Elaboration of Ionic Liquids on the Anti-Wear Performance of the Reinforced Steel-Steel Contact Surface
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
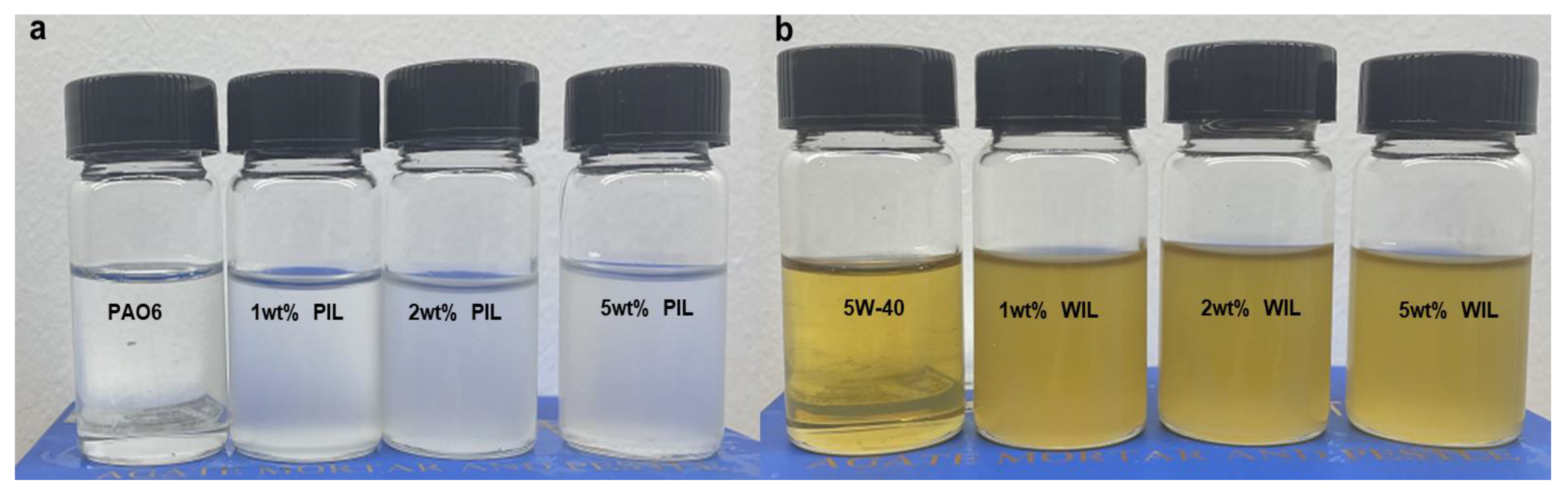
2.2. Preparation Details of Lubricants
2.3. Tribological Test Condition
2.4. Experiment Devices Utilized
3. Results and Discussion
