Serum Adiponectin, a Novel Biomarker Correlates with Skin Thickness in Systemic Sclerosis
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Clinical Assessment
2.3. Laboratory Procedures
2.4. Statistical Analysis
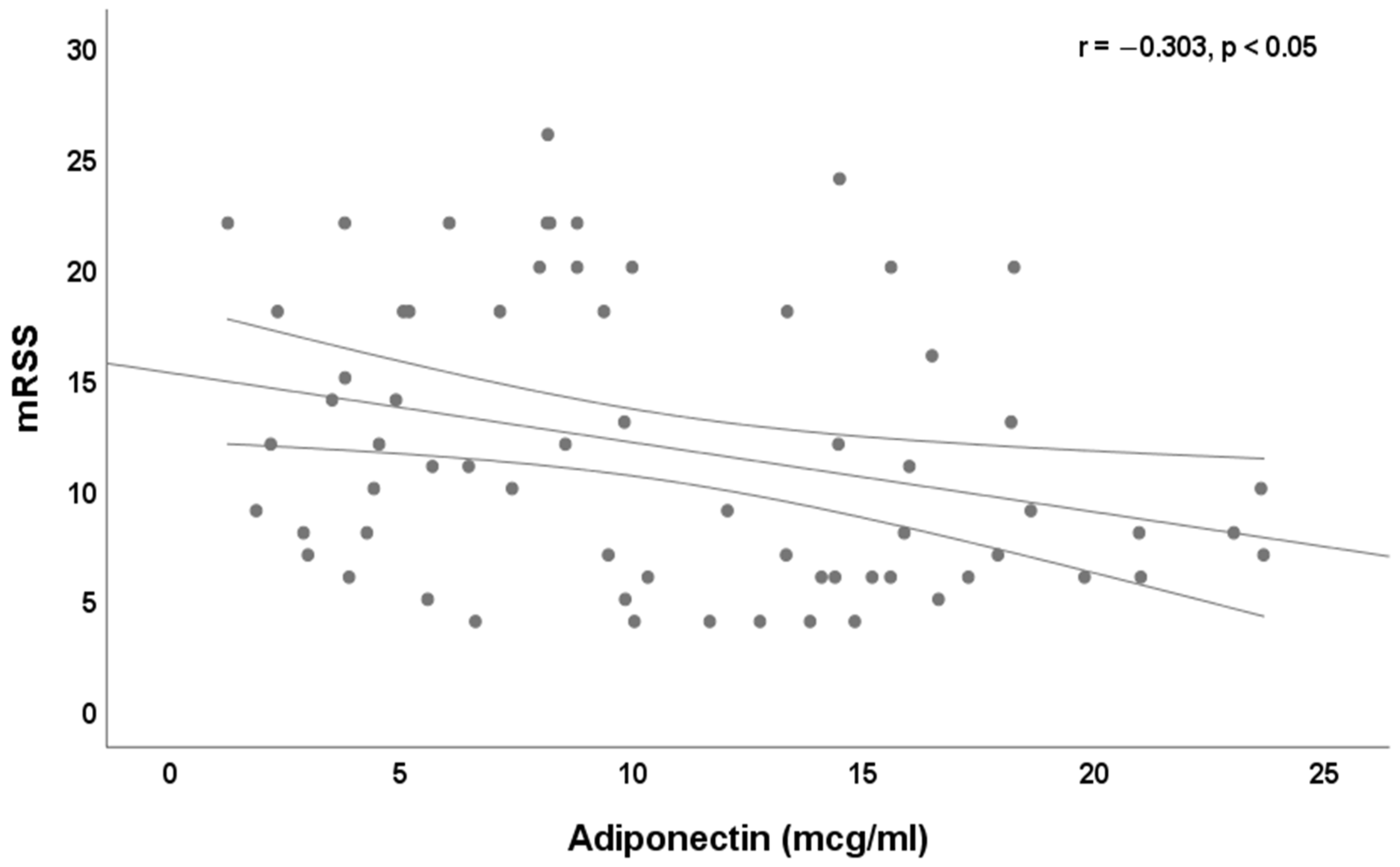
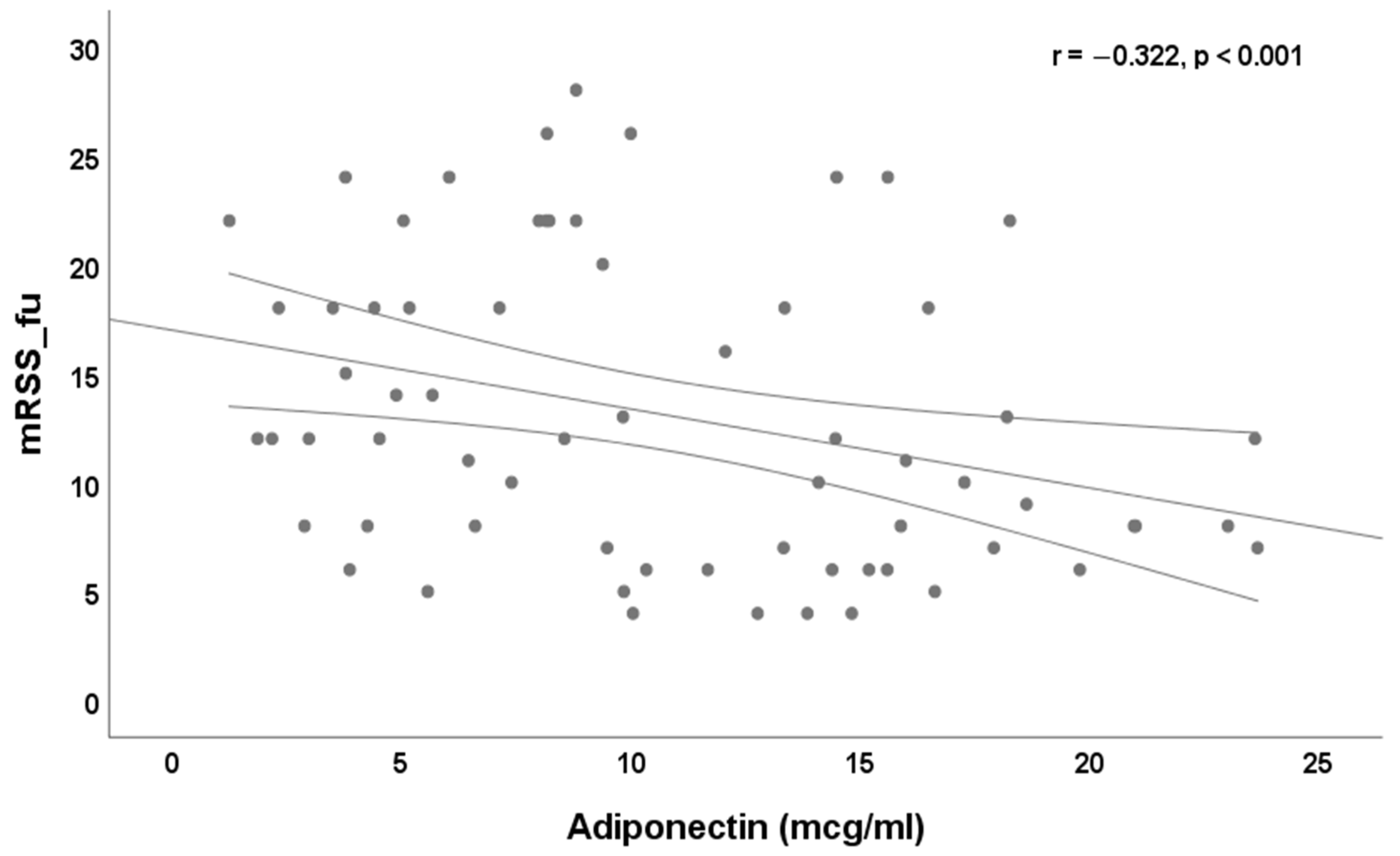
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabrielli, A.; Avvedimento, E.V.; Krieg, T. Scleroderma. N. Engl. J. Med. 2009, 360, 1989–2003. [Google Scholar] [CrossRef]
- Varga, J.; Abraham, D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Investig. 2007, 117, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.R.; Jahoda, C.A.; Chuong, C.M.; Watt, F.M.; Horsley, V. Defining dermal adipose tissue. Exp. Dermatol. 2014, 23, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Bogatkevich, G.S. Editorial: Fate of fat tissue adipocytes: Do they transform into myofibroblasts in scleroderma? Arthritis Rheumatol. 2015, 67, 860–861. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Melichian, D.S.; Chang, E.; Warner-Blankenship, M.; Ghosh, A.K.; Varga, J. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. Am. J. Pathol. 2009, 174, 519–533. [Google Scholar] [CrossRef]
- Zheng, F.; Fornoni, A.; Elliot, S.J.; Guan, Y.; Breyer, M.D.; Striker, L.J.; Striker, G.E. Upregulation of type I collagen by TGF-beta in mesangial cells is blocked by PPARgamma activation. Am. J. Physiol. Renal Physiol. 2002, 282, F639–F648. [Google Scholar] [CrossRef]
- Miyahara, T.; Schrum, L.; Rippe, R.; Xiong, S.; Yee, H.F., Jr.; Motomura, K.; Anania, F.A.; Willson, T.M.; Tsukamoto, H. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J. Biol. Chem. 2000, 275, 35715–35722. [Google Scholar] [CrossRef] [PubMed]
