Genetic Heterogeneity of SLC22 Family of Transporters in Drug Disposition
Abstract
:1. Introduction
2. Carriers for Organic Cations
2.1. Role of Organic Cation Transporters in Drug Disposition
2.2. Impact of Genetic Variability in Pharmacokinetics
3. Carriers for Organic Anions
3.1. Role of Organic Anion Transporters in Drug Disposition
3.2. Impact of Genetic Variability in Pharmacokinetics
4. SLC22 Genetic Heterogeneity in Cancer Pharmacology
5. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Nigam, S.K. The SLC22 transporter family: A paradigm for the impact of drug transporters on metabolic pathways, signaling, and disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 663–687. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H.; Endou, H. The SLC22 drug transporter family. Pflugers Arch. 2004, 447, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Nigam, K.B.; Date, R.C.; Bush, K.T.; Springer, S.A.; Saier, M.H., Jr.; Wu, W.; Nigam, S.K. Evolutionary analysis and classification of OATs, OCTs, OCTNs, and other SLC22 transporters: Structure-function implications and analysis of sequence motifs. PLoS ONE 2015, 10, e0140569. [Google Scholar] [CrossRef] [PubMed]
- Kaler, G.; Truong, D.M.; Khandelwal, A.; Nagle, M.; Eraly, S.A.; Swaan, P.W.; Nigam, S.K. Structural variation governs substrate specificity for organic anion transporter (OAT) homologs. Potential remote sensing by OAT family members. J. Biol. Chem. 2007, 282, 23841–23853. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.C.; Zur, A.A.; Maurer, T.S.; Yee, S.W.; Tolsma, J.; Jasper, P.; Scott, D.O.; Giacomini, K.M. Rapid method to determine intracellular drug concentrations in cellular uptake assays: Application to metformin in organic cation transporter 1-transfected human embryonic kidney 293 cells. Drug Metab. Dispos. 2016, 44, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, B.C.; Burckhardt, G. Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev. Physiol. Biochem. Pharmacol. 2003, 146, 95–158. [Google Scholar] [PubMed]
- Engstrom, K.; Ameer, S.; Bernaudat, L.; Drasch, G.; Baeuml, J.; Skerfving, S.; Bose-O’Reilly, S.; Broberg, K. Polymorphisms in genes encoding potential mercury transporters and urine mercury concentrations in populations exposed to mercury vapor from gold mining. Environ. Health Perspect. 2013, 121, 85–91. [Google Scholar] [PubMed]
- Han, Y.F.; Fan, X.H.; Wang, X.J.; Sun, K.; Xue, H.; Li, W.J.; Wang, Y.B.; Chen, J.Z.; Zhen, Y.S.; Zhang, W.L.; et al. Association of intergenic polymorphism of organic anion transporter 1 and 3 genes with hypertension and blood pressure response to hydrochlorothiazide. Am. J. Hypertens. 2011, 24, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Bhatnagar, V.; Wen, G.; Hamilton, B.A.; Eraly, S.A.; Nigam, S.K. Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)]. Kidney Int. 2005, 68, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Arimany-Nardi, C.; Koepsell, H.; Pastor-Anglada, M. Role of SLC22A1 polymorphic variants in drug disposition, therapeutic responses, and drug-drug interactions. Pharmacogenomics J. 2015, 15, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. Polyspecific organic cation transporters: Their functions and interactions with drugs. Trends Pharmacol. Sci. 2004, 25, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Sala-Rabanal, M.; Li, D.C.; Dake, G.R.; Kurata, H.T.; Inyushin, M.; Skatchkov, S.N.; Nichols, C.G. Polyamine transport by the polyspecific organic cation transporters OCT1, OCT2, and OCT3. Mol. Pharm. 2013, 10, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Lemos, C.; Faria, A.; Meireles, M.; Martel, F.; Monteiro, R.; Calhau, C. Thiamine is a substrate of organic cation transporters in Caco-2 cells. Eur. J. Pharmacol. 2012, 682, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Gorboulev, V.; Ulzheimer, J.C.; Akhoundova, A.; Ulzheimer-Teuber, I.; Karbach, U.; Quester, S.; Baumann, C.; Lang, F.; Busch, A.E.; Koepsell, H. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997, 16, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 2007, 24, 1227–1251. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Takeda, M.; Narikawa, S.; Enomoto, A.; Ichida, K.; Endou, H. Human organic anion transporters and human organic cation transporters mediate renal transport of prostaglandins. J. Pharmacol. Exp. Ther. 2002, 301, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Sakurai, Y.; Saito, H.; Masuda, S.; Urakami, Y.; Goto, M.; Fukatsu, A.; Ogawa, O.; Inui, K. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J. Am. Soc. Nephrol. 2002, 13, 866–874. [Google Scholar] [PubMed]
- Busch, A.E.; Karbach, U.; Miska, D.; Gorboulev, V.; Akhoundova, A.; Volk, C.; Arndt, P.; Ulzheimer, J.C.; Sonders, M.S.; Baumann, C.; et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol. Pharmacol. 1998, 54, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Lips, K.S.; Volk, C.; Schmitt, B.M.; Pfeil, U.; Arndt, P.; Miska, D.; Ermert, L.; Kummer, W.; Koepsell, H. Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am. J. Respir. Cell Mol. Biol. 2005, 33, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, W.; Ganapathy, M.E.; Wang, H.; Kekuda, R.; Conway, S.J.; Leibach, F.H.; Ganapathy, V. Structure, function, and regional distribution of the organic cation transporter OCT3 in the kidney. Am. J. Physiol. Renal Physiol. 2000, 279, F449–F458. [Google Scholar] [CrossRef] [PubMed]
- Sata, R.; Ohtani, H.; Tsujimoto, M.; Murakami, H.; Koyabu, N.; Nakamura, T.; Uchiumi, T.; Kuwano, M.; Nagata, H.; Tsukimori, K.; et al. Functional analysis of organic cation transporter 3 expressed in human placenta. J. Pharmacol. Exp. Ther. 2005, 315, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Duan, H.; Hebert, M.F.; Liang, C.J.; Rice, K.M.; Wang, J. Taste of a pill: Organic cation transporter-3 (OCT3) mediates metformin accumulation and secretion in salivary glands. J. Biol. Chem. 2014, 289, 27055–27064. [Google Scholar] [CrossRef] [PubMed]
- Breining, P.; Pedersen, S.B.; Pikelis, A.; Rolighed, L.; Sundelin, E.I.O.; Jessen, N.; Richelsen, B. High expression of organic cation transporter 3 in human bat-like adipocytes. Implications for extraneuronal norepinephrine uptake. Mol. Cell. Endocrinol. 2017, 443, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I.; Yabuuchi, H.; Nezu, J.; Sai, Y.; Oku, A.; Shimane, M.; Tsuji, A. Cloning and characterization of a novel human pH-dependent organic cation transporter, OCTN1. FEBS Lett. 1997, 419, 107–111. [Google Scholar] [CrossRef]