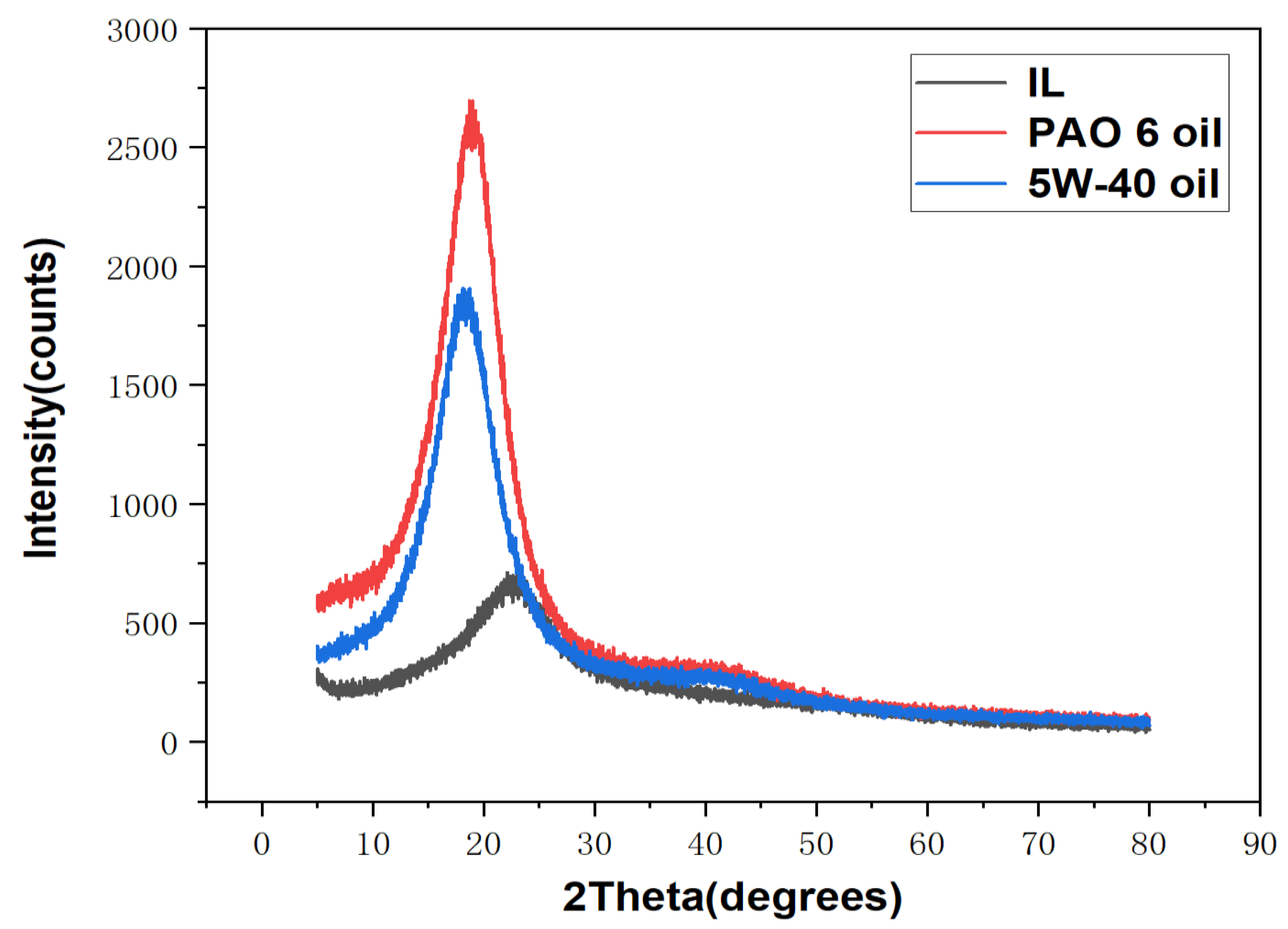
3.1. Characterization of Base Oil and IL
3.2. The Rheological Behavior of Lubricants
3.3. Tribology Characteristics
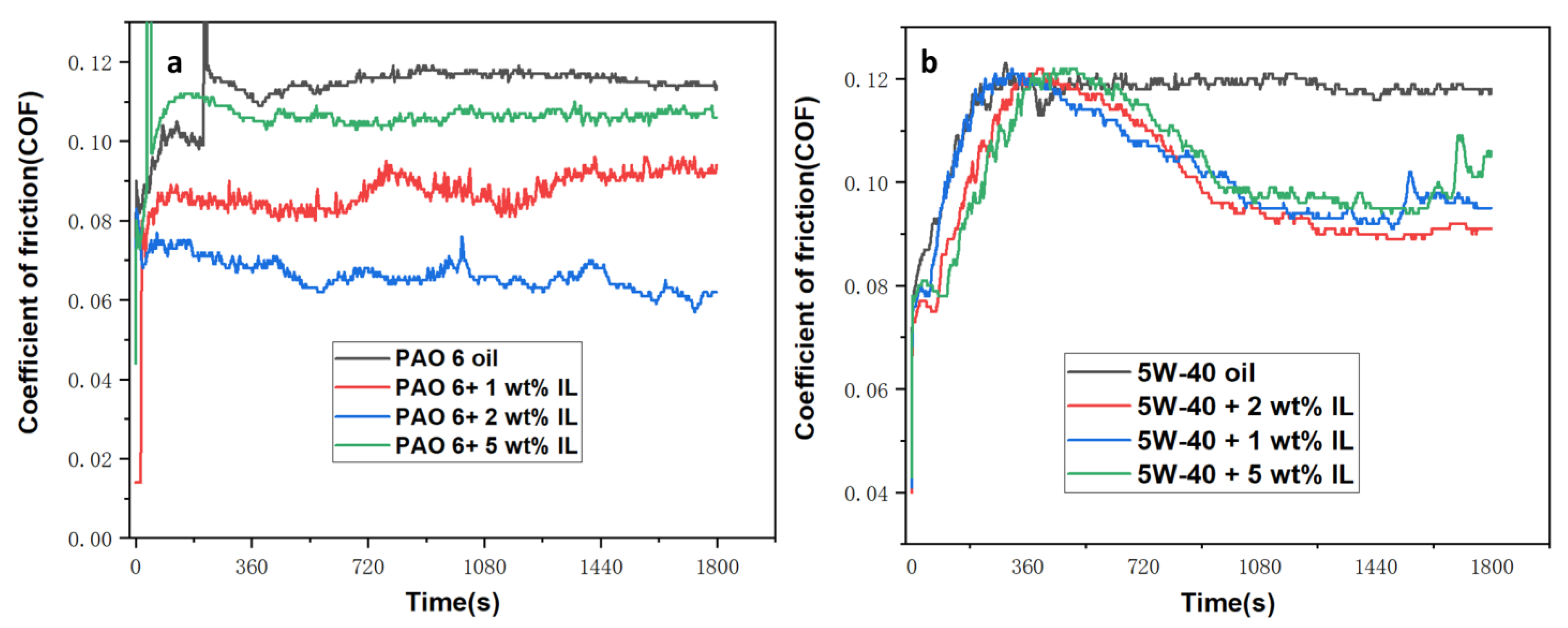
3.3.1. The COF and WSD Measurement
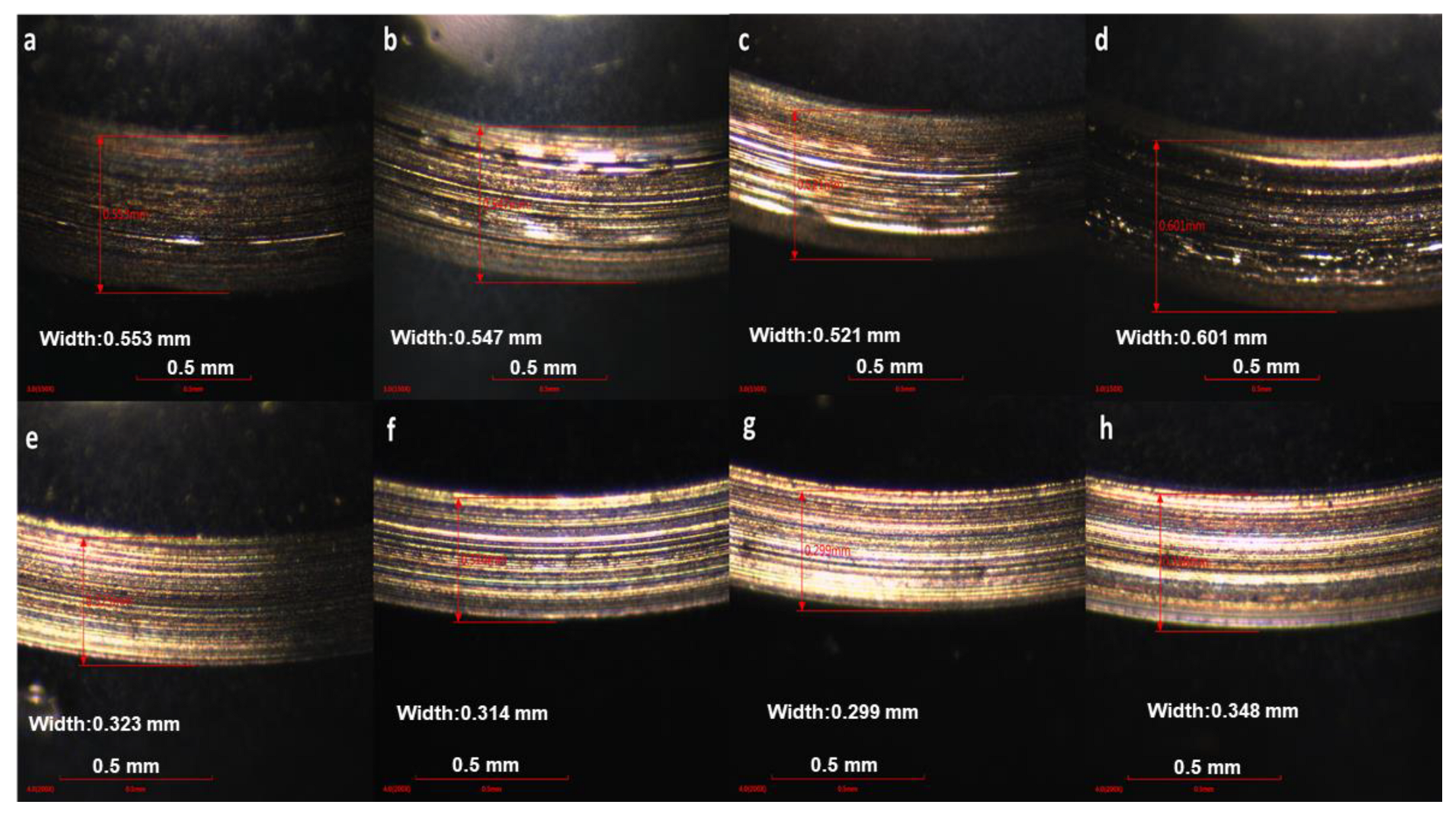
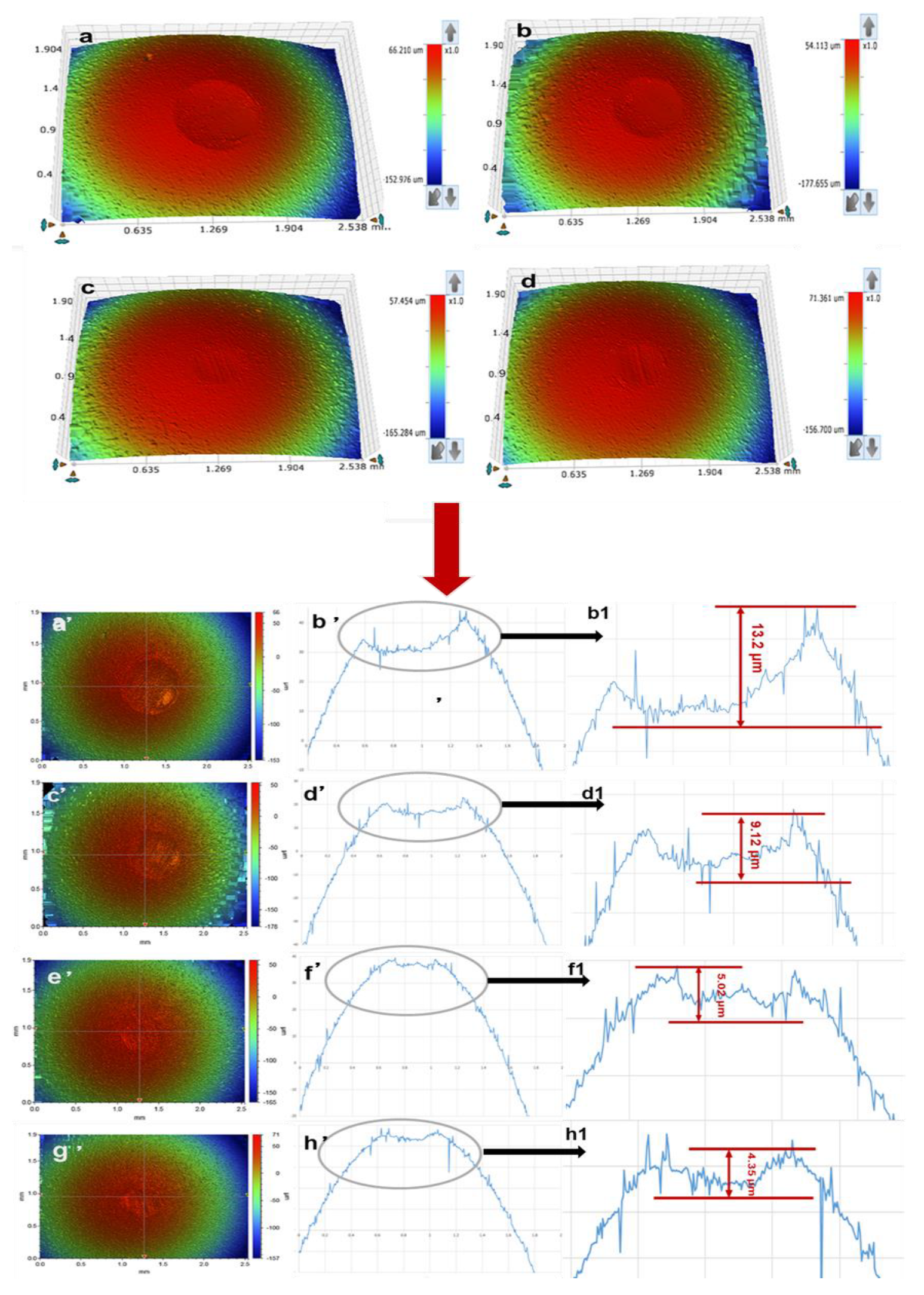
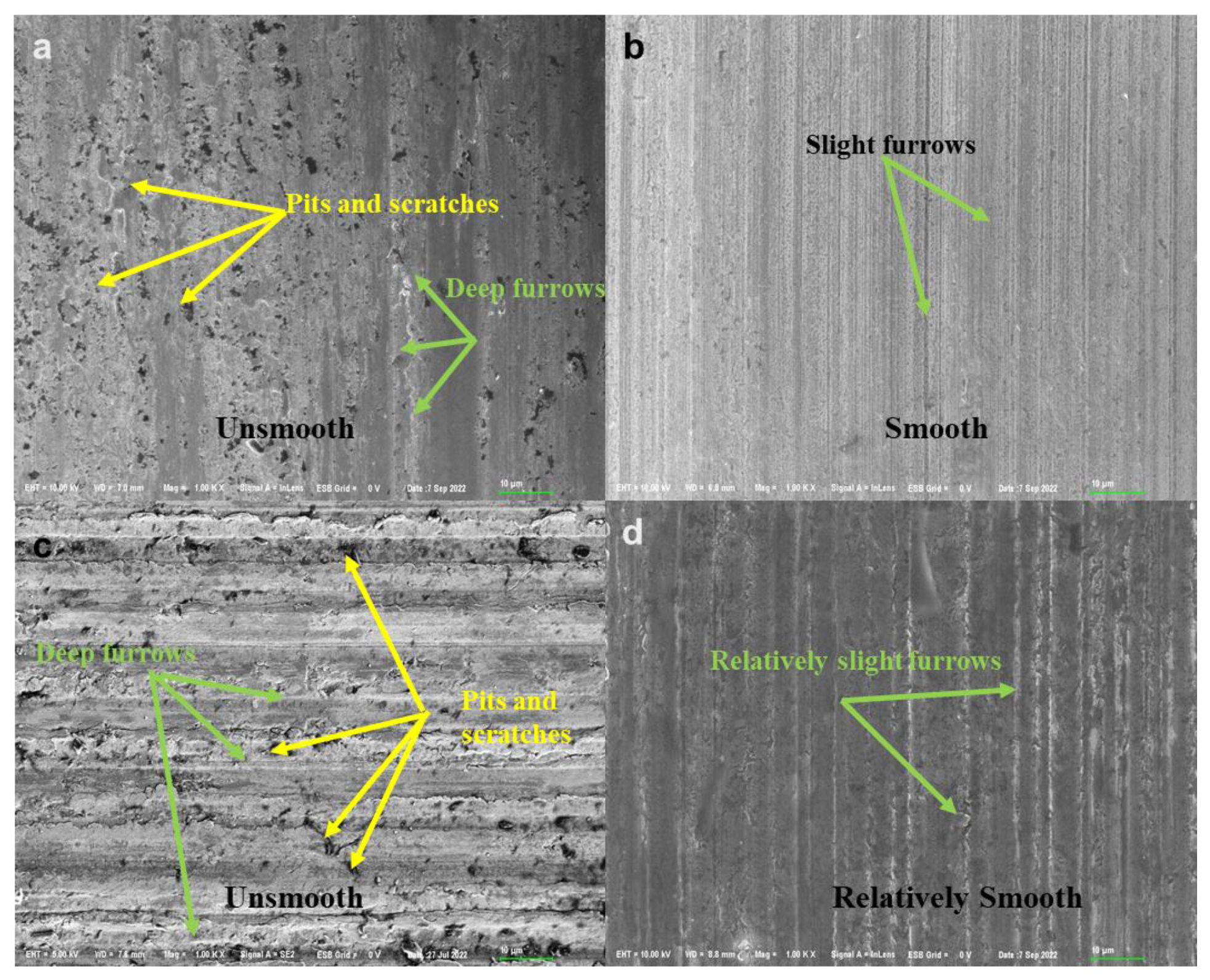
