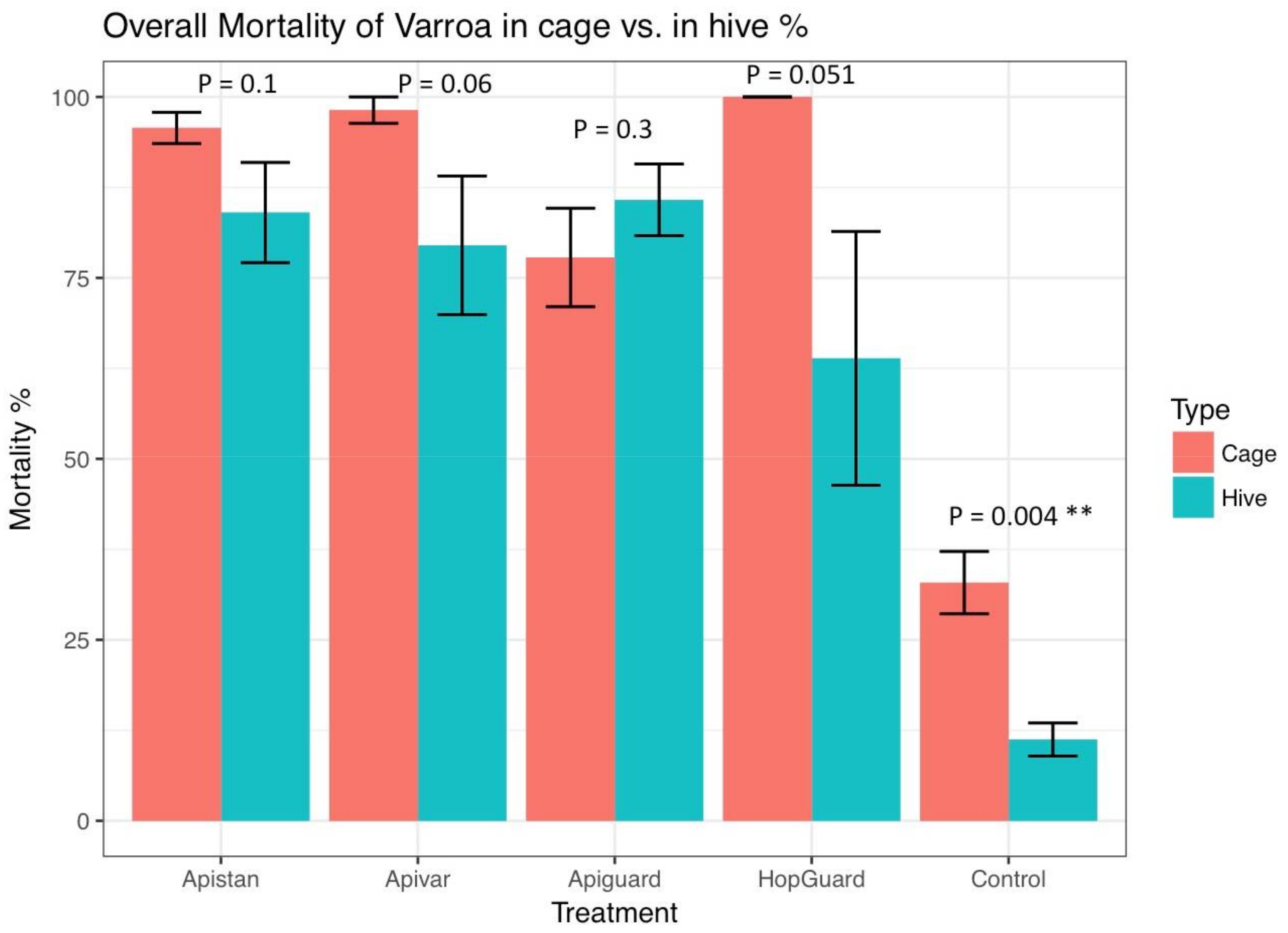
3. Results
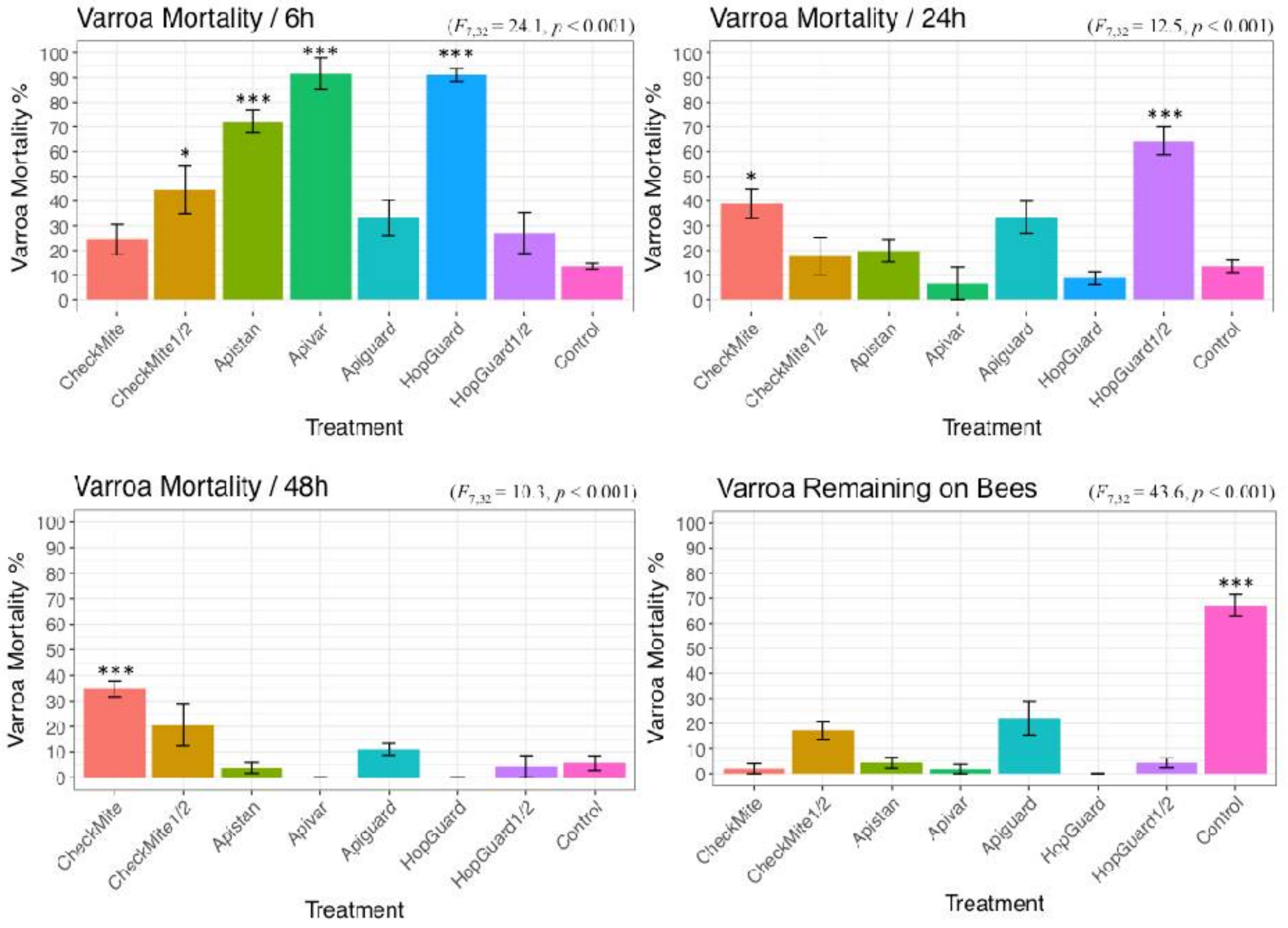
3.1. Laboratory Toxicity Experiment
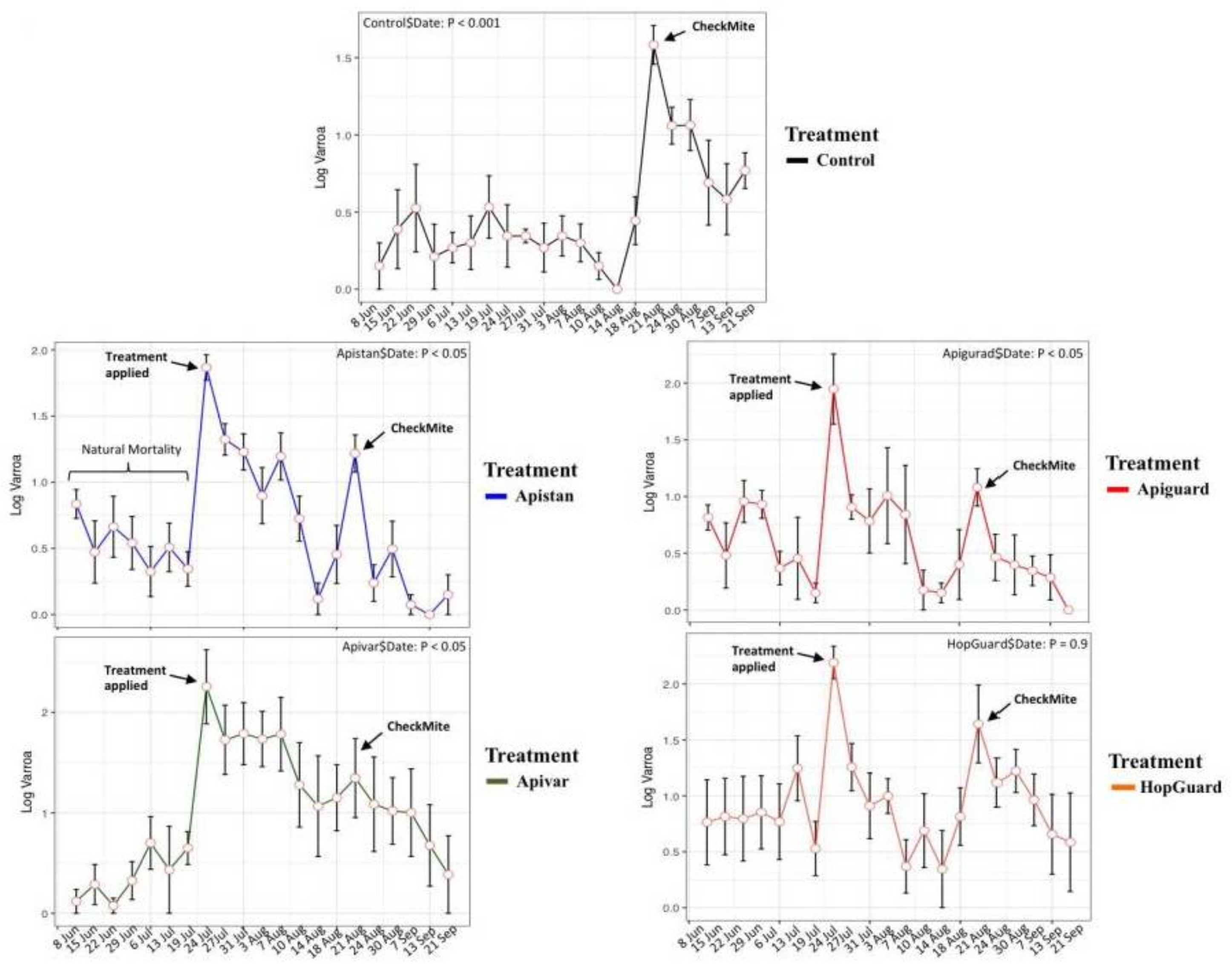
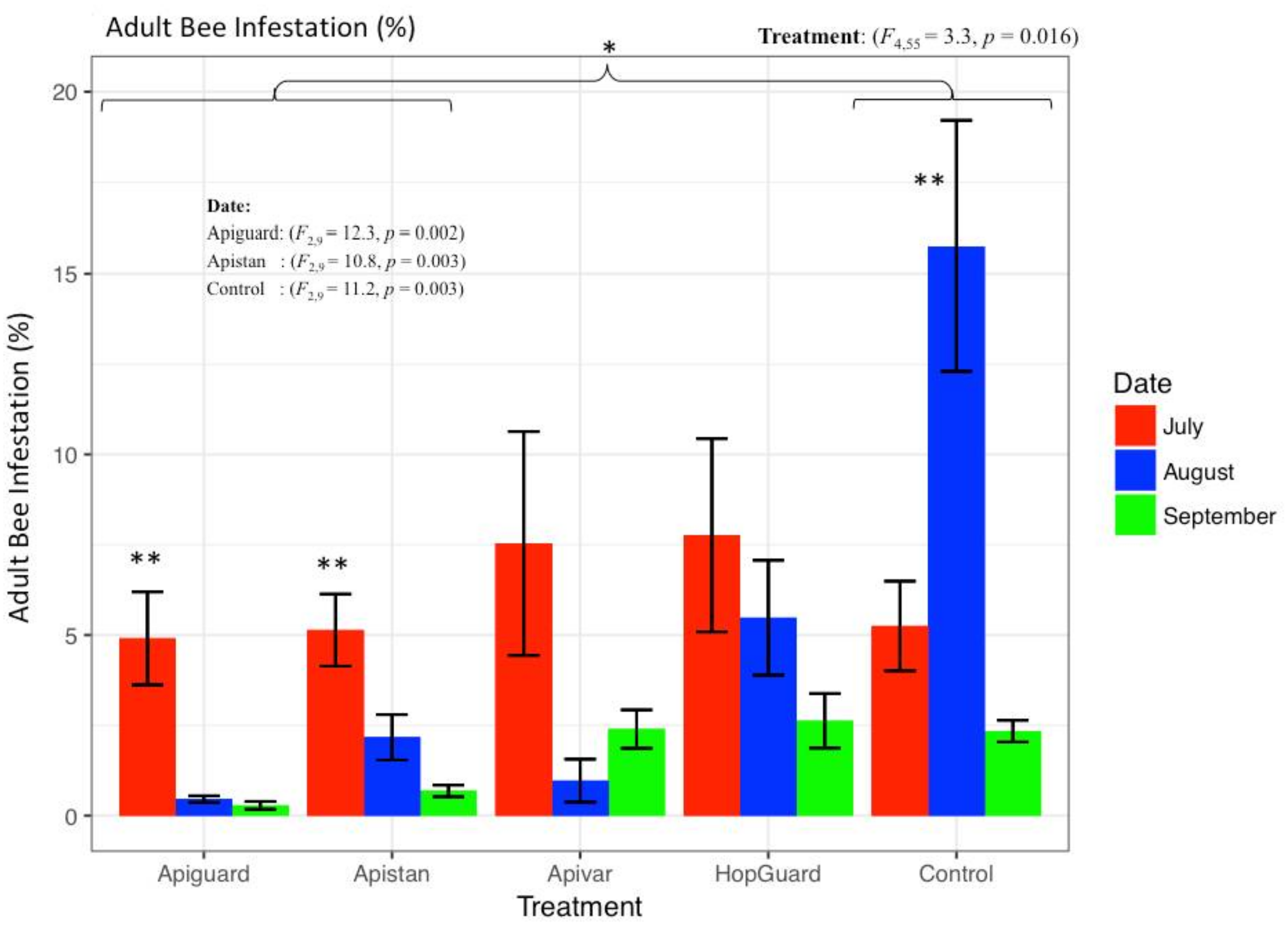
3.2. Field Experiment
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Anderson, D.L.; Trueman, J.W. Varroa jacobsoni (acari: Varroidae) is more than one species. Exp. Appl. Acarol. 2000, 24, 165–189. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103 (Suppl. 1), S96–S119. [Google Scholar] [CrossRef] [PubMed]
- Miozes-Koch, R.; Slabezki, Y.; Efrat, H.; Kalev, H.; Kamer, Y.; Yakobson; Dag, A. First detection in israel of fluvalinate resistance in the varroa mite using bioassay and biochemical methods. Exp. Appl. Acarol. 2000, 24, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Floris, I.; Satta, A.; Cabras, P.; Garau, V.L.; Angioni, A. Comparison between two thymol formulations in the control of Varroa destructor: Effectiveness, persistence, and residues. J. Econ. Entomol. 2004, 97, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Spreafico, M.; Eordegh, F.R.; Bernardinelli, I.; Colombo, M. First detection of strains of Varroa destructor resistant to coumaphos. Results of laboratory tests and field trials. Apidologie 2001, 32, 49–55. [Google Scholar] [CrossRef]
- Gregorc, A.; Poklukar, J. Rotenone and oxalic acid as alternative acaricidal treatments for Varroa destructor in honeybee colonies. Vet. Parasitol. 2003, 111, 351–360. [Google Scholar] [CrossRef]
- Melathopoulos, A.P.; Gates, J. Comparison of two thymol-based acaricides, api life var (R) and apiguard (TM), for the control of varroa mites. Am. Bee J. 2003, 143, 489–493. [Google Scholar]
- Bogdanov, S.; Kilchenmann, V.; Imdorf, A.; Fluri, P. Residues in honey after application of thymol against varroa using the frakno thymol frame. Am. Bee J. 1998, 138, 610–611. [Google Scholar]
- Wallner, K. Varroacides and their residues in bee products. Apidologie 1999, 30, 235–248. [Google Scholar] [CrossRef]
- Baxter, J.; Ellis, M.; Wilson, W. Field evaluation of apistan and five candidate compounds for parasitic mite control in honey bees. Am. Bee J. 2000, 11, 898–900. [Google Scholar]
- Elzen, P.J.; Baxter, J.R.; Spivak, M.; Wilson, W.T. Amitraz resistance in varroa: New discovery in North America. Am. Bee J. 1999, 139, 362–362. [Google Scholar]
- Elzen, P.J.; Westervelt, D. Detection of coumaphos resistance in Varroa destructor in florida. Am. Bee J. 2002, 142, 291–292. [Google Scholar]
- Gracia-Salinas, M.J.; Ferrer-Dufol, M.; Latorre-Castro, E.; Monero-Manera, C.; Castillo-Hernandez, J.A.; Lucientes-Curd, J.; Peribanez-Lopez, M.A. Detection of fluvalinate resistance in Varroa destructor in spanish apiaries. J. Apic. Res. 2006, 45, 101–105. [Google Scholar] [CrossRef]
- Bak, B.; Wilde, J.; Siuda, M. Characteristics of north-eastern population of Varroa destructor resistant to synthetic pyrethroids. Med. Weter. 2012, 68, 603–606. [Google Scholar]
- Thompson, H.M.; Brown, M.A.; Ball, R.F.; Bew, M.H. First report of Varroa destructor resistance to pyrethroids in the UK. Apidologie 2002, 33, 357–366. [Google Scholar] [CrossRef]
- Lindberg, C.M.; Melathopoulos, A.P.; Winston, M.L. Laboratory evaluation of miticides to control Varroa jacobsoni (acari: Varroidae), a honey bee (hymenoptera: Apidae) parasite. J. Econ. Entomol. 2000, 93, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Gregorc, A.; Jelenc, J. Control of Varroa jacobsoni oud. In honeybee colonies using apilife-VAR. Zb. Vet. Fak. Univ. Ljublj. 1996, 33, 231–235. [Google Scholar]
- Fassbinder, C.; Grodnitzky, J.; Coats, J. Monoterpenoids as possible control agents for Varroa destructor. J. Apic. Res. 2002, 41, 83–88. [Google Scholar] [CrossRef]
- Mattila, H.R.; Otis, G.W. The efficacy of apiguard against varroa and tracheal mites, and its effect on honey production: 1999 trial. Am. Bee J. 2000, 140, 969–973. [Google Scholar]
- Gregorc, A.; Planinc, I. The control of Varroa destructor in honey bee colonies using the thymol-based acaricide-apiguard. Am. Bee J. 2005, 145, 672–675. [Google Scholar]
- Rademacher, E.; Marika, H.; Saskia, S. The development of hopguard® as a winter treatment against Varroa destructor in colonies of Apis mellifera. Apidologie 2015, 6, 748–759. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Ahumada, F.; Curry, R.; Probasco, G.; Schantz, L. Population growth of Varroa destructor (acari: Varroidae) in commercial honey bee colonies treated with beta plant acids. Exp. Appl. Acarol. 2014, 64, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Gregorc, A.; Knight, P.R.; Adamczyk, J. Powdered sugar shake to monitor and oxalic acid treatments to control varroa mites (Varroa destructor anderson and trueman) in honey bee (Apis mellifera) colonies. J. Apic. Res. 2017, 56, 71–75. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Van Buren, N.W.M.; Mariën, A.G.H.; Velthuis, H.H.W. The role of trophallaxis in the distribution of perizin in a honeybee colony with regard to the control of the varroa mite. Entomol. Exp. Appl. 1992, 65, 157–164. [Google Scholar] [CrossRef]
- Van Buren, N.W.M.; Mariën, A.G.H.; Velthuis, H.H.W. The effectiveness of systemic agents used to control the mite, Varroa jacobsoni, in colonies of the honey bee, Apis mellifera depends on food distribution patterns. Apidologie 1993, 24, 33–43. [Google Scholar] [CrossRef]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; Vanengelsdorp, D.; Pettis, J.S. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef] [PubMed]
- Tremolada, P.; Bernardinelli, I.; Colombo, M.; Spreacfico, M.; Vighi, M. Coumaphos distribution in the hive ecosystem: Case study for modelling applications. Ecotoxicology 2004, 13, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Martell, A.-C.; Zeggane, S.; Aurieres, C.; Drahnudel, P.; Faucon, J.-P.; Aubert, M. Acaricide residues in honey and wax after treatment of honey bee colonies with apivar® or asuntol® 50. Apidologie 2007, 6, 534–544. [Google Scholar] [CrossRef]
- Premrov Bajuk, B.; Babnik, K.; Snoj, T.; Milčinski, L.; Pislak Ocepek, M.; Škof, M.; Jenčič, V.; Filazi, A.; Štajnbaher, D.; Kobal, S. Coumaphos residues in honey, bee brood, and beeswax after Varroa treatment. Apidologie 2017, 48, 588–598. [Google Scholar] [CrossRef]