- Garrett, Q.; Xu, S.; Simmons, P.A.; Vehige, J.; Flanagan, J.L.; Willcox, M.D. Expression and localization of carnitine/organic cation transporter OCTN1 and OCTN2 in ocular epithelium. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4844–4849. [Google Scholar] [CrossRef] [PubMed]
- Grundemann, D.; Harlfinger, S.; Golz, S.; Geerts, A.; Lazar, A.; Berkels, R.; Jung, N.; Rubbert, A.; Schomig, E. Discovery of the ergothioneine transporter. Proc. Natl. Acad. Sci. USA 2005, 102, 5256–5261. [Google Scholar] [CrossRef] [PubMed]
- Pochini, L.; Scalise, M.; Galluccio, M.; Pani, G.; Siminovitch, K.A.; Indiveri, C. The human OCTN1 (SLC22A4) reconstituted in liposomes catalyzes acetylcholine transport which is defective in the mutant L503F associated to the Crohn’s disease. Biochim. Biophys. Acta 2012, 1818, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Lamhonwah, A.M.; Tein, I. Novel localization of OCTN1, an organic cation/carnitine transporter, to mammalian mitochondria. Biochem. Biophys. Res. Commun. 2006, 345, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I.; Ohashi, R.; Nezu, J.; Yabuuchi, H.; Oku, A.; Shimane, M.; Sai, Y.; Tsuji, A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J. Biol. Chem. 1998, 273, 20378–20382. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, W.; Prasad, P.D.; Seth, P.; Rajan, D.P.; Leibach, F.H.; Chen, J.; Conway, S.J.; Ganapathy, V. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. J. Pharmacol. Exp. Ther. 1999, 290, 1482–1492. [Google Scholar] [PubMed]
- Lamhonwah, A.M.; Skaug, J.; Scherer, S.W.; Tein, I. A third human carnitine/organic cation transporter (OCTN3) as a candidate for the 5q31 Crohn’s disease locus (IBD5). Biochem. Biophys. Res. Commun. 2003, 301, 98–101. [Google Scholar] [CrossRef]
- Lamhonwah, A.M.; Ackerley, C.A.; Tilups, A.; Edwards, V.D.; Wanders, R.J.; Tein, I. OCTN3 is a mammalian peroxisomal membrane carnitine transporter. Biochem. Biophys. Res. Commun. 2005, 338, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Lu, X.; Xu, Y.; Swiderski, C.F.; Jordan, C.T.; Moscow, J.A. Identification of OCT6 as a novel organic cation transporter preferentially expressed in hematopoietic cells and leukemias. Exp. Hematol. 2002, 30, 1162–1169. [Google Scholar] [CrossRef]
- Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [PubMed]
- Wang, D.S.; Jonker, J.W.; Kato, Y.; Kusuhara, H.; Schinkel, A.H.; Sugiyama, Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J. Pharmacol. Exp. Ther. 2002, 302, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.; Lehmann, C.; Rubbert, A.; Knispel, M.; Hartmann, P.; van Lunzen, J.; Stellbrink, H.J.; Faetkenheuer, G.; Taubert, D. Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab. Dispos. 2008, 36, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkov, M.V.; Saadatmand, A.R.; Lotsch, J.; Tegeder, I.; Stingl, J.C.; Brockmoller, J. Genetically polymorphic OCT1: Another piece in the puzzle of the variable pharmacokinetics and pharmacodynamics of the opioidergic drug tramadol. Clin. Pharmacol. Ther. 2011, 90, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, J.; Kuron, D.; Faltraco, F.; Knoch, T.; Dos Santos Pereira, J.N.; Abu Abed, M.; Prukop, T.; Brockmoller, J.; Tzvetkov, M.V. OCT1 mediates hepatic uptake of sumatriptan and loss-of-function oct1 polymorphisms affect sumatriptan pharmacokinetics. Clin. Pharmacol. Ther. 2016, 99, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkov, M.V.; Saadatmand, A.R.; Bokelmann, K.; Meineke, I.; Kaiser, R.; Brockmoller, J. Effects of OCT1 polymorphisms on the cellular uptake, plasma concentrations and efficacy of the 5-HT(3) antagonists tropisetron and ondansetron. Pharmacogenomics J. 2012, 12, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkov, M.V.; dos Santos Pereira, J.N.; Meineke, I.; Saadatmand, A.R.; Stingl, J.C.; Brockmoller, J. Morphine is a substrate of the organic cation transporter OCT1 and polymorphisms in OCT1 gene affect morphine pharmacokinetics after codeine administration. Biochem. Pharmacol. 2013, 86, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Bourdet, D.L.; Pritchard, J.B.; Thakker, D.R. Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3). J. Pharmacol. Exp. Ther. 2005, 315, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Umehara, K.I.; Iwatsubo, T.; Noguchi, K.; Kamimura, H. Functional involvement of organic cation transporter1 (OCT1/Oct1) in the hepatic uptake of organic cations in humans and rats. Xenobiotica 2007, 37, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Van Montfoort, J.E.; Muller, M.; Groothuis, G.M.; Meijer, D.K.; Koepsell, H.; Meier, P.J. Comparison of “Type I” and “Type II” organic cation transport by organic cation transporters and organic anion-transporting polypeptides. J. Pharmacol. Exp. Ther. 2001, 298, 110–115. [Google Scholar] [PubMed]
- Mulgaonkar, A.; Venitz, J.; Grundemann, D.; Sweet, D.H. Human organic cation transporters 1 (SLC22A1), 2 (SLC22A2), and 3 (SLC22A3) as disposition pathways for fluoroquinolone antimicrobials. Antimicrob. Agents Chemother. 2013, 57, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Dickens, D.; Owen, A.; Alfirevic, A.; Giannoudis, A.; Davies, A.; Weksler, B.; Romero, I.A.; Couraud, P.O.; Pirmohamed, M. Lamotrigine is a substrate for OCT1 in brain endothelial cells. Biochem. Pharmacol. 2012, 83, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Okuda, M.; Inui, K. Metformin transport by renal basolateral organic cation transporter hOCT2. Pharm. Res. 2005, 22, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Weng, Y.; Wang, W.; Bai, M.; Lei, H.; Zhou, H.; Jiang, H. Multiple organic cation transporters contribute to the renal transport of sulpiride. Biopharm. Drug Dispos. 2017, 38, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, M.; Bilos, A.; van den Heuvel, J.; Rijpma, S.R.; Hurkmans, E.G.E.; Sauerwein, R.W.; Russel, F.G.M.; Koenderink, J.B. Proguanil and cycloguanil are organic cation transporter and multidrug and toxin extrusion substrates. Malar. J. 2017, 16, 422. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, Z.; Zhou, S.; Weng, Y.; Lei, H.; Zeng, S.; Li, L.; Jiang, H. Multiple drug transporters are involved in renal secretion of entecavir. Antimicrob. Agents Chemother. 2016, 60, 6260–6270. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Duan, H.; Shirasaka, Y.; Prasad, B.; Wang, J. Atenolol renal secretion is mediated by human organic cation transporter 2 and multidrug and toxin extrusion proteins. Drug Metab. Dispos. 2015, 43, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Minematsu, T.; Iwai, M.; Umehara, K.; Usui, T.; Kamimura, H. Characterization of human organic cation transporter 1 (OCT1/SLC22A1)- and oct2 (SLC22A2)-mediated transport of 1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)-4,9-dihydro-1H-naphtho[2,3-d]imidazolium bromide (YM155 monobromide), a novel small molecule survivin suppressant. Drug Metab. Dispos. 2010, 38, 1–4. [Google Scholar] [PubMed]