3.3.2. The Micro Morphology Characterization of Wear Scar
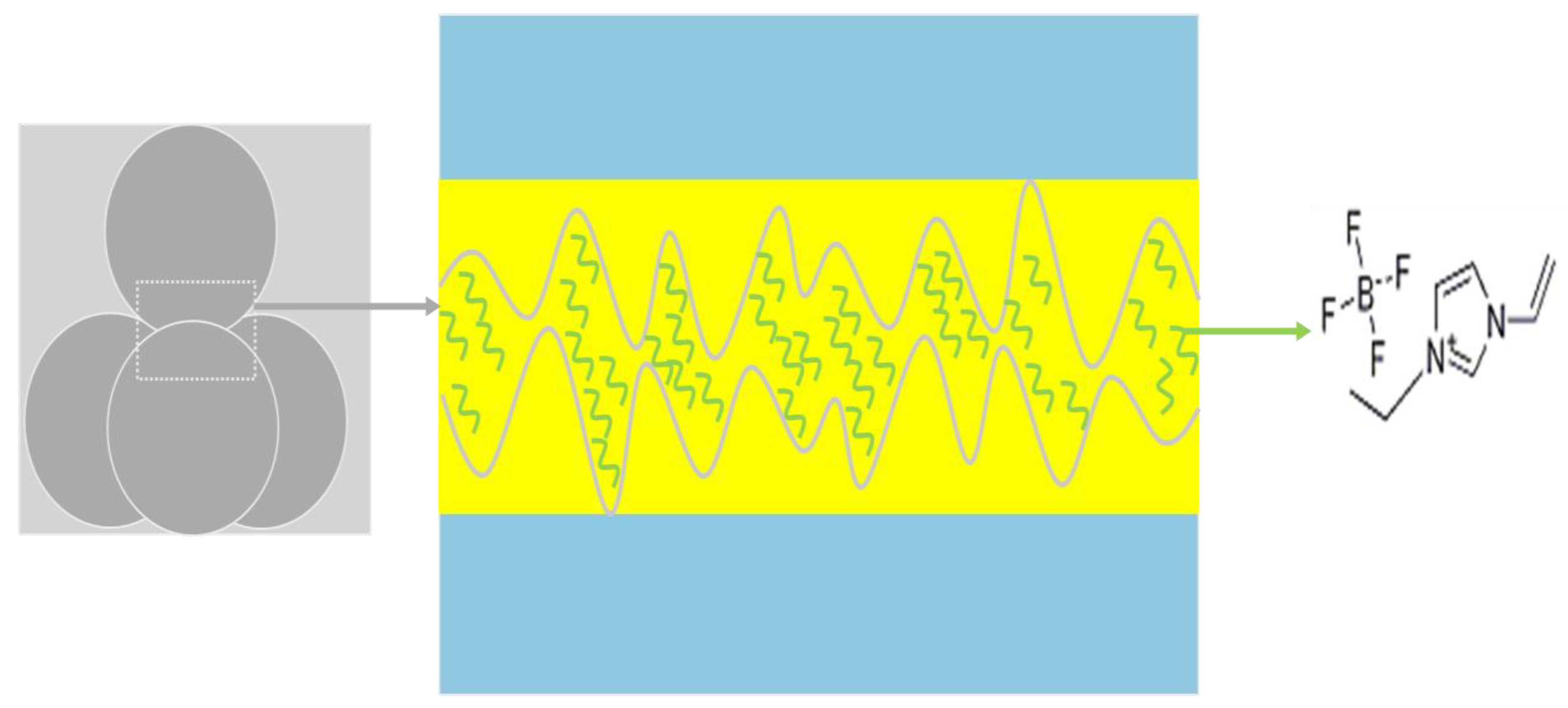
3.3.3. Lubrication Mechanism
4. Conclusions
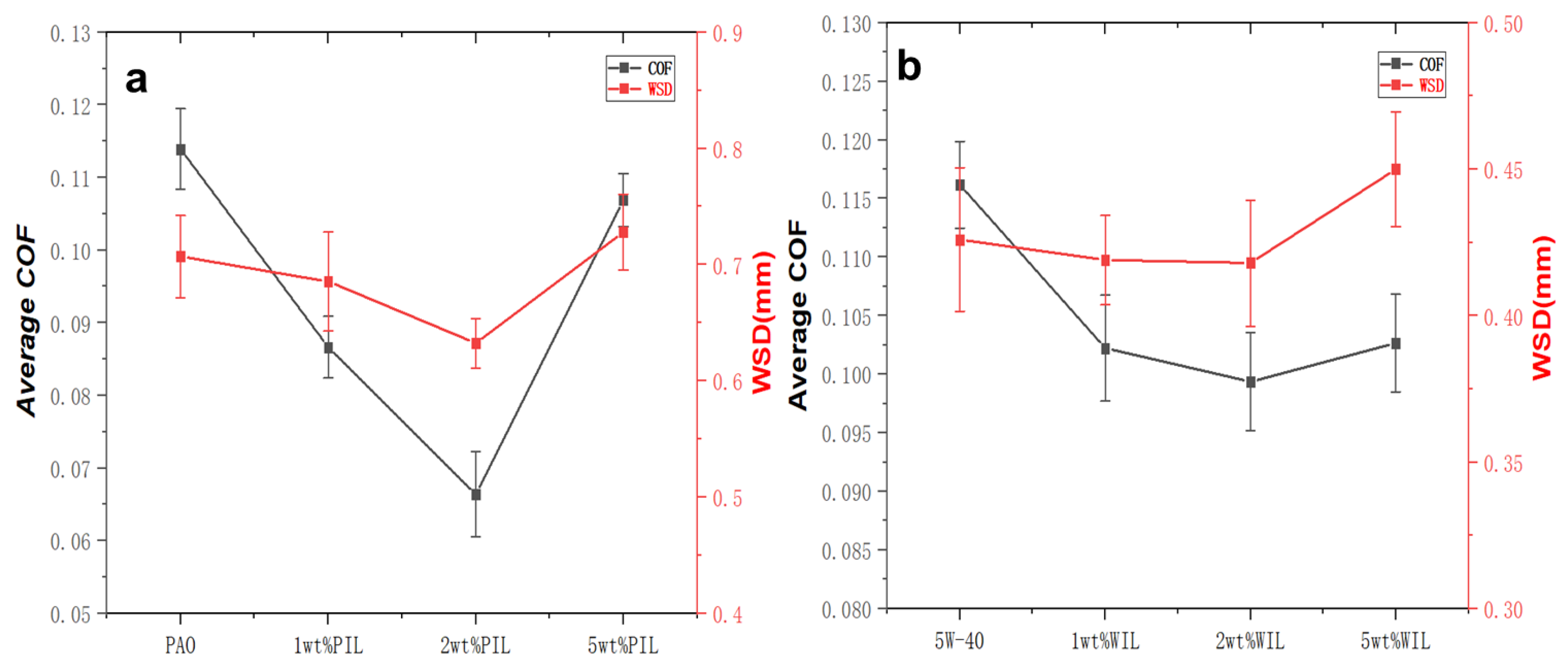
- The COF and WSD values of 2 wt% PIL were decreased by 41.7% and 10.5% while 14.5% and 2.8% for 2 wt% WIL compared with their corresponding base oils.
- IL addition is conducive to the viscosity enhancement of base oil, especially the 5W 40 oil. In addition, all lubricants behave like non-Newtonian fluids.
- The COF and WSD values of base oil can be greatly reduced by the modification of IL (within suitable concentration), especially the PAO6 oil. In this study, 2 wt% PIL and WIL showed the best tribological performance. An amount of 5 wt% PIL and WIL will in turn undermine the anti-friction properties due to the rising viscosity.
- Although IL can also improve the friction properties of 5W 40, the improvement effect of WIL is not as significant as that of PIL, which can be attributed to the higher viscosity of 5W 40, especially after being modified by IL.
- EDS characterization has confirmed that the key elements such as F, B, and N from IL in the surface of the wear scar were detected, strongly indicating the IL does work in the process of friction lubrication and is responsible for the generation of tribo-film in the contact surface due to the polar effect of IL.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, M.K.A.; Hou, X. Exploring the lubrication mechanism of CeO2 nanoparticles dispersed in engine oil by bis(2-ethylhexyl) phosphate as a novel antiwear additive. Tribol. Int. 2021, 165, 107321. [Google Scholar] [CrossRef]
- Jia, J.; Yang, G.; Zhang, C.; Zhang, S.; Zhang, Y.; Zhang, P. Effects of magnetic ionic liquid as a lubricant on the friction and wear behavior of a steel-steel sliding contact under elevated temperatures. Friction 2019, 9, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Iglesias, P. Tribological behavior of ammonium-based protic ionic liquid as lubricant additive. Friction 2020, 9, 169–178. [Google Scholar] [CrossRef]
- Otero, I.; López, E.R.; Reichelt, M.; Fernández, J. Friction and anti-wear properties of two tris(pentafluoroethyl)trifluorophosphate ionic liquids as neat lubricants. Tribol. Int. 2014, 70, 104–111. [Google Scholar] [CrossRef]
- Blanco, D.; González, R.; Viesca, J.L.; Fernández-González, A.; Bartolomé, M.; Battez, A.H. Antifriction and Antiwear Properties of an Ionic Liquid with Fluorine-Containing Anion Used as Lubricant Additive. Tribol. Lett. 2017, 65, 1–10. [Google Scholar] [CrossRef]
- Battez, A.H.; Fernandes, C.M.; Martins, R.C.; Bartolomé, M.; González, R.; Seabra, J.H. Two phosphonium cation-based ionic liquids used as lubricant additive: Part I: Film thickness and friction characteristics. Tribol. Int. 2017, 107, 233–239. [Google Scholar] [CrossRef]
- Grace, J.; Vysochanska, S.; Lodge, J.; Iglesias, P. Ionic Liquids as Additives of Coffee Bean Oil in Steel-Steel Contacts. Lubricants 2015, 3, 637. [Google Scholar] [CrossRef]
- Ploss, M.; Tian, Y.; Yoshikawa, S.; Westbroek, R.; Leckner, J.; Glavatskih, S. Tribological Performance of Non-halogenated Phosphonium Ionic Liquids as Additives to Polypropylene and Lithium-Complex Greases. Tribol. Lett. 2019, 68, 3. [Google Scholar] [CrossRef] [Green Version]
- Bermúdez, M.D.; Jiménez, A.E.; Sanes, J.; Carrión, F.J. Ionic liquids as advanced lubricant fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef]
- Rivera, N.; Blanco, D.; Viesca, J.L.; Fernández-González, A.; González, R.; Battez, A.H. Tribological performance of three fatty acid anion-based ionic liquids (FAILs) used as lubricant additive. J. Mol. Liq. 2019, 296, 111881. [Google Scholar] [CrossRef]
- Weng, L.; Liu, X.; Liang, Y.; Xue, Q. Effect of tetraalkylphosphonium based ionic liquids as lubricants on the tribological performance of a steel-on-steel system. Tribol. Lett. 2007, 26, 11–17. [Google Scholar] [CrossRef]
- Yu, B.; Liu, Z.; Zhou, F.; Liu, W.; Liang, Y. A novel lubricant additive based on carbon nanotubes for ionic liquids. Mater. Lett. 2008, 62, 2967–2969. [Google Scholar] [CrossRef]
- Pejakovic, V.; Kronberger, M.; Mahrova, M.; Vilas, M.; Tojo, E.; Kalin, M. Pyrrolidinium sulfate and ammonium sulfate ionic liquids as lubricant additives for steel/steel contact lubrication. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2012, 226, 923–932. [Google Scholar] [CrossRef]
- Huang, G.; Yu, Q.; Ma, Z.; Cai, M. Probing the lubricating mechanism of oil-soluble ionic liquids additives. Tribol. Int. 2017, 107, 152–162. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Luo, T.; Wang, F.; Xiao, G.; Chen, Z.; Yi, M.; Sheng, C.; Xu, C. Tribological properties of 1-octyl–3-methylimidazolium lactate ionic liquid as a lubricant additive. J. Mol. Liq. 2021, 332, 115828. [Google Scholar] [CrossRef]
- Batteza, A.H.; González, R.; Viesca, J.L.; Blanco, D.; Asedegbega, E.; Osorio, A. Tribological behaviour of two imidazolium ionic liquids as lubricant additives for steel/steel contacts. Wear 2009, 266, 1224–1228. [Google Scholar] [CrossRef]
- Jiang, H.; Hou, X.; Dearn, K.D.; Su, D.; Ali, M.K.A. Thermal stability enhancement mechanism of engine oil using hybrid MoS2/h-BN nano-additives with ionic liquid modification. Adv. Powder Technol. 2021, 32, 4658–4669. [Google Scholar] [CrossRef]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Harith, M.H.; Yusoff, M.N.A.M.; Zulfattah, Z.M.; Jamshaid, M. Tribological Improvement Using Ionic Liquids as Additives in Synthetic and Bio-Based Lubricants for Steel–Steel Contacts. Tribol. Trans. 2020, 63, 235–250. [Google Scholar] [CrossRef]