- Bogatkevich, G.S.; Highland, K.B.; Akter, T.; Silver, R.M. The PPARgamma Agonist Rosiglitazone Is Antifibrotic for Scleroderma Lung Fibroblasts: Mechanisms of Action and Differential Racial Effects. Pulm. Med. 2012, 2012, 545172. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Vadacca, M.; Margiotta, D.; Rigon, A.; Cacciapaglia, F.; Coppolino, G.; Amoroso, A.; Afeltra, A. Adipokines and systemic lupus erythematosus: Relationship with metabolic syndrome and cardiovascular disease risk factors. J. Rheumatol. 2009, 36, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Vadacca, M.; Zardi, E.M.; Margiotta, D.; Rigon, A.; Cacciapaglia, F.; Arcarese, L.; Buzzulini, F.; Amoroso, A.; Afeltra, A. Leptin, adiponectin and vascular stiffness parameters in women with systemic lupus erythematosus. Intern. Emerg. Med. 2013, 8, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Liu, L.; Yang, Y.; Tamaki, Z.; Wei, J.; Marangoni, R.G.; Bhattacharyya, S.; Summer, R.S.; Ye, B.; Varga, J. The adipokine adiponectin has potent anti-fibrotic effects mediated via adenosine monophosphate-activated protein kinase: Novel target for fibrosis therapy. Arthritis Res. Ther. 2012, 14, R229. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Jinnin, M.; Muchemwa, F.C.; Makino, T.; Kajihara, I.; Makino, K.; Honda, N.; Sakai, K.; Fukushima, S.; Ihn, H. Adiponectin expression is decreased in the involved skin and sera of diffuse cutaneous scleroderma patients. Exp. Dermatol. 2011, 20, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Lakota, K.; Wei, J.; Carns, M.; Hinchcliff, M.; Lee, J.; Whitfield, M.L.; Sodin-Semrl, S. Levels of adiponectin, a marker for PPAR-gamma activity, correlate with skin fibrosis in systemic sclerosis: Potential utility as biomarker? Arthritis Res. Ther. 2012, 14, R102. [Google Scholar] [CrossRef] [PubMed]
- Masui, Y.; Asano, Y.; Shibata, S.; Noda, S.; Aozasa, N.; Akamata, K.; Yamada, D.; Tamaki, Z.; Tada, Y.; Sugaya, M.; et al. Serum adiponectin levels inversely correlate with the activity of progressive skin sclerosis in patients with diffuse cutaneous systemic sclerosis. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Tomcik, M.; Arima, K.; Hulejova, H.; Kuklova, M.; Filkova, M.; Braun, M.; Beláček, J.; Novák, M.; Bečvář, R.; Vencovský, J.; et al. Adiponectin relation to skin changes and dyslipidemia in systemic sclerosis. Cytokine 2012, 58, 165–168. [Google Scholar] [CrossRef]