- Mulgaonkar, A.; Venitz, J.; Sweet, D.H. Fluoroquinolone disposition: Identification of the contribution of renal secretory and reabsorptive drug transporters. Expert Opin. Drug Metab. Toxicol. 2012, 8, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.C.; Liang, X.; Yee, S.W.; Geier, E.G.; Stocker, S.L.; Chen, L.; Giacomini, K.M. Targeted disruption of organic cation transporter 3 attenuates the pharmacologic response to metformin. Mol. Pharmacol. 2015, 88, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Z.; Zheng, J.; Gee Cheung, F.S.; Chan, T.; Zhu, L.; Zhuge, H.; Zhou, F. The inhibitory effects of the bioactive components isolated from Scutellaria baicalensis on the cellular uptake mediated by the essential solute carrier transporters. J. Pharm. Sci. 2013, 102, 4205–4211. [Google Scholar] [CrossRef] [PubMed]
- Chiappori, A.; Folli, C.; Riccio, A.M.; Caci, E.; Descalzi, D.; De Ferrari, L.; Ingrassia, E.; Nicolini, G.; Canonica, G.W. Salbutamol: How does it enter smooth muscle cells? Int. J. Immunopathol. Pharmacol. 2012, 25, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Urban, T.J.; Brown, C.; Castro, R.A.; Shah, N.; Mercer, R.; Huang, Y.; Brett, C.M.; Burchard, E.G.; Giacomini, K.M. Effects of genetic variation in the novel organic cation transporter, OCTN1, on the renal clearance of gabapentin. Clin. Pharmacol. Ther. 2008, 83, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Oguri, T.; Kunii, E.; Fukuda, S.; Sone, K.; Uemura, T.; Takakuwa, O.; Kanemitsu, Y.; Ohkubo, H.; Takemura, M.; Maeno, K.; et al. Organic cation transporter 6 directly confers resistance to anticancer platinum drugs. Biomed. Rep. 2016, 5, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Hayer, M.; Bonisch, H.; Bruss, M. Molecular cloning, functional characterization and genomic organization of four alternatively spliced isoforms of the human organic cation transporter 1 (hOCT1/SLC22A1). Ann. Hum. Genet. 1999, 63, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Sheardown, S.A.; Brown, C.; Owen, R.P.; Zhang, S.; Castro, R.A.; Ianculescu, A.G.; Yue, L.; Lo, J.C.; Burchard, E.G.; et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Investig. 2007, 117, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.L.; Visser, L.E.; van Schaik, R.H.; Hofman, A.; Uitterlinden, A.G.; Stricker, B.H. Genetic variation in the organic cation transporter 1 is associated with metformin response in patients with diabetes mellitus. Pharmacogenomics J. 2009, 9, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, L.; Kalnina, I.; Geldnere, K.; Bumbure, A.; Ritenberga, R.; Nikitina-Zake, L.; Fridmanis, D.; Vaivade, I.; Pirags, V.; Klovins, J. Association of genetic variation in the organic cation transporters OCT1, OCT2 and multidrug and toxin extrusion 1 transporter protein genes with the gastrointestinal side effects and lower BMI in metformin-treated type 2 diabetes patients. Pharmacogenetics Genom. 2012, 22, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, R.; Fukuda, T.; Niu, J.; Mizuno, T.; Chidambaran, V.; Vinks, A.A.; Sadhasivam, S. ABCC3 and OCT1 genotypes influence pharmacokinetics of morphine in children. Pharmacogenomics 2014, 15, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Chidambaran, V.; Mizuno, T.; Venkatasubramanian, R.; Ngamprasertwong, P.; Olbrecht, V.; Esslinger, H.R.; Vinks, A.A.; Sadhasivam, S. OCT1 genetic variants influence the pharmacokinetics of morphine in children. Pharmacogenomics 2013, 14, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.L.; Visser, L.E.; van Schaik, R.H.; Hofman, A.; Uitterlinden, A.G.; Stricker, B.H. OCT1 polymorphism is associated with response and survival time in anti-Parkinsonian drug users. Neurogenetics 2011, 12, 79–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martel, F.; Grundemann, D.; Calhau, C.; Schomig, E. Apical uptake of organic cations by human intestinal Caco-2 cells: Putative involvement of ASF transporters. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 363, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.H.; Zhang, Y.X.; Lu, R.Y.; Jin, B.; Wang, S.; Liu, Z.R.; Tang, Y.L.; Ding, M.P. Specific OCT1 and ABCG2 polymorphisms are associated with lamotrigine concentrations in Chinese patients with epilepsy. Epilepsy Res. 2016, 127, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Pereira, J.N.; Tadjerpisheh, S.; Abu Abed, M.; Saadatmand, A.R.; Weksler, B.; Romero, I.A.; Couraud, P.O.; Brockmoller, J.; Tzvetkov, M.V. The poorly membrane permeable antipsychotic drugs amisulpride and sulpiride are substrates of the organic cation transporters from the SLC22 family. AAPS J. 2014, 16, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Song, I.S. Genetic variants of organic cation transporter 1 (OCT1) and OCT2 significantly reduce lamivudine uptake. Biopharm. Drug Dispos. 2012, 33, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Saadatmand, A.R.; Tadjerpisheh, S.; Brockmoller, J.; Tzvetkov, M.V. The prototypic pharmacogenetic drug debrisoquine is a substrate of the genetically polymorphic organic cation transporter OCT1. Biochem. Pharmacol. 2012, 83, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Song, I.S.; Shin, H.J.; Shim, E.J.; Jung, I.S.; Kim, W.Y.; Shon, J.H.; Shin, J.G. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin. Pharmacol. Ther. 2008, 84, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Urakami, Y.; Akazawa, M.; Saito, H.; Okuda, M.; Inui, K. cDNA cloning, functional characterization, and tissue distribution of an alternatively spliced variant of organic cation transporter hOCT2 predominantly expressed in the human kidney. J. Am. Soc. Nephrol. 2002, 13, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Anzai, N.; Kimura, T.; Miura, D.; Fukutomi, T.; Takeda, M.; Sakurai, H.; Endou, H. Functional analysis of human organic cation transporter OCT3 (SLC22A3) polymorphisms. J. Pharmacol. Sci. 2010, 113, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Kato, Y.; Sai, Y.; Tsuji, A. Functional characterization of human organic cation transporter OCTN1 single nucleotide polymorphisms in the Japanese population. J. Pharm. Sci. 2004, 93, 2920–2926. [Google Scholar] [CrossRef] [PubMed]
- Urban, T.J.; Yang, C.; Lagpacan, L.L.; Brown, C.; Castro, R.A.; Taylor, T.R.; Huang, C.C.; Stryke, D.; Johns, S.J.; Kawamoto, M.; et al. Functional effects of protein sequence polymorphisms in the organic cation/ergothioneine transporter OCTN1 (SLC22A4). Pharmacogenetics Genom. 2007, 17, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Toh, D.S.; Cheung, F.S.; Murray, M.; Pern, T.K.; Lee, E.J.; Zhou, F. Functional analysis of novel variants in the organic cation/ergothioneine transporter 1 identified in Singapore populations. Mol. Pharm. 2013, 10, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhu, L.; Wang, K.; Murray, M. Recent advance in the pharmacogenomics of human solute carrier transporters (SLCS) in drug disposition. Adv. Drug Deliv. Rev. 2017, 116, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. The SLC22 family with transporters of organic cations, anions and zwitterions. Mol. Aspects Med. 2013, 34, 413–435. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015, 95, 83–123. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, G.; Burckhardt, B.C. In vitro and in vivo evidence of the importance of organic anion transporters (OATS) in drug therapy. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 29–104. [Google Scholar]
- Hilgendorf, C.; Ahlin, G.; Seithel, A.; Artursson, P.; Ungell, A.L.; Karlsson, J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab. Dispos. Biol. Fate Chem. 2007, 35, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Bush, K.T.; Liu, H.C.; Zhu, C.; Abagyan, R.; Nigam, S.K. Shared ligands between organic anion transporters (OAT1 and OAT6) and odorant receptors. Drug Metab. Dispos. Biol. Fate Chem. 2015, 43, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Ueo, H.; Motohashi, H.; Katsura, T.; Inui, K. Human organic anion transporter hOAT3 is a potent transporter of cephalosporin antibiotics, in comparison with hOAT1. Biochem. Pharmacol. 2005, 70, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Maeda, K.; Kamiyama, E.; Sugiyama, D.; Kondo, T.; Shiroyanagi, Y.; Nakazawa, H.; Okano, T.; Adachi, M.; Schuetz, J.D.; et al. Multiple human isoforms of drug transporters contribute to the hepatic and renal transport of olmesartan, a selective antagonist of the angiotensinII AT1-receptor. Drug Metab. Dispos. 2007, 35, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Hasannejad, H.; Takeda, M.; Taki, K.; Shin, H.J.; Babu, E.; Jutabha, P.; Khamdang, S.; Aleboyeh, M.; Onozato, M.L.; Tojo, A.; et al. Interactions of human organic anion transporters with diuretics. J. Pharmacol. Exp. Ther. 2004, 308, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Cihlar, T.; Lin, D.C.; Pritchard, J.B.; Fuller, M.D.; Mendel, D.B.; Sweet, D.H. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol. Pharmacol. 1999, 56, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Vapurcuyan, A.; Shahidullah, M.; Aleksunes, L.M.; Pelis, R.M. Expression of organic anion transporter 2 in the human kidney and its potential role in the tubular secretion of guanine-containing antiviral drugs. Drug Metab. Dispos. 2012, 40, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Bleasby, K.; Hall, L.A.; Perry, J.L.; Mohrenweiser, H.W.; Pritchard, J.B. Functional consequences of single nucleotide polymorphisms in the human organic anion transporter hOAT1 (SLC22A6). J. Pharmacol. Exp. Ther. 2005, 314, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Babu, E.; Narikawa, S.; Endou, H. Interaction of human organic anion transporters with various cephalosporin antibiotics. Eur. J. Pharmacol. 2002, 438, 137–142. [Google Scholar] [CrossRef]
- Burckhardt, B.C.; Brai, S.; Wallis, S.; Krick, W.; Wolff, N.A.; Burckhardt, G. Transport of cimetidine by flounder and human renal organic anion transporter 1. Am. J. Physiol. Renal Physiol. 2003, 284, F503–F509. [Google Scholar] [CrossRef] [PubMed]
- Uwai, Y.; Taniguchi, R.; Motohashi, H.; Saito, H.; Okuda, M.; Inui, K. Methotrexate-loxoprofen interaction: Involvement of human organic anion transporters hOAT1 and hOAT3. Drug Metab. Pharmacokinet. 2004, 19, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Cihlar, T.; Ho, E.S. Fluorescence-based assay for the interaction of small molecules with the human renal organic anion transporter 1. Anal. Biochem. 2000, 283, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.Y.; Bleasby, K.; Yabut, J.; Cai, X.; Chan, G.H.; Hafey, M.J.; Xu, S.; Bergman, A.J.; Braun, M.P.; Dean, D.C.; et al. Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by human organic anion transporter 3, organic anion transporting polypeptide 4C1, and multidrug resistance P-glycoprotein. J. Pharmacol. Exp. Ther. 2007, 321, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Khamdang, S.; Takeda, M.; Noshiro, R.; Narikawa, S.; Enomoto, A.; Anzai, N.; Piyachaturawat, P.; Endou, H. Interactions of human organic anion transporters and human organic cation transporters with nonsteroidal anti-inflammatory drugs. J. Pharmacol. Exp. Ther. 2002, 303, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Mulato, A.S.; Ho, E.S.; Cihlar, T. Nonsteroidal anti-inflammatory drugs efficiently reduce the transport and cytotoxicity of adefovir mediated by the human renal organic anion transporter 1. J. Pharmacol. Exp. Ther. 2000, 295, 10–15. [Google Scholar] [PubMed]
- Morrissey, K.M.; Stocker, S.L.; Wittwer, M.B.; Xu, L.; Giacomini, K.M. Renal transporters in drug development. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 503–529. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Anderson, G.D.; Wang, J. Drug-drug interactions involving membrane transporters in the human kidney. Expert Opin. Drug Metab. Toxicol. 2006, 2, 505–532. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wu, R.R.; van Poelje, P.D.; Erion, M.D. Isolation of a family of organic anion transporters from human liver and kidney. Biochem. Biophys. Res. Commun. 2001, 283, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Cha, S.H.; Tsuda, M.; Apiwattanakul, N.; Nakajima, N.; Kanai, Y.; Endou, H. Identification of multispecific organic anion transporter 2 expressed predominantly in the liver. FEBS Lett. 1998, 429, 179–182. [Google Scholar] [CrossRef]
- Marada, V.V.; Florl, S.; Kuhne, A.; Muller, J.; Burckhardt, G.; Hagos, Y. Interaction of human organic anion transporter 2 (OAT2) and sodium taurocholate cotransporting polypeptide (NTCP) with antineoplastic drugs. Pharmacol. Res. 2015, 91, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Ohshiro, N.; Sakai, R.; Ohbayashi, M.; Kohyama, N.; Yamamoto, T. Transport mechanism and substrate specificity of human organic anion transporter 2 (hOAT2 [SLC22A7]). J. Pharm. Pharmacol. 2005, 57, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, A.; Tatsumi, S.; Takeda, R.; Naka, A.; Matsuoka, H.; Hashimoto, Y.; Hatta, K.; Maeda, K.; Kamoshida, S. High expression of organic anion transporter 2 and organic cation transporter 2 is an independent predictor of good outcomes in patients with metastatic colorectal cancer treated with FOLFOX--based chemotherapy. Am. J. Cancer Res. 2014, 4, 528–536. [Google Scholar] [PubMed]
- Tahara, H.; Kusuhara, H.; Endou, H.; Koepsell, H.; Imaoka, T.; Fuse, E.; Sugiyama, Y. A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J. Pharmacol. Exp. Ther. 2005, 315, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Sakai, R.; Ohshiro, N.; Ohbayashi, M.; Kohyama, N.; Yamamoto, T. Possible involvement of organic anion transporter 2 on the interaction of theophylline with erythromycin in the human liver. Drug Metab. Dispos. 2005, 33, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Babu, E.; Takeda, M.; Narikawa, S.; Kobayashi, Y.; Yamamoto, T.; Cha, S.H.; Sekine, T.; Sakthisekaran, D.; Endou, H. Human organic anion transporters mediate the transport of tetracycline. Jpn. J. Pharmacol. 2002, 88, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, G. Drug transport by organic anion transporters (OATs). Pharmacol. Ther. 2012, 136, 106–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, Y.H.; Putluru, S.P.; Matta, M.K.; Kole, P.; Mandlekar, S.; Furlong, M.T.; Liu, T.; Iyer, R.A.; Marathe, P.; et al. Diclofenac and its ACYL glucuronide: Determination of in vivo exposure in human subjects and characterization as human drug transporter substrates in vitro. Drug Metab. Dispos. 2016, 44, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Nagle, M.A.; Truong, D.M.; Dnyanmote, A.V.; Ahn, S.Y.; Eraly, S.A.; Wu, W.; Nigam, S.K. Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J. Biol. Chem. 2011, 286, 243–251. [Google Scholar] [CrossRef] [PubMed]
- VanWert, A.L.; Sweet, D.H. Impaired clearance of methotrexate in organic anion transporter 3 (SLC22A8) knockout mice: A gender specific impact of reduced folates. Pharm. Res. 2008, 25, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Vanwert, A.L.; Bailey, R.M.; Sweet, D.H. Organic anion transporter 3 (Oat3/Slc22a8) knockout mice exhibit altered clearance and distribution of penicillin G. Am. J. Physiol. Ren. Physiol. 2007, 293, F1332–F1341. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Tian, Y.; Fujita, T.; Ikeda, Y.; Kumagai, Y.; Kondo, T.; Tanabe, K.; Nakayama, H.; Horita, S.; Kusuhara, H.; et al. Inhibitory effects of p-aminohippurate and probenecid on the renal clearance of adefovir and benzylpenicillin as probe drugs for organic anion transporter (OAT) 1 and OAT3 in humans. Eur. J. Pharm. Sci. 2014, 59, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Windass, A.S.; Lowes, S.; Wang, Y.; Brown, C.D. The contribution of organic anion transporters OAT1 and OAT3 to the renal uptake of rosuvastatin. J. Pharmacol. Exp. Ther. 2007, 322, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Khamdang, S.; Takeda, M.; Shimoda, M.; Noshiro, R.; Narikawa, S.; Huang, X.L.; Enomoto, A.; Piyachaturawat, P.; Endou, H. Interactions of human- and rat-organic anion transporters with pravastatin and cimetidine. J. Pharmacol. Sci. 2004, 94, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Khamdang, S.; Narikawa, S.; Kimura, H.; Hosoyamada, M.; Cha, S.H.; Sekine, T.; Endou, H. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J. Pharmacol. Exp. Ther. 2002, 302, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Shimada, H.; Irino, Y.; Take, M.; Egashira, S. Inhibition of methotrexate uptake via organic anion transporters OAT1 and OAT3 by glucuronides of nonsteroidal anti-inflammatory drugs. Biol. Pharm. Bull. 2017, 40, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Honjo, H.; Uwai, Y.; Aoki, Y.; Iwamoto, K. Stereoselective inhibitory effect of flurbiprofen, ibuprofen and naproxen on human organic anion transporters hOAT1 and hOAT3. Biopharm. Drug Dispos. 2011, 32, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C.; Liu, Q.; Meng, Q.; Cang, J.; Sun, H.; Peng, J.; Ma, X.; Huo, X.; Liu, K. Aspirin and probenecid inhibit organic anion transporter 3-mediated renal uptake of cilostazol and probenecid induces metabolism of cilostazol in the rat. Drug Metab. Dispos. 2014, 42, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Vanwert, A.L.; Srimaroeng, C.; Sweet, D.H. Organic anion transporter 3 (Oat3/Slc22a8) interacts with carboxyfluoroquinolones, and deletion increases systemic exposure to ciprofloxacin. Mol. Pharmacol. 2008, 74, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Reese, M.J.; Bowers, G.D.; Humphreys, J.E.; Gould, E.P.; Ford, S.L.; Webster, L.O.; Polli, J.W. Drug interaction profile of the HIV integrase inhibitor cabotegravir: Assessment from in vitro studies and a clinical investigation with midazolam. Xenobiotica 2016, 46, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Nagle, M.A.; Kouznetsova, V.L.; Tsigelny, I.F.; Nigam, S.K. Untargeted metabolomics identifies enterobiome metabolites and putative uremic toxins as substrates of organic anion transporter 1 (OAT1). J. Proteome Res. 2011, 10, 2842–2851. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.T.; Wu, W.; Lun, C.; Nigam, S.K. The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut-liver-kidney axis. J. Biol. Chem. 2017, 292, 15789–15803. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Sekine, T.; Kusuhara, H.; Yu, E.; Kim, J.Y.; Kim, D.K.; Sugiyama, Y.; Kanai, Y.; Endou, H. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J. Biol. Chem. 2000, 275, 4507–4512. [Google Scholar] [CrossRef] [PubMed]
- Hagos, Y.; Bahn, A.; Vormfelde, S.V.; Brockmoller, J.; Burckhardt, G. Torasemide transport by organic anion transporters contributes to hyperuricemia. J. Am. Soc. Nephrol. 2007, 18, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Anzai, N.; Jutabha, P.; Enomoto, A.; Yokoyama, H.; Nonoguchi, H.; Hirata, T.; Shiraya, K.; He, X.; Cha, S.H.; Takeda, M.; et al. Functional characterization of rat organic anion transporter 5 (SLC22A19) at the apical membrane of renal proximal tubules. J. Pharmacol. Exp. Ther. 2005, 315, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Malnic, B.; Gonzalez-Kristeller, D.C.; Gutiyama, L.M. Odorant receptors. In The Neurobiology of Olfaction; Menini, A., Ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Zimmerman, E.I.; Gibson, A.A.; Hu, S.; Vasilyeva, A.; Orwick, S.J.; Du, G.; Mascara, G.P.; Ong, S.S.; Chen, T.; Vogel, P.; et al. Multikinase inhibitors induce cutaneous toxicity through OAT6-mediated uptake and MAP3K7-driven cell death. Cancer Res. 2016, 76, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Anzai, N.; Enomoto, A.; He, X.; Kim, D.K.; Endou, H.; Kanai, Y. Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology 2007, 45, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Emami Riedmaier, A.; Burk, O.; van Eijck, B.A.; Schaeffeler, E.; Klein, K.; Fehr, S.; Biskup, S.; Muller, S.; Winter, S.; Zanger, U.M.; et al. Variability in hepatic expression of organic anion transporter 7/SLC22A9, a novel pravastatin uptake transporter: Impact of genetic and regulatory factors. Pharmacogenomics J. 2016, 16, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Bahn, A.; Hagos, Y.; Reuter, S.; Balen, D.; Brzica, H.; Krick, W.; Burckhardt, B.C.; Sabolic, I.; Burckhardt, G. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J. Biol. Chem. 2008, 283, 16332–16341. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Takeda, M.; Enomoto, A.; Fujimura, M.; Miyazaki, H.; Anzai, N.; Endou, H. Interactions of urate transporter URAT1 in human kidney with uricosuric drugs. Nephrology 2011, 16, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shi, Y.; Zhuang, S.; Liu, N. Recent advances on uric acid transporters. Oncotarget 2017, 8, 100852–100862. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, V.; Richard, E.L.; Wu, W.; Nievergelt, C.M.; Lipkowitz, M.S.; Jeff, J.; Maihofer, A.X.; Nigam, S.K. Analysis of ABCG2 and other urate transporters in uric acid homeostasis in chronic kidney disease: Potential role of remote sensing and signaling. Clin. Kidney J. 2016, 9, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Zair, Z.M.; Eloranta, J.J.; Stieger, B.; Kullak-Ublick, G.A. Pharmacogenetics of OATP (SLC21/SLCO), OAT and OCT (SLC22) and PEPT (SLC15) transporters in the intestine, liver and kidney. Pharmacogenomics 2008, 9, 597–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, T.; Brown, C.; Carlson, E.J.; Taylor, T.; de la Cruz, M.; Johns, S.J.; Stryke, D.; Kawamoto, M.; Fujita, K.; Castro, R.; et al. Functional analysis of polymorphisms in the organic anion transporter, SLC22A6 (OAT1). Pharmacogenetics Genom. 2005, 15, 201–209. [Google Scholar] [CrossRef]
- Shen, H.; Lai, Y.; Rodrigues, A.D. Organic anion transporter 2: An enigmatic human solute carrier. Drug Metab. Dispos. Biol. Fate Chem. 2017, 45, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Cropp, C.D.; Komori, T.; Shima, J.E.; Urban, T.J.; Yee, S.W.; More, S.S.; Giacomini, K.M. Organic anion transporter 2 (SLC22A7) is a facilitative transporter of CGMP. Mol. Pharmacol. 2008, 73, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Erdman, A.R.; Mangravite, L.M.; Urban, T.J.; Lagpacan, L.L.; Castro, R.A.; de la Cruz, M.; Chan, W.; Huang, C.C.; Johns, S.J.; Kawamoto, M.; et al. The human organic anion transporter 3 (OAT3; SLC22A8): Genetic variation and functional genomics. Am. J. Physiol. Ren. Physiol. 2006, 290, F905–F912. [Google Scholar] [CrossRef] [PubMed]
- Yee, S.W.; Nguyen, A.N.; Brown, C.; Savic, R.M.; Zhang, Y.; Castro, R.A.; Cropp, C.D.; Choi, J.H.; Singh, D.; Tahara, H.; et al. Reduced renal clearance of cefotaxime in Asians with a low-frequency polymorphism of OAT3 (SLC22A8). J. Pharm. Sci. 2013, 102, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Ivanyuk, A.; Livio, F.; Biollaz, J.; Buclin, T. Renal drug transporters and drug interactions. Clin. Pharmacokinet. 2017, 56, 825–892. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhu, L.; Cui, P.H.; Church, W.B.; Murray, M. Functional characterization of nonsynonymous single nucleotide polymorphisms in the human organic anion transporter 4 (hOAT4). Br. J. Pharmacol. 2010, 159, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Vormfelde, S.V.; Schirmer, M.; Hagos, Y.; Toliat, M.R.; Engelhardt, S.; Meineke, I.; Burckhardt, G.; Nurnberg, P.; Brockmoller, J. Torsemide renal clearance and genetic variation in luminal and basolateral organic anion transporters. Br. J. Clin. Pharmacol. 2006, 62, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Hosoyamada, M.; Hisatome, I.; Enomoto, A.; Hikita, M.; Endou, H.; Hosoya, T. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J. Am. Soc. Nephrol. JASN 2004, 15, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, K.; Terada, T.; Motohashi, H.; Asaka, J.; Aoki, M.; Katsura, T.; Kamba, T.; Ogawa, O.; Inui, K. Analysis of regulatory polymorphisms in organic ion transporter genes (SLC22A) in the kidney. J. Hum. Genet. 2008, 53, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Lee, C.H.; Lee, S.S.; Song, I.S.; Shin, J.G. Identification of genetic polymorphisms of human oat1 and OAT2 genes and their relationship to hOAT2 expression in human liver. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Visscher, H.; Rassekh, S.R.; Sandor, G.S.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; van der Pal, H.J.; Rogers, P.C.; Rieder, M.J.; Carleton, B.C.; et al. Genetic variants in SLC22A17 and SLC22A7 are associated with anthracycline-induced cardiotoxicity in children. Pharmacogenomics 2015, 16, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Pellicer, M.; Garcia-Gonzalez, X.; Garcia, M.I.; Robles, L.; Gravalos, C.; Garcia-Alfonso, P.; Pachon, V.; Longo, F.; Martinez, V.; Blanco, C.; et al. Identification of new SNPs associated with severe toxicity to capecitabine. Pharmacol. Res. 2017, 120, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shu, Y. Role of solute carriers in response to anticancer drugs. Mol. Cell. Ther. 2014, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- El-Gebali, S.; Bentz, S.; Hediger, M.A.; Anderle, P. Solute carriers (SLCs) in cancer. Mol. Aspects Med. 2013, 34, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Angelini, S.; Pantaleo, M.A.; Ravegnini, G.; Zenesini, C.; Cavrini, G.; Nannini, M.; Fumagalli, E.; Palassini, E.; Saponara, M.; Di Battista, M.; et al. Polymorphisms in OCTN1 and OCTN2 transporters genes are associated with prolonged time to progression in unresectable gastrointestinal stromal tumours treated with imatinib therapy. Pharmacol. Res. 2013, 68, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kerb, R.; Brinkmann, U.; Chatskaia, N.; Gorbunov, D.; Gorboulev, V.; Mornhinweg, E.; Keil, A.; Eichelbaum, M.; Koepsell, H. Identification of genetic variations of the human organic cation transporter hOCT1 and their functional consequences. Pharmacogenetics 2002, 12, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Herraez, E.; Lozano, E.; Macias, R.I.; Vaquero, J.; Bujanda, L.; Banales, J.M.; Marin, J.J.; Briz, O. Expression of SLC22A1 variants may affect the response of hepatocellular carcinoma and cholangiocarcinoma to sorafenib. Hepatology 2013, 58, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Leabman, M.K.; Feng, B.; Mangravite, L.M.; Huang, C.C.; Stryke, D.; Kawamoto, M.; Johns, S.J.; DeYoung, J.; Carlson, E.; et al. Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1. Proc. Natl. Acad. Sci. USA 2003, 100, 5902–5907. [Google Scholar] [CrossRef] [PubMed]
- Lozano, E.; Herraez, E.; Briz, O.; Robledo, V.S.; Hernandez-Iglesias, J.; Gonzalez-Hernandez, A.; Marin, J.J. Role of the plasma membrane transporter of organic cations OCT1 and its genetic variants in modern liver pharmacology. Biomed. Res. Int. 2013, 2013, 692071. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, A.; Polillo, M.; Capecchi, M.; Cervetti, G.; Barate, C.; Angelini, S.; Guerrini, F.; Fontanelli, G.; Arici, R.; Ciabatti, E.; et al. The c.480C>G polymorphism of hOCT1 influences imatinib clearance in patients affected by chronic myeloid leukemia. Pharmacogenomics J. 2014, 14, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, S.; Bucelli, C.; Arrigoni, E.; Barate, C.; Grassi, S.; Ricci, F.; Guerrini, F.; Ciabatti, E.; Fava, C.; D’Avolio, A.; et al. The hOCT1 and ABCB1 polymorphisms do not influence the pharmacodynamics of nilotinib in chronic myeloid leukemia. Oncotarget 2017, 8, 88021–88033. [Google Scholar] [CrossRef] [PubMed]
- Arimany-Nardi, C.; Montraveta, A.; Lee-Verges, E.; Puente, X.S.; Koepsell, H.; Campo, E.; Colomer, D.; Pastor-Anglada, M. Human organic cation transporter 1 (hOCT1) as a mediator of bendamustine uptake and cytotoxicity in chronic lymphocytic leukemia (CLL) cells. Pharmacogenomics J. 2015, 15, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, Y.; Yu, Q.; Wang, H.; Tan, F.; Zhu, Q.; Yuan, L.; Jiang, H.; Zeng, S.; Yu, L. Response to comment on “epigenetic activation of the drug transporter OCT2 sensitizes renal cell carcinoma to oxaliplatin”. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, M.; Schirripa, M.; Cao, S.; Zhang, W.; Yang, D.; Dadduzio, V.; Salvatore, L.; Borelli, B.; Pietrantonio, F.; Ning, Y.; et al. Potential role of polymorphisms in the transporter genes ent1 and MATE1/OCT2 in predicting TAS-102 efficacy and toxicity in patients with refractory metastatic colorectal cancer. Eur. J. Cancer 2017, 86, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hong, C.; Chen, E.C.; Yee, S.W.; Xu, L.; Almof, E.U.; Wen, C.; Fujii, K.; Johns, S.J.; Stryke, D.; et al. Genetic and epigenetic regulation of the organic cation transporter 3, SLC22A3. Pharmacogenomics J. 2013, 13, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Okada, Y.; Jang, S.G.; Ku, J.L.; Park, J.G.; Kamatani, Y.; Hosono, N.; Tsunoda, T.; Kumar, V.; Tanikawa, C.; et al. Common variant in 6q26-q27 is associated with distal colon cancer in an Asian population. Gut 2011, 60, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Grisanzio, C.; Werner, L.; Takeda, D.; Awoyemi, B.C.; Pomerantz, M.M.; Yamada, H.; Sooriakumaran, P.; Robinson, B.D.; Leung, R.; Schinzel, A.C.; et al. Genetic and functional analyses implicate the NUDT11, HNF1B, and SLC22A3 genes in prostate cancer pathogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 11252–11257. [Google Scholar] [CrossRef] [PubMed]
- Angelini, S.; Soverini, S.; Ravegnini, G.; Barnett, M.; Turrini, E.; Thornquist, M.; Pane, F.; Hughes, T.P.; White, D.L.; Radich, J.; et al. Association between imatinib transporters and metabolizing enzymes genotype and response in newly diagnosed chronic myeloid leukemia patients receiving imatinib therapy. Haematologica 2013, 98, 193–200. [Google Scholar] [CrossRef] [PubMed]
- VanWert, A.L.; Gionfriddo, M.R.; Sweet, D.H. Organic anion transporters: Discovery, pharmacology, regulation and roles in pathophysiology. Biopharm. Drug Dispos. 2010, 31, 1–71. [Google Scholar] [CrossRef] [PubMed]
- Srimaroeng, C.; Perry, J.L.; Pritchard, J.B. Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica 2008, 38, 889–935. [Google Scholar] [CrossRef] [PubMed]
- Pelis, R.M.; Wright, S.H. SLC22, SLC44, and SLC47 transporters—Organic anion and cation transporters: Molecular and cellular properties. Curr. Top. Membr. 2014, 73, 233–261. [Google Scholar] [PubMed]
- Schaeffeler, E.; Hellerbrand, C.; Nies, A.T.; Winter, S.; Kruck, S.; Hofmann, U.; van der Kuip, H.; Zanger, U.M.; Koepsell, H.; Schwab, M. DNA methylation is associated with downregulation of the organic cation transporter OCT1 (SLC22A1) in human hepatocellular carcinoma. Genome Med. 2011, 3, 82. [Google Scholar] [CrossRef] [PubMed]
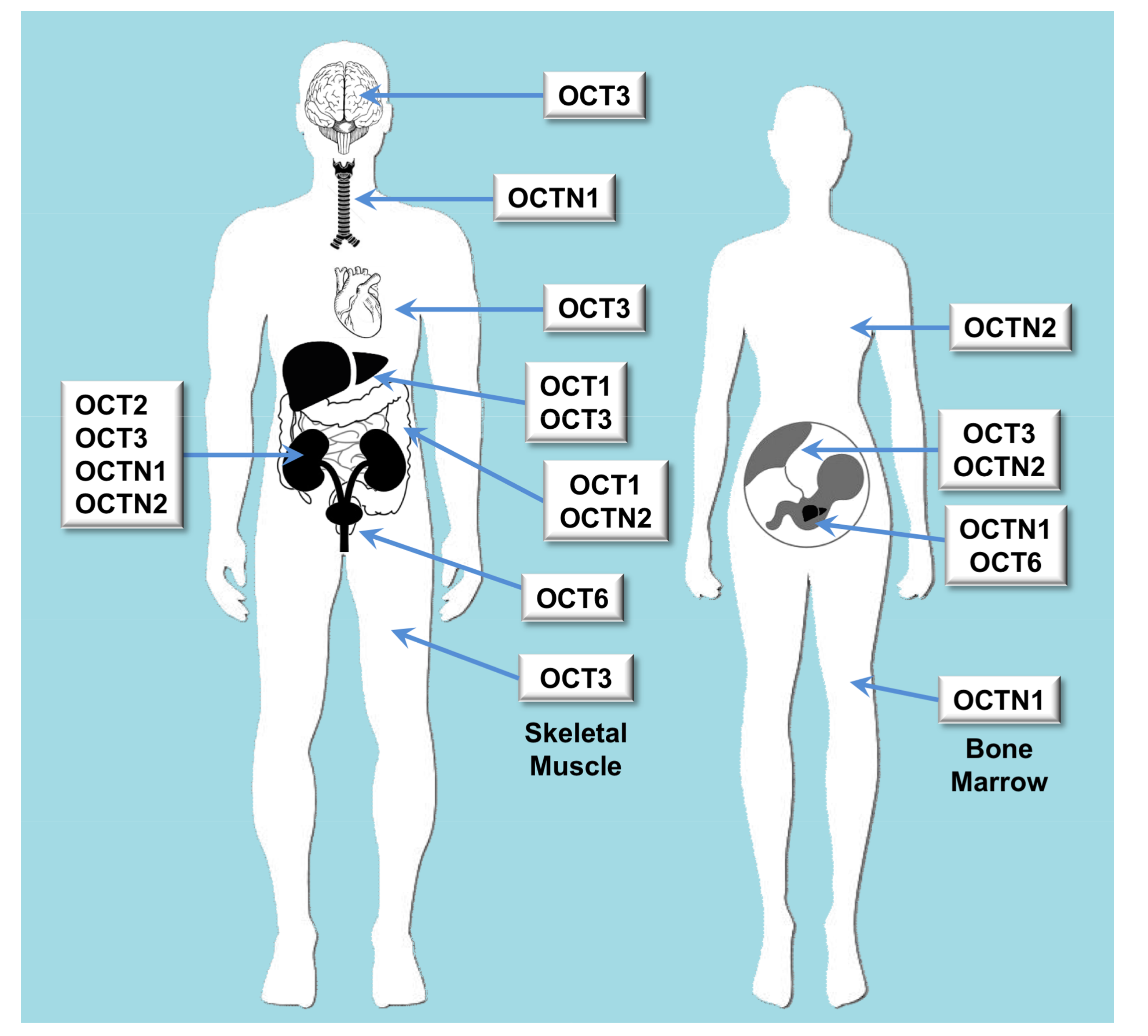
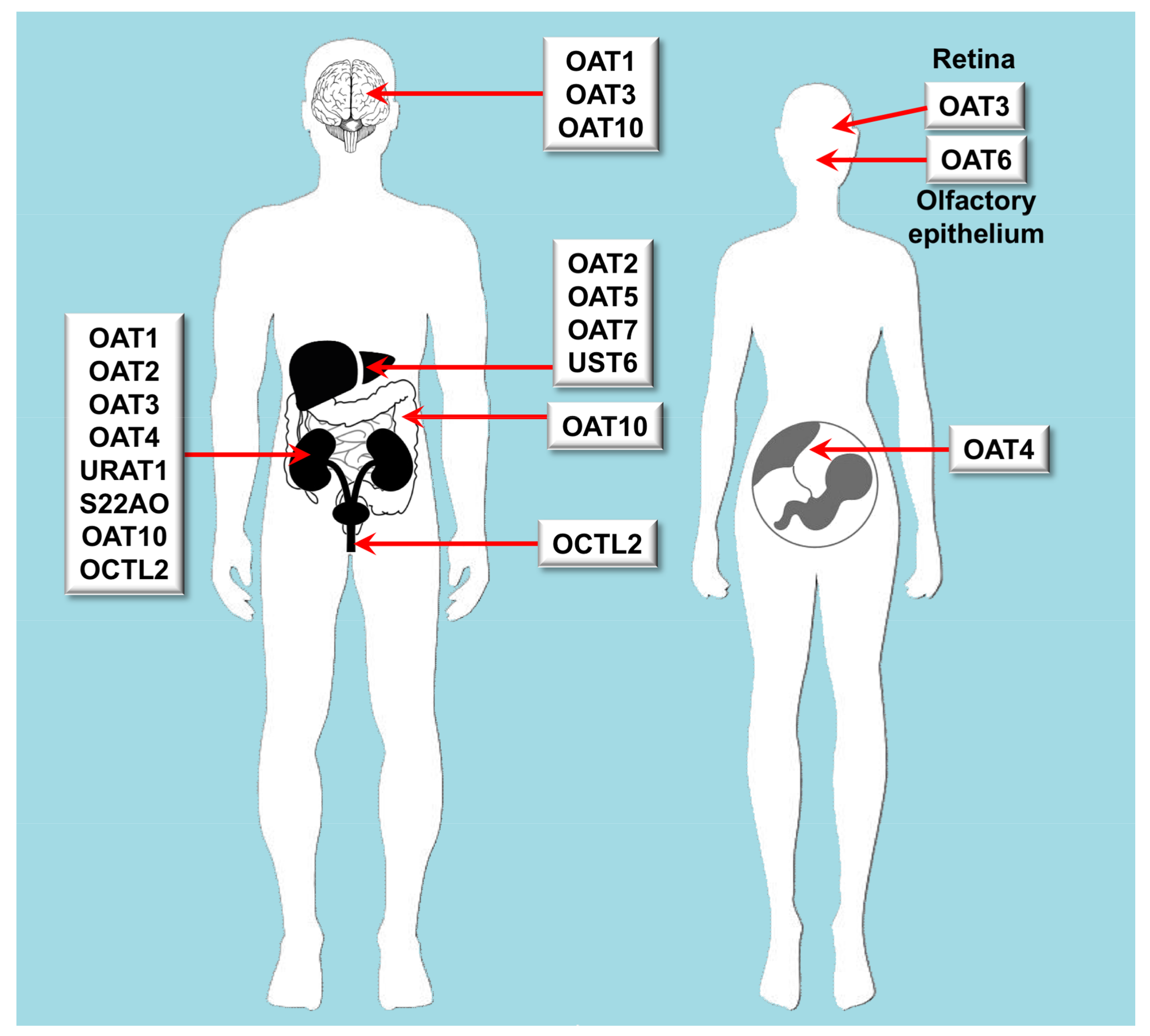
| Major Clade | Subclade | Gene | Protein | Tissue Distribution | Endogenous Substrates | Xenobiotic Substrates/Inhibitors |
|---|---|---|---|---|---|---|
| OCT | Oct | SLC22A1 | OCT1 | Liver and to a less extent also in brain, heart, immune cells, intestine, kidney and lung | Biogenic monoamines, biogenic polyamines, catecholamines, ethanolamines, neuromodulators, vitamins, prostaglandins | Cimetidine, ciprofloxacin, famotidine, lamivudine, lamotrigine, metformin, O-desmethyltramadol, ondansetron, quinidine, ranitidine, sumatriptan, tropisetron, zalcitabine |
| OCT | Oct | SLC22A2 | OCT2 | Kidney, brain, lung | Acetylcholine, dopamine, serotonin | Amantadine, anti-malarials, atenolol, cimetidine, entecavir, fluoroquinolones, metformin, procainamide, quinidine, sulpiride |
| OCT | Oct | SLC22A3 | OCT3 | Widely expressed | Norepinephrine | Metformin, wogonin |
| OCT | Octn | SLC22A4 | OCTN1 | Kidney, bone marrow, trachea, fetal liver (at lower levels in many tissues) | Acetylcholine, carnitine | Ergothioneine, entecavir, fluoroquinolones, gabapentin, sulpiride |
| OCT | Octn | SLC22A5 | OCTN2 | Intestine, kidney, placenta, mammary gland | Carnitine | Entecavir, fluoroquinolones, sulpiride |
| Major Clade | Subclade | Gene | Protein | Tissue Distribution | Endogenous Substrates | Xenobiotic Substrates/Inhibitors |
|---|---|---|---|---|---|---|
| OAT | Oat | SLC22A6 | OAT1 | Kidney, brain | cGMP, C5- and C6-mono and dicarboxylates, prostaglandins, urate | Acyclovir, adefovir, captopril, cidofovir, cimetidine, furosemide, ganciclovir, methotrexate, olmesartan, tenofovir, ranitidine |
| OAT | Oat | SLC22A7 | OAT2 | Liver, kidney | Conjugated steroid hormones, cGMP, nucleobases, nucleosides, nucleotides, prostaglandins | Acyclovir, cimetidine, diclofenac, entecavir, erythromycin, ganciclovir, irinotecan, methotrexate, penciclovir, ranitidine, tetracycline, 5-FU |
| OAT | Oat | SLC22A8 | OAT3 | Kidney, retina, brain | Acidic neurotransmitter metabolites, bile acids, C5-dicarboxylates, cAMP, cGMP, conjugated steroid hormones, prostaglandins, urate | Cimetidine, benzylpenicillin, bumetanide, cephalosporins, ciprofloxacin, furosemide, methotrexate, NSAIDs, pravastatin, probenecid, rosuvastatin, zidovudine |
| OAT | Oat | SLC22A9 | OAT7 | Liver | Conjugated steroid hormones, monocarboxylates, short chain fatty acids | Pravastatin |
| OAT | Oat | SLC22A10 | OAT5 | Liver | Unknown | |
| OAT | Oat | SLC22A11 | OAT4 | Kidney, placenta, adrenal gland | Bile acids, C5-dicarboxylates, conjugated steroid hormones prostaglandins, urate | Bumetanide, hydrochlorothiazide, ketoprofen, methotrexate, salicylate, tetracycline, torsemide, zidovudine |
| OAT | Oat | SLC22A12 | URAT1 | Kidney | Urate | |
| OAT | Oat | SLC22A20 | OAT6 | Nasal epithelial cells | Conjugated steroid hormones, C5-dicarboxylates, short chain fatty acids | Sorafenib |
| OAT | Oat | SLC22A24 | S22AO | Kidney | Unknown | |
| OAT | Oat | SLC22A25 | UST6 | Liver | Unknown | |
| OAT | Oat-like | SLC22A13 | OAT10 | Kidney, brain, colon | C4-dicarboxylates, glutathione, nicotinate, urate | |
| OAT | Oat-like | SLC22A14 | OCTL2 | Testis, kidney | Unknown |
| Transporter (Gene) | Genetic Polymorphism | Amino Acid Change | Effect on Expression | Activity | Effect on Drug Disposition |
|---|---|---|---|---|---|
| OCT1 (SLC22A1) | c.181C>T | R61C | = | Reduced | Reduced intestinal uptake of metformin |
| Higher plasma concentrations of O-desmethyltramadol, sumatriptan, morphine, tropisetron, ondansetron | |||||
| c.252T>C | C88R | = | Reduced | Increased systemic exposure to sumatriptan | |
| c.1201G>A | G401S | = | Reduced | Reduced intestinal uptake of metformin | |
| Higher plasma concentrations of O-desmethyltramadol, sumatriptan, morphine, tropisetron, ondansetron | |||||
| c.1222A>G | M408V | = | Similar | Gastrointestinal side-effects of metformin | |
| Alteration of lamotrigine serum concentration | |||||
| c.1258_1260delATG | M420del | = | Reduced | Reduced intestinal uptake of metformin | |
| Higher plasma concentrations of O-desmethyltramadol, morphine, tropisetron, ondansetron | |||||
| c.1386A>C (rs622342) | - | ↓Expected | Reduced | Low effect of metformin, levodopa | |
| c.1393G>C | G465R | = | Reduced | Reduced intestinal uptake of metformin | |
| Increased systemic exposure to sumatriptan | |||||
| OCT*5 | G465R + M420del | = | Reduced | Higher plasma concentrations of O-desmethyltramadol, morphine, tropisetron and ondansetron | |
| OCT1*6 | C88R + M420del | = | Reduced | Higher plasma concentrations of O-desmethyltramadol, morphine, tropisetron and ondansetron | |
| rs36056065 (c.1276 + 1ins GTAAGTTG) | - | Aberrant splicing | Gastrointestinal side-effects of metformin | ||
| OCT2 (SLC22A2) | c.495G>A | M165I | Reduced | ||
| c.596C>G | T199I | Reduced | Reduced renal clearance of metformin | ||
| c.602C>T | T201M | Reduced | Reduced renal clearance of metformin | ||
| c.808G>T | A270S | Reduced | Reduced renal clearance of metformin | ||
| c.1198C>T | R400C | Reduced | |||
| c.1294A>C | K432Q | Similar | |||
| OCT3 (SLC22A3) | A116S | = | Reduced | ||
| T400I | = | Reduced | |||
| A439V | = | Reduced | |||
| OCTN1 (SLC22A4) | c.188G>A | R63H | ↓Membrane | Reduced | |
| c.248G>C | R83P | ↓Membrane | Reduced | ||
| c.400C>A | L134M | Similar | |||
| c.475G>A | V159M | Similar | |||
| c.494A>G | D165G | ↓Membrane | Lost | ||
| c.615G>A | M205I | ↓Membrane | Reduced | ||
| c.774G>C | M258I | Similar | |||
| c.844C>T | K282X | Lost | |||
| c.917C>T | T306I | = | Similar | ||
| c.1031T>A | M344K | Similar | |||
| c.1445G>A | G482D | = | Reduced | ||
| c.1460T>C | M487T | Similar | |||
| c.1499T>A | I500N | = | Reduced | ||
| c.1507G>A | L503F | = | Reduced | Reduced tubular secretion of gabapentin | |
| c.1531G>A | = | Reduced | |||
| OCTN2 (SLC22A5) | c.51C>G | F17L | Reduced | ||
| c.325G>C | E109Q | Similar | |||
| c.364G>T | D122Y | ↓Membrane | Reduced | ||
| c.430C>T | L144F | Similar | |||
| c.523G>A | V175M | Similar | |||
| c.573G>T | K191N | Similar | |||
| c.614C>T | A214V | Similar | |||
| c.791C>T | T264M | Reduced | |||
| c.904A>G | K302E | ↓Membrane | Reduced | ||
| c.934A>G | I312V | Similar | |||
| c.949G>A | E317K | Higher | |||
| M352R | = | Lost | |||
| c.1345T>G | Y449D | Reduced | |||
| P478L | = | Lost | |||
| c.1341TG>T | V481F | Reduced | |||
| V481I | Similar | ||||
| c.1463G>A | R488H | Similar | |||
| c.1522T>C | F508L | Similar | |||
| c.1588A>G | M530V | Similar | |||
| c.1645C>T | P549S | Similar |
| Transporter (Gene) | Genetic Polymorphism | Amino Acid Change | Effect on Expression | Activity | Effect on Drug Disposition |
|---|---|---|---|---|---|
| OAT1 (SLC22A6) | c.149G>A/C | R50H | = | Higher affinity for adefovir, cidofovir and tenofovir | No effect on renal clearance |
| c.1361G>A | R454Q | = | Loss of activity | No effect on renal clearance | |
| OAT2 (SLC22A7) | c.492_493insTCCCAG | E131_W132insSQ | = | Lost | Not investigated |
| c.1592 + 206A>G | – | ↑ | Higher cell uptake | Anthracycline-induced cardiotoxicity and severe toxicity to capecitabine | |
| OAT3 (SLC22A8) | c.445C>A | R149S | = | Lost | Not investigated |
| c.715C>T | N239X | = | Lost | Not investigated | |
| c.779T>G | I260R | = | Lost | Not investigated | |
| c.829C>T | R277W | = | Reduced | Not investigated | |
| c.913A>T | I305F | = | Reduced | Lower renal clearance of cefotaxime | |
| OAT4 (SLC22A11) | c.86T>C | L29P | = | Lower renal clearance of torsemide | |
| c.142C>T | R48Y | = | Reduced | ||
| c.464T>G | V155G | = | Reduced | ||
| c.1175C>T | T392I | = | Reduced | ||
| OAT7 (SLC22A9) | c.268C>T | R90C | = | Reduced | Reduced hepatic uptake of pravastatin |
| c.1298C>G | T433R | = | Reduced | ||
| c.1298C>T | T433M | = | Reduced | ||
| c.1437A>G | I479M | = | Reduced | ||
| URAT1 (SLC22A12) | c.269G>A | R90H | = | Lost | Lower renal clearance of drugs substrates of the transporter |
| c.412G>A | V138M | = | Lost | ||
| c.490G>A | G164S | = | Reduced | ||
| c.650C>T | T217M | = | Lost | ||
| c.774G>A | W258X | = | Lost | ||
| c.889C>T | Q297X | = | Lost | ||
| c.894G>T | E298D | = | Lost | ||
| c.1145A>T | Q382L | = | Reduced | ||
| c.1289T>C | M430T | = | Reduced | ||
| c.1639_1643del | V547Kfs | = | Lost | ||
| IVS2 + 1G>A | – | Aberrant splicing | Lost |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano, E.; Briz, O.; Macias, R.I.R.; Serrano, M.A.; Marin, J.J.G.; Herraez, E. Genetic Heterogeneity of SLC22 Family of Transporters in Drug Disposition. J. Pers. Med. 2018, 8, 14. https://doi.org/10.3390/jpm8020014
Lozano E, Briz O, Macias RIR, Serrano MA, Marin JJG, Herraez E. Genetic Heterogeneity of SLC22 Family of Transporters in Drug Disposition. Journal of Personalized Medicine. 2018; 8(2):14. https://doi.org/10.3390/jpm8020014
Chicago/Turabian StyleLozano, Elisa, Oscar Briz, Rocio I. R. Macias, Maria A. Serrano, Jose J. G. Marin, and Elisa Herraez. 2018. "Genetic Heterogeneity of SLC22 Family of Transporters in Drug Disposition" Journal of Personalized Medicine 8, no. 2: 14. https://doi.org/10.3390/jpm8020014