- Peng, R.; He, X.; Tang, X.; Tong, J.; Zhao, L.; Peng, X. An investigation into the synergistic strengthening mechanism of ionic liquid and nanoparticles as a hybrid nanofluid in friction interface. Tribol. Int. 2022, 165, 107298. [Google Scholar] [CrossRef]
- Rohlmann, P.; Munavirov, B.; Furó, I.; Antzutkin, O.; Rutland, M.W.; Glavatskih, S. Non-halogenated Ionic Liquid Dramatically Enhances Tribological Performance of Biodegradable Oils. Front. Chem. 2019, 7, 98. [Google Scholar] [CrossRef]
- Rahman, M.H.; Khajeh, A.; Panwar, P.; Patel, M.; Martini, A.; Menezes, P.L. Recent progress on phosphonium-based room temperature ionic liquids: Synthesis, properties, tribological performances and applications. Tribol. Int. 2022, 167, 107331. [Google Scholar] [CrossRef]
- Faes, J.; González, R.; Blanco, D.; Fernández-González, A.; Hernández-Battez, A.; Iglesias, P.; Viesca, J.L. Methyltrioctylammonium Octadecanoate as Lubricant Additive to Different Base Oils. Lubricants 2022, 10, 128. [Google Scholar] [CrossRef]
- Huang, X.; Wu, J.; Lu, X.; Feng, X.; Shi, Y. Tribological Properties of Porous PEEK Composites Containing Ionic Liquid under Dry Friction Condition. Lubricants 2017, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Somers, A.E.; Howlett, P.C.; Macfarlane, D.R.; Forsyth, M. A Review of Ionic Liquid Lubricants. Lubricants 2013, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Palacio, M.; Bhushan, B. A review of ionic liquids for green molecular lubrication in nanotechnology. Tribol. Lett. 2010, 40, 247–268. [Google Scholar] [CrossRef]
- Reeves, C.J.; Siddaiah, A.; Menezes, P.L. Tribological study of imidazolium and phosphonium ionic liquid-based lubricants as additives in carboxylic acid-based natural oil: Advancements in environmentally friendly lubricants. J. Clean. Prod. 2018, 176, 241–250. [Google Scholar] [CrossRef]
- Pejakovic, V.; Totolin, V.; Ristic, A.; Gabler, C.; Kalin, M.; Dörr, N. Tribological performance and degradation of 1-n-butyl-1-methylpyrrolidinium methylsulfate ionic liquid in glycerol as lubricant for steel-steel sliding contacts. Lubr. Sci. 2019, 31, 137–149. [Google Scholar] [CrossRef]
- Sani, A.S.A.; Rahim, E.A.; Samion, S. Tribological performance of modified jatropha oil containing oil-miscible ionic liquid for machining applications. J. Mech. Sci. Technol. 2017, 31, 5675–5685. [Google Scholar] [CrossRef]
- Qu, M.; Yang, Z.; Zhang, C.; Yu, Q.; Cai, M.; Zhou, F. Significantly enhancing lubricity and anti-wear performances of glycerol lubricant with urea-functionalized imidazolium-organophosphate ionic liquid as additive. Tribol. Int. 2021, 153, 106602. [Google Scholar] [CrossRef]
- Viesca, J.; Mallada, M.; Blanco, D.; Fernández-González, A.; Espina-Casado, J.; González, R.; Battez, A.H. Lubrication performance of an ammonium cation-based ionic liquid used as an additive in a polar oil. Tribol. Int. 2017, 116, 422–430. [Google Scholar] [CrossRef]
- González, R.; Ramos, D.; Blanco, D.; Fernández-González, A.; Viesca, J.L.; Hadfield, M.; Battez, A.H. Tribological performance of tributylmethylammonium bis(trifluoromethylsulfonyl)amide as neat lubricant and as an additive in a polar oil. Friction 2018, 7, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Khemchandani, B.; Somers, A.; Howlett, P.; Jaiswal, A.; Sayanna, E.; Forsyth, M. A biocompatible ionic liquid as an antiwear additive for biodegradable lubricants. Tribol. Int. 2014, 77, 171–177. [Google Scholar] [CrossRef]
- Zheng, Z.; Yu, H.; Chen, H.; Liu, X.; Wang, H.; Feng, D.; Qiao, D. Guanidine-phosphate ionic liquids as antiwear additives in two basic oils: Different lubrication mechanisms. Tribol. Int. 2021, 160, 106942. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Guo, B.; Jiang, N.; Chu, J.; Liu, J.; Liang, Y.; Yu, Q.; Yang, S.; Guo, F.; et al. Functionalized phosphate ionic liquids as additives in PEG with excellent tribo-logical properties for boundary/mixed/elastohydrodynamic lubrication. Tribol. Int. 2021, 164, 107242. [Google Scholar] [CrossRef]
- Guo, H.; Pang, J.; Adukure, A.R.; Iglesias, P. Influence of Hydrogen Bonding and Ionicity of Protic Ionic Liquids on Lubricating Steel–Steel and Steel–Aluminum Contacts: Potential Ecofriendly Lubricants and Additives. Tribol. Lett. 2020, 68, 1–10. [Google Scholar] [CrossRef]
- Blanco, D.; Battez, A.H.; Viesca, J.L.; González, R.; Fernández-González, A. Lubrication of CrN coating with ethyl-dimethyl-2-methoxyethylammonium tris (pentafluoroethyl) trifluorophosphate ionic liquid as additive to PAO 6. Tribol. Lett. 2011, 41, 295–302. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D.; Iglesias, P.; Carrión, F.J.; Martínez-Nicolás, G. 1-N-alkyl-3-methylimidazolium ionic liquids as neat lubricants and lubricant additives in steel–aluminium con-tacts. Wear 2006, 260, 766–782. [Google Scholar] [CrossRef]
