Social Behavioral Deficits in Krushinsky-Molodkina Rats, an Animal Model of Audiogenic Epilepsy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavioral Tests
2.2.1. Habituation
2.2.2. Elevated Plus Maze
2.2.3. Three-Chambered Social Preference/Novelty Tests
2.2.4. Off-Line Analysis
2.2.5. Verification of Audiogenic Seizure Susceptibility (AGS)
2.2.6. Statistical Analysis
3. Results
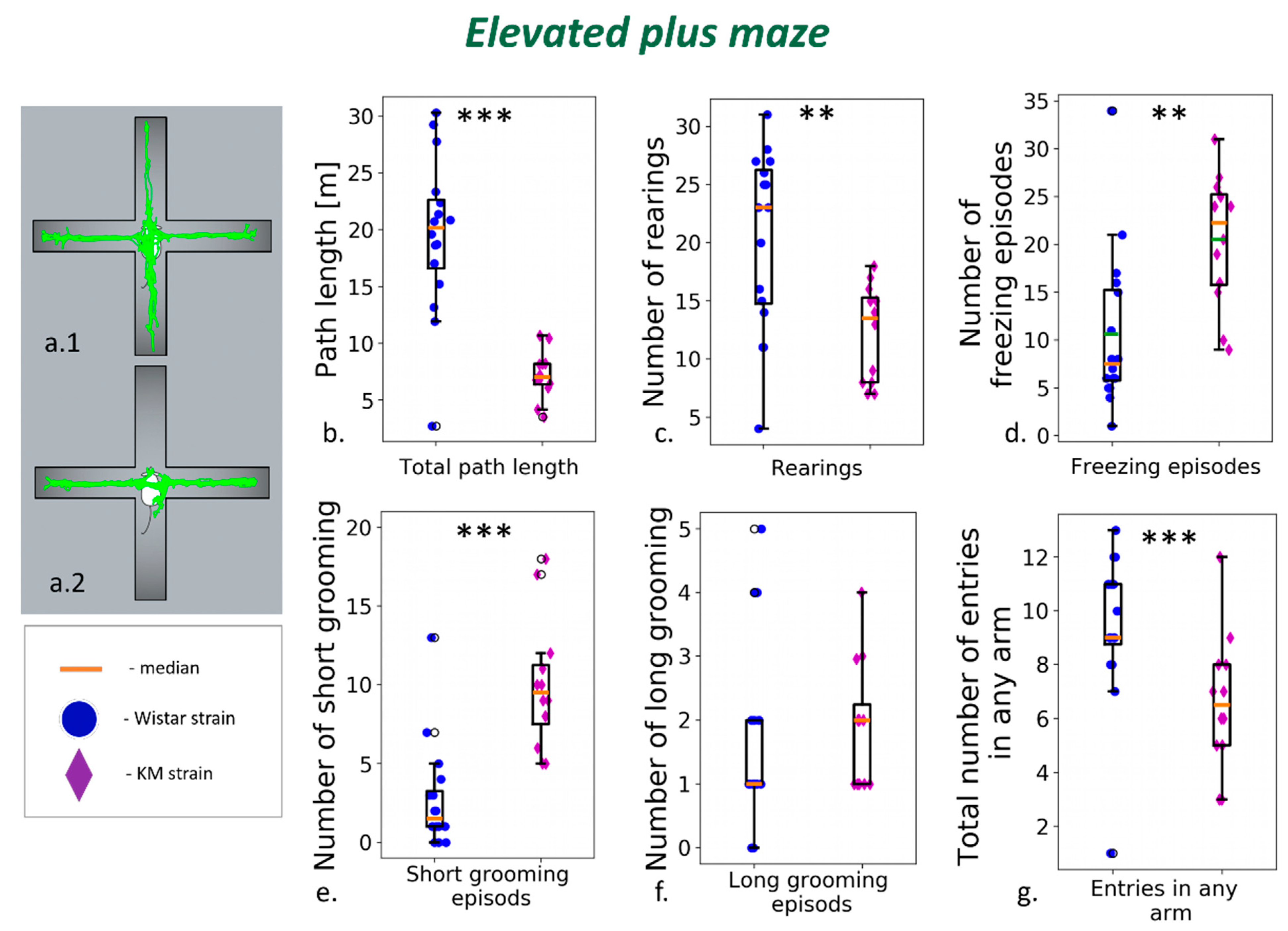
3.1. Elevated Plus Maze
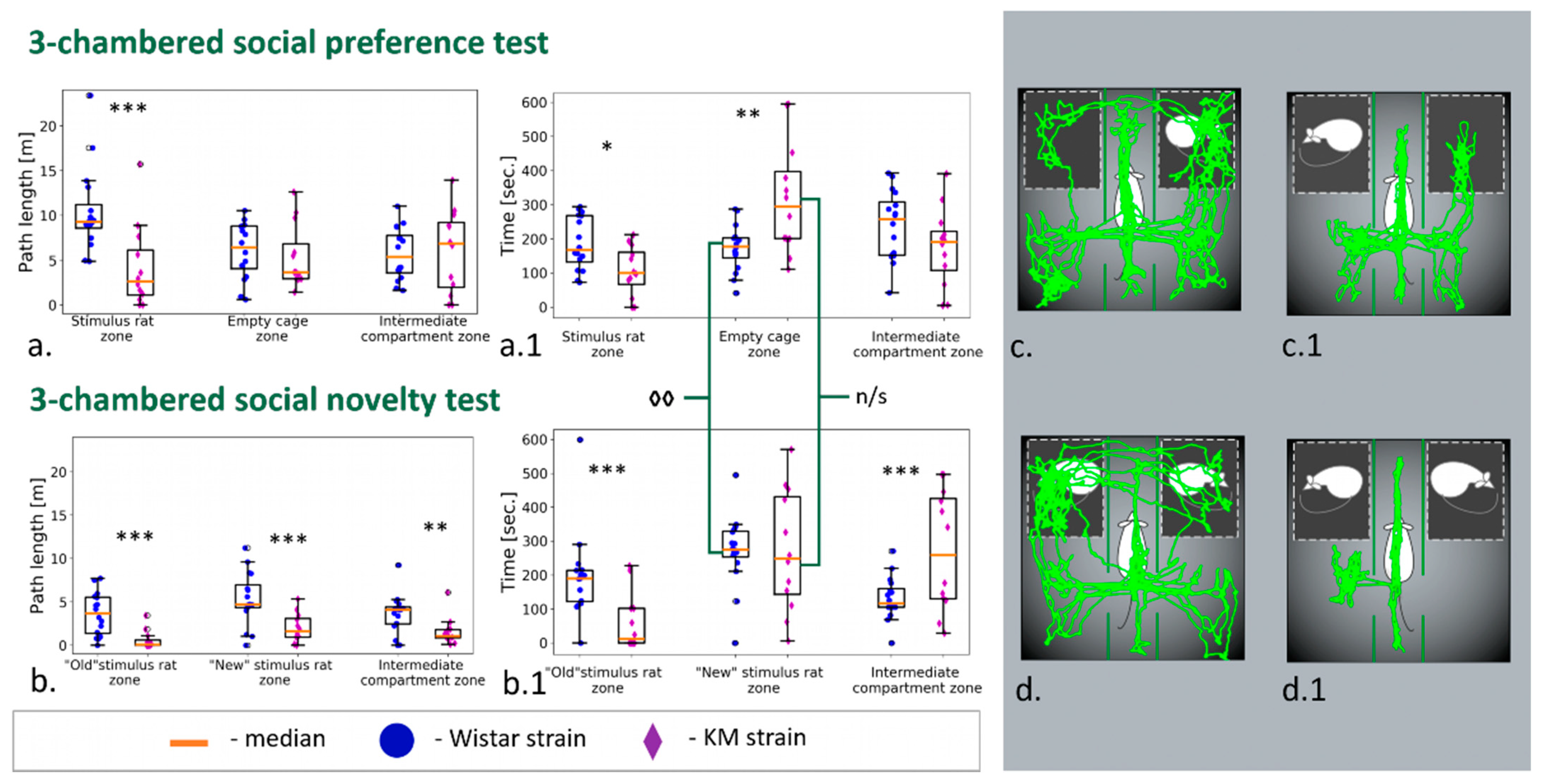
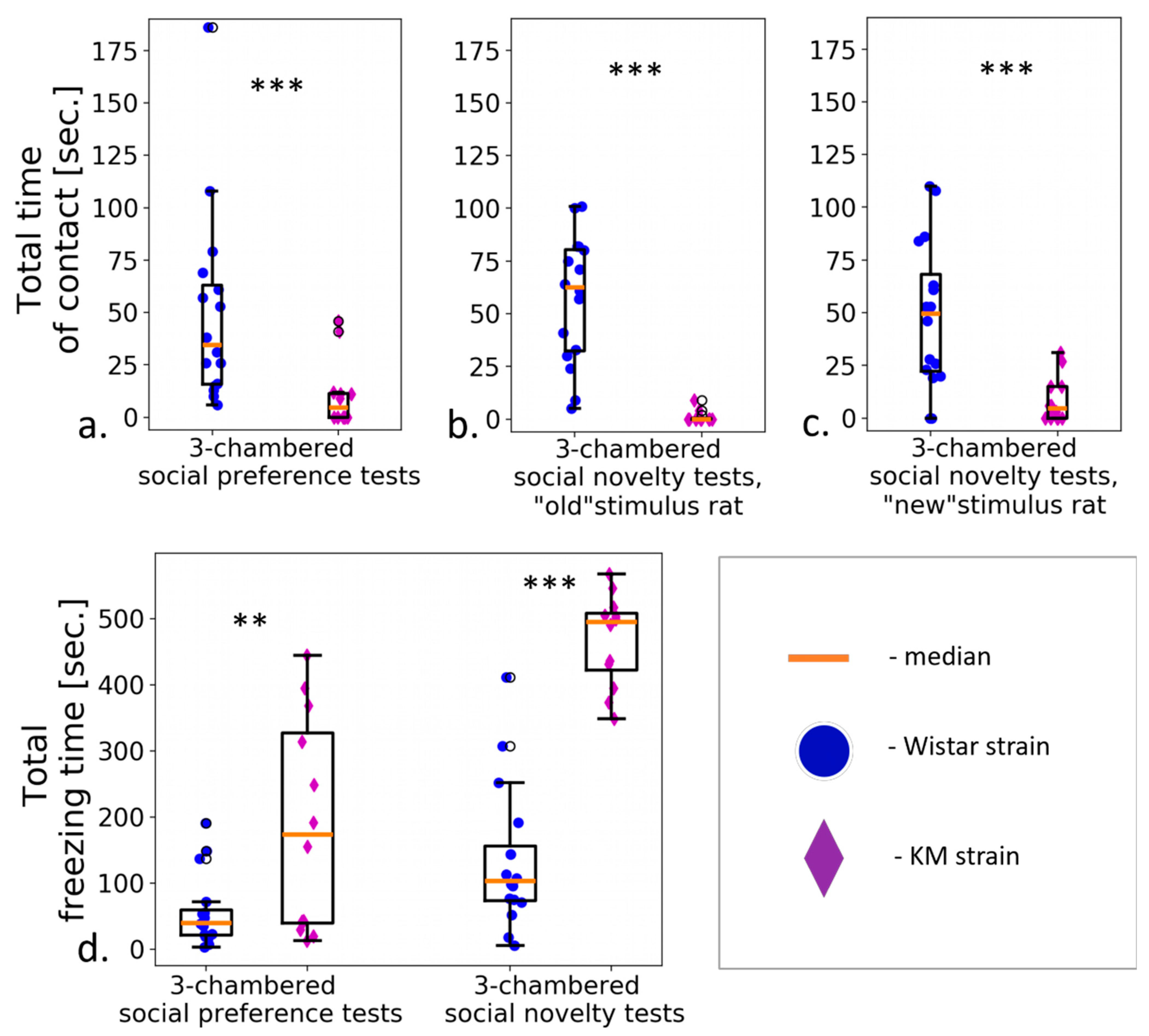
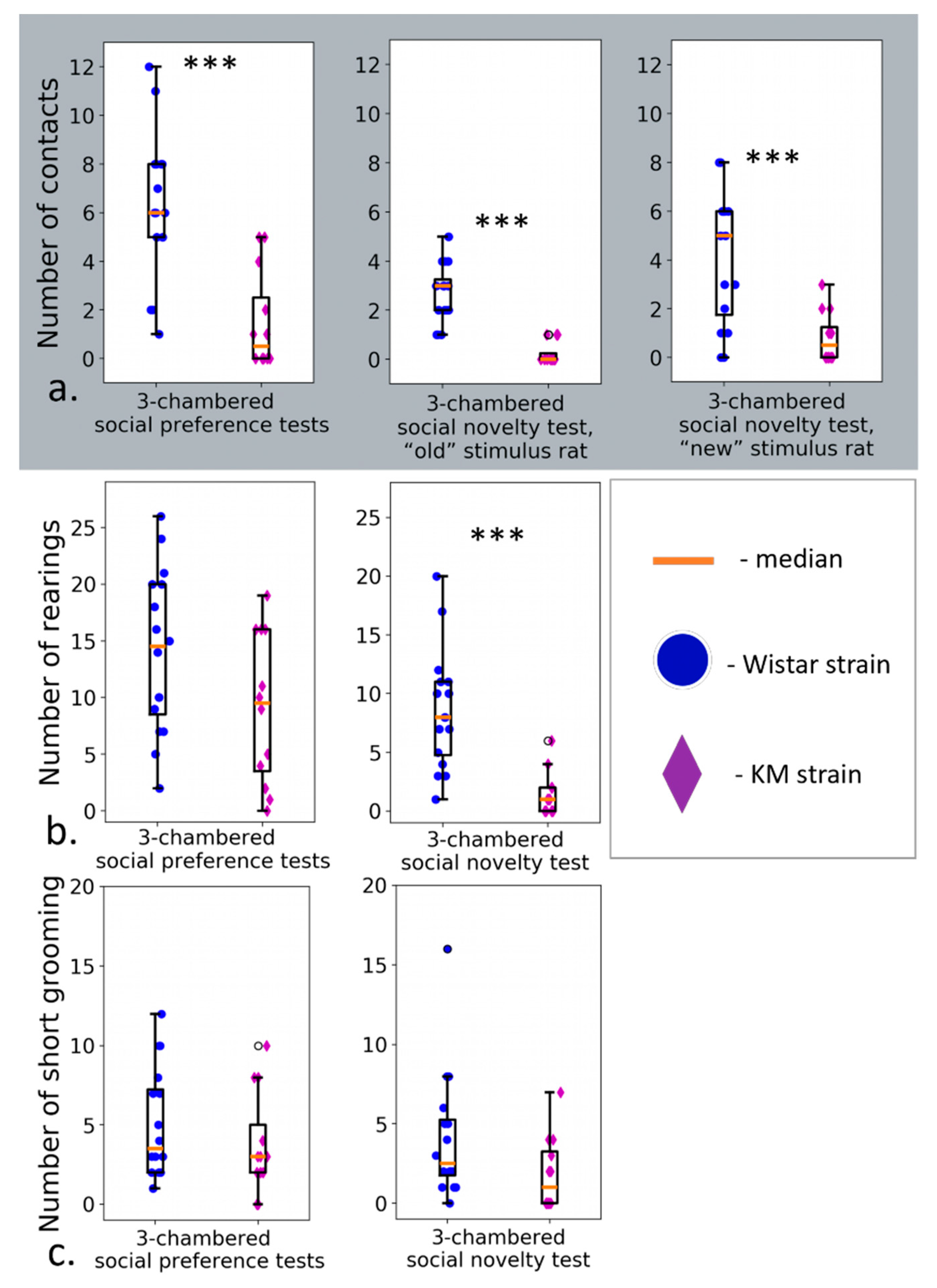
3.2. Three-Chambered Social Preference/Social Novelty Tests
3.3. AGS Provocation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lukmanji, S.; Manji, S.A.; Kadhim, S.; Sauro, K.M.; Wirrell, E.C.; Kwon, C.S.; Jetté, N. The co-occurrence of epilepsy and autism: A systematic review. Epilepsy Behav. 2019, 98, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Frith, U.; Happé, F. Autism spectrum disorder. Curr. Biol. 2005, 15, R786–R790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilton, C.L.; Harper, J.D.; Kueker, R.H.; Lang, A.R.; Abbacchi, A.M.; Todorov, A.; Lavesser, P.D. Sensory responsiveness as a predictor of social severity in children with high functioning autism spectrum disorders. J. Autism Dev. Disord. 2010, 40, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Gilby, K.L. A new rat model for vulnerability to epilepsy and autism spectrum disorders. Epilepsia 2008, 49, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Gilby, K.L.; O’Brien, T.J. Epilepsy, autism, and neurodevelopment: Kindling a shared vulnerability? Epilepsy Behav. 2013, 26, 370–374. [Google Scholar] [CrossRef]
- Castro, G.P.; Medeiros, D.d.C.; Guarnieri, L.d.O.; Mourão, F.A.G.; Pinto, H.P.P.; Pereira, G.S.; Moraes, M.F.D. Wistar audiogenic rats display abnormal behavioral traits associated with artificial selection for seizure susceptibility. Epilepsy Behav. 2017, 71, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Sarkisova, K.Y.; Midzianovskaia, I.S.; Kulikov, M.A. Depressive-like behavioral alterations and c-fos expression in the dopaminergic brain regions in WAG/Rij rats with genetic absence epilepsy. Behav. Brain Res. 2003, 144, 211–226. [Google Scholar] [CrossRef]
- Poletaeva, I.I.; Surina, N.M.; Kostina, Z.A.; Perepelkina, O.V.; Fedotova, I.B. The Krushinsky-Molodkina rat strain: The study of audiogenic epilepsy for 65 years. Epilepsy Behav. 2017, 71, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acikgoz, B.; Dalkiran, B.; Dayi, A. An overview of the currency and usefulness of behavioral tests used from past to present to assess anxiety, social behavior and depression in rats and mice. Behav. Process. 2022, 200, 104670. [Google Scholar] [CrossRef]
- Beery, A.K.; Shambaugh, K.L. Comparative Assessment of Familiarity/Novelty Preferences in Rodents. Front. Behav. Neurosci. 2021, 15, 648830. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Zhang, H.; Klaminder, J.; Brodin, T.; Andersson, P.L.; Andersson, M. ToxTrac: A fast and robust software for tracking organisms. Methods Ecol. Evol. 2018, 9, 460–464. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsova, G.D. Audiogenic seizures in rats of different genetical strains. Zhurnal Vyss. Nervn. Deyatelnosti Im. I.P. Pavlov. 1998, 48, 143–152. [Google Scholar]
- Midzyanovskaya, I.S.; Kuznetsova, G.D.; Vinogradova, L.v.; Shatskova, A.B.; Coenen AM, L.; van Luijtelaar, G. Mixed forms of epilepsy in a subpopulation of WAG/Rij rats. Epilepsy Behav. 2004, 5, 655–661. [Google Scholar] [CrossRef]