- Extension Toxicology Network. Pesticide Information Proþle: Coumaphos. Extoxnet. 2001. Available online: http://extoxnet.orst.edu/pips/coumapho.htm (Accessed on 10 December 2015).
- Gregorc, A. A clinical case of honey bee intoxication after using coumaphos strips against Varroa destructor. J. Apic. Res. 2012, 51, 142–143. [Google Scholar] [CrossRef]
- Koumad, S.; Haddad, N. Resistance of Varroa destructor to apistan© and bayvarol. J. Zool. Res. 2015, 1, 35–42. [Google Scholar]
- Merrington, O. Bibliography on the Use of Amitraz for Varroa Control in bees (Apis Spp.) (1979–1989); Cambridge Animal and Public Health Ltd.: Cambridge, UK, 1990; p. 36. [Google Scholar]
- Maggi, M.D.; Ruffinengo, S.R.; Negri, P.; Eguaras, M.J. Resistance phenomena to amitraz from populations of the ectoparasitic mite Varroa destructor of Argentina. Parasitol. Res. 2010, 107, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Milani, N. The resistance of Varroa jacobsoni oud. To acaricides. Apidologie 1999, 30, 229–234. [Google Scholar] [CrossRef]
- Al Toufailia, H.M.; Ratnieks, F.L.W. How effective is apistan at killing Varroa? Bee Craft 2016, 98, 7–11. [Google Scholar]
- Floris, I.; Cabras, P.; Garau, V.L.; Minelli, E.V.; Satta, A.; Troullier, J. Persistence and effectiveness of pyrethroids in plastic strips against Varroa jacobsoni (acari: Varroidae) and mite resistance in a mediterranean area. J. Econ. Entomol. 2001, 94, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Al Naggar, Y.; Tan, Y.; Rutherford, C.; Connor, W.; Griebel, P.; Giesy, J.P.; Robertson, A.J. Effects of treatments with apivar((r)) and thymovar((r)) on v-destructor populations, virus infections and indoor winter survival of canadian honey bee (Apis mellifera L.) colonies. J. Apic. Res. 2015, 54, 548–554. [Google Scholar] [CrossRef]
- Vallon, J.; Salvary, F.; Jourdan, P. Suivi de l’efficacité des traitements contre Varroa destructor bénéficiant d’une amm au cours de l’automne et l’hiver 2006/2007. Bull. Tech. Apic. 2007, 2, 49–54. [Google Scholar]
- Pires, S.; Murilhas, A.; Pereira, O.; Maia, M. Current Effectiveness of Amitraz against Varroa in Portugal. In Proceedings of the 39th Apimondia International Apicultural Congress, Dublin, Ireland, 21 November 2005. [Google Scholar]
- Lodesani, M.; Costa, C. Maximizing the efficacy of a thymol based product against the mite Varroa destructor by increasing the air space in the hive. J. Apic. Res. 2008, 47, 113–117. [Google Scholar] [CrossRef]
- Degrandi-Hoffman, G.; Ahumada, F.; Probasco, G.; Schantz, L. The effects of beta acids from hops (Humulus lupulus) on mortality of Varroa destructor (acari: Varroidae). Exp. Appl. Acarol. 2012, 58, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Smith, G.C. A model of the mite parasite, Varroa destructor, on honeybees (Apis mellifera) to investigate parameters important to mite population growth. Ecol. Model. 2002, 148, 263–275. [Google Scholar] [CrossRef]
- Frey, E.; Schnell, H.; Rosenkranz, P. Invasion of Varroa destructor mites into mite-free honey bee colonies under the controlled conditions of a military training area. J. Apic. Res. 2011, 50, 138–144. [Google Scholar] [CrossRef]
- Imdorf, A.; Charriere, J.D.; Bachofen, B. Efficiency checking of the Varroa jacobsoni control methods by means of oxalic acid. Apiacta 1997, 3, 89–91. [Google Scholar]
- Higes, M.; Meana, A.; Suarez, M.; Llorente, J. Negative long-term effects on bee colonies treated with oxalic acid against Varroa jacobsoni oud. Apidologie 1999, 30, 289–292. [Google Scholar] [CrossRef]
- Fakhimzadeh, K.; Ellis, J.D.; Hayes, J.W. Physical control of varroa mites (Varroa destructor): The effects of various dust materials on varroa mite fall from adult honey bees (Apis mellifera) in vitro. J. Apic. Res. 2011, 50, 203–211. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregorc, A.; Alburaki, M.; Sampson, B.; Knight, P.R.; Adamczyk, J. Toxicity of Selected Acaricides to Honey Bees (Apis mellifera) and Varroa (Varroa destructor Anderson and Trueman) and Their Use in Controlling Varroa within Honey Bee Colonies. Insects 2018, 9, 55. https://doi.org/10.3390/insects9020055
Gregorc A, Alburaki M, Sampson B, Knight PR, Adamczyk J. Toxicity of Selected Acaricides to Honey Bees (Apis mellifera) and Varroa (Varroa destructor Anderson and Trueman) and Their Use in Controlling Varroa within Honey Bee Colonies. Insects. 2018; 9(2):55. https://doi.org/10.3390/insects9020055
Chicago/Turabian StyleGregorc, Aleš, Mohamed Alburaki, Blair Sampson, Patricia R. Knight, and John Adamczyk. 2018. "Toxicity of Selected Acaricides to Honey Bees (Apis mellifera) and Varroa (Varroa destructor Anderson and Trueman) and Their Use in Controlling Varroa within Honey Bee Colonies" Insects 9, no. 2: 55. https://doi.org/10.3390/insects9020055