- Liu, W.; Ye, C.; Chen, Y.; Ou, Z.; Sun, D.C. Tribological behavior of sialon ceramics sliding against steel lubricated by fluorine-containing oils. Tribol. Int. 2002, 35, 503–509. [Google Scholar] [CrossRef]





| Lubricants Information | Conditions | Reduction | Friction Pair | |||||
|---|---|---|---|---|---|---|---|---|
| Ref./Year | Base Oil | Ionic Liquids | Wt% | Speed | Temperature (°C) | Load (N) | COF(%) | |
| [2]/2019 | MF (DIOS-Fe3O4) | MIL ([C6mim]5[Dy(SCN)8]) | 1.22 g/cm3 | \ | 25–150 | 100–200 | Reduced | Ball on disk |
| [3]/2020 | Mineral oil | DCi | 1 | 0.05 m/s | 25–100 | 2 | 29–35.5 | Ball on flat |
| [5]/2017 | Priolube 1936(A2) | [P66614][NTf2] | 0.25–1 | \ | 25 | 80 | 1.37 | Ball on plate |
| [6]/2017 | Yubase4/Group III | [P66614] [(iC8)2PO2], [P66614][BEHP] | 0.5–1 | 0.01–3 m/s | 40–100 | \ | Reduced | Ball on disc |
| [7]/2015 | Coffee bean oil | [THTDP][Deca], [THTDP][NTf2] | 1–5 | \ | 25 | 2 | Reduced | Block on flat |
| [8]/2020 | PP and LiX greases | [P66614][BOB], [P66614][BMB], [P66614][BMPP], [P66614][DCA] | 2–10 | 0.2 m/s | 25–130 | \ | 60 | Pin on disk |
| [10]/2019 | Priolube 3970 (BO) | [N8881][C8:0], ([N8881][C12:0], ([N8881][C16:0] | 0.5–2 | 10–2500 mm/s | 25–100 | 30–50 | 4 | Ball on disc |
| [13]/2012 | Glycerol | BuMepyr-MeSO4, Et3MeN-MeSO4 | 0.625–8 | 0.1 m/s | 100 | 10 | Reduced | Ball on flat |
| [15]/2021 | SAE 10W-40, PEG400 | L-L108, L-P108, L-B108 | 0.5–4 | 200 rpm | 25 | 5 | 6–24.3 | Ball on disk |
| [18]/2020 | PAO8, TMPTO | [P14,6,6,6][TMPP], [P14,6,6,6][Deca], [BMIM][BF4] | 0.5–1.5 | \ | 75 | 100 | 28 | Four ball |
| [20]/2019 | OPAG, Estisol 240(ME) | P-BMB | 0–20 | 0.2 m/s | 90 | 21 | 10 | Ball on plate |
| [26]/2018 | Avocado oil | P66614Tf2N, C10mimTf2N | 0–100 | 36 mm/s | 23 | 10 | 11.24–68.88 | Pin on disk |
| [27]/2019 | Glycerol | 1-n-butyl-1-methylpyrrolidinium methylsulfate | 0.6–8 | 0.1 m/s | 25–100 | 10 | 20–56 | Ball on disc |
| [28]/2017 | Jatropha Trimethylolpropane ester (MJO) | Methyltrioctylammonium bis(trifluoromethylsulfonyl)imide (AIL) | 1–10 | 1200 rpm | 75 | 392 | 48 | Four ball |
| [29]/2021 | Glycerol | M-16-EDHPA | 0.3–1.5 | \ | 25 | 100 | Reduced | Ball on disk |
| [30]/2017 | Priolube 1936(A2) | [N1888][NTf2] | 1.25–5 | \ | Room temperature | 40–120 | 1.45 | Ball on disk |
| [31]/2019 | diester oil(A1) | [N4441][NTf2] | 1.5 | \ | 25–100 | 30–70 | Reduced | Ball on disk |
| [32]/2014 | Safflower oil (SO) | P1444DPP | 0.005 mol/kg | 0.1 m/s | 50 | 50-350 | Reduced | Pin on disc |
| [33]/2021 | DOP, PEG200 | TMG-DMP, TMG-DEP, TMG-DPP, TMG-DBP | 0.5–5 | \ | 25, 100 | 300 | Reduced | Ball on disc |
| [34]/2021 | PEG400 | NP44, NP88 | 0.5–3 | 7–3200 mm/s | 25–100 | 20 | Reduced | Ball on disc |
| [35]/2020 | Mineral oil | Eet, Met, Det | 1 | 0.03 m/s | 25 | 3 | 63.3 | Ball on flat |
| [36]/2011 | PAO6 | ethyl-dimethyl-2-methoxyethylammonium tris(pentafluoroethyl)trifluorophosphate | 1 | 0.02 m/s | Room temperature | 20–40 | \ | Ball on plate |
| [37]/2006 | Mineral oil | 1-n-alkyl-3-methylimidazolium | 1 | \ | \ | 2.45 | 70 | Pin on disc |
| [38]/2002 | \ | PEPE, X1P, L108 | \ | 0.1 m/s | 20 | 0.5–400 | \ | Dy–sialon disc |
| Base Oil | Viscosity Index | IL Concentration | Sample Label |
|---|---|---|---|
| PAO6 | 138 | 1, 2, 5 wt% | PIL |
| 5W-40 | 174 | 1, 2, 5 wt% | WIL |
| IL Used | Molecular Weight | Molecular Formula | CAS Number | Melting Point | Density |
|---|---|---|---|---|---|
| (1-vinyl-3-ethyliMidazoliumtetrafluoroborate) | 209.9 | C7H11BF4N2 | 936030-51-2 | −3.7 | 1.25 (50 °C) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Hou, X.; Ma, Y.; Guan, W.; Liu, H.; Qian, Y. Elaboration of Ionic Liquids on the Anti-Wear Performance of the Reinforced Steel-Steel Contact Surface. Lubricants 2022, 10, 260. https://doi.org/10.3390/lubricants10100260
Jiang H, Hou X, Ma Y, Guan W, Liu H, Qian Y. Elaboration of Ionic Liquids on the Anti-Wear Performance of the Reinforced Steel-Steel Contact Surface. Lubricants. 2022; 10(10):260. https://doi.org/10.3390/lubricants10100260
Chicago/Turabian StyleJiang, Hua, Xianjun Hou, Yuxin Ma, Weiwei Guan, Haijun Liu, and Yucong Qian. 2022. "Elaboration of Ionic Liquids on the Anti-Wear Performance of the Reinforced Steel-Steel Contact Surface" Lubricants 10, no. 10: 260. https://doi.org/10.3390/lubricants10100260