- Poletaeva, I.I.; Kostyna, Z.A.; Surina, N.M.; Fedotova, I.B.; Zorina, Z.A. The Krushinsky-Molodkina genetic rat strain as a unique experimental model of seizure states. Vavilovskii Zhurnal Genet. I Sel. 2017, 21, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Surina, N.M.; Kalinina, T.S.; Volkova, A.v.; Malikova, L.A.; Poletaeva, I.I.; Rayevsky, K.S.; Fedotova, I.B. Anxiety and predisposition to audiogenic epilepsy in rats of different genotypes. Bull. Exp. Biol. Med. 2011, 151, 68–75. [Google Scholar] [CrossRef]
- Bambini-Junior, V.; Zanatta, G.; della Flora Nunes, G.; Mueller de Melo, G.; Michels, M.; Fontes-Dutra, M.; Nogueira Freire, V.; Riesgo, R.; Gottfried, C. Resveratrol prevents social deficts in animal model of autism induced by valproic acid. Neurosci. Lett. 2014, 583, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Ergaz, Z.; Weinstein-Fudim, L.; Ornoy, A. Genetic and non-genetic animal models for autism spectrum disorders (ASD). Reprod. Toxicol. 2016, 64, 116–140. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Asadi-Shekaari, M.; Basiri, M.; Parvan, M.; Shabani, M.; Nozari, M. Improvement of autistic-like behaviors in adult rats prenatally exposed to valproic acid through early suppression of NMDA receptor function. Psychopharmacology 2020, 237, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.; Millette, A.; Devine, D.P. Sensory and motor characterization in the postnatal valproate rat model of autism. Dev. Neurosci. 2012, 34, 258–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servadio, M.; Vanderschuren, L.J.M.J.; Trezza, V. Modeling autism-relevant behavioral phenotypes in rats and mice: Do “autistic” rodents exist? Behav. Pharmacol. 2015, 26, 522–540. [Google Scholar] [CrossRef] [PubMed]
- Posserud, M.; Hysing, M.; Helland, W.; Gillberg, C.; Lundervold, A.J. Autism traits: The importance of “co-morbid” problems for impairment and contact with services. Data from the Bergen Child Study. Res. Dev. Disabil. 2018, 72, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelakos, C.C.; Tudor, J.C.; Ferri, S.L.; Jongens, T.A.; Abel, T. Home-cage hypoactivity in mouse genetic models of autism spectrum disorder. Neurobiol. Learn. Mem. 2019, 165, 107000. [Google Scholar] [CrossRef]
- Neklyudova, A.; Smirnov, K.; Rebreikina, A.; Martynova, O.; Sysoeva, O. Electrophysiological and Behavioral Evidence for Hyper- and Hyposensitivity in Rare Genetic Syndromes Associated with Autism. Genes 2022, 13, 671. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, K.; Stroganova, T.; Molholm, S.; Sysoeva, O. Reviewing evidence for the relationship of eeg abnormalities and rtt phenotype paralleled by insights from animal studies. Int. J. Mol. Sci. 2021, 22, 5308. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebik, A.A.; Riga, V.D.; Smirnov, K.S.; Sysoeva, O.V.; Midzyanovskaya, I.S. Social Behavioral Deficits in Krushinsky-Molodkina Rats, an Animal Model of Audiogenic Epilepsy. J. Pers. Med. 2022, 12, 2062. https://doi.org/10.3390/jpm12122062
Rebik AA, Riga VD, Smirnov KS, Sysoeva OV, Midzyanovskaya IS. Social Behavioral Deficits in Krushinsky-Molodkina Rats, an Animal Model of Audiogenic Epilepsy. Journal of Personalized Medicine. 2022; 12(12):2062. https://doi.org/10.3390/jpm12122062
Chicago/Turabian StyleRebik, Anastasiya A., Vyacheslav D. Riga, Kirill S. Smirnov, Olga V. Sysoeva, and Inna S. Midzyanovskaya. 2022. "Social Behavioral Deficits in Krushinsky-Molodkina Rats, an Animal Model of Audiogenic Epilepsy" Journal of Personalized Medicine 12, no. 12: 2062. https://doi.org/10.3390/jpm12122062