- Zhao, J.H.; Huang, X.L.; Duan, Y.; Wang, Y.J.; Chen, S.Y.; Wang, J. Serum adipokines levels in patients with systemic sclerosis: A meta-analysis. Mod. Rheumatol. 2017, 27, 298–305. [Google Scholar] [CrossRef]
- Masui, Y.; Asano, Y.; Takahashi, T.; Shibata, S.; Akamata, K.; Aozasa, N.; Noda, S.; Taniguchi, T.; Ichimura, Y.; Toyama, T.; et al. Clinical significance of monitoring serum adiponectin levels during intravenous pulse cyclophosphamide therapy in interstitial lung disease associated with systemic sclerosis. Mod. Rheumatol. 2013, 23, 323–329. [Google Scholar] [CrossRef]
- Marangoni, R.G.; Korman, B.D.; Wei, J.; Wood, T.A.; Graham, L.V.; Whitfield, M.L.; Scherer, P.E.; Tourtellotte, W.G.; Varga, J. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015, 67, 1062–1073. [Google Scholar] [CrossRef]
- Marangoni, R.G.; Masui, Y.; Fang, F.; Korman, B.; Lord, G.; Lee, J.; Lakota, K.; Wei, J.; Scherer, P.E.; Otvos, L.; et al. Adiponectin is an endogenous anti-fibrotic mediator and therapeutic target. Sci. Rep. 2017, 7, 4397. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Lakota, K.; Taniguchi, T.; Yoshizaki, A.; Sato, S.; Hong, W.; Zhou, X.; Sodin-Semrl, S.; Fang, F.; Asano, Y.; et al. An orally-active adiponectin receptor agonist mitigates cutaneous fibrosis, inflammation and microvascular pathology in a murine model of systemic sclerosis. Sci. Rep. 2018, 8, 11843. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Carreira, P.E. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Maurer, B.; Graf, N.; Michel, B.A.; Müller-Ladner, U.; Czirják, L.; Denton, C.P.; Tyndall, A.; Metzig, C.; Lanius, V.; Khanna, D.; et al. Prediction of worsening of skin fibrosis in patients with diffuse cutaneous systemic sclerosis using the EUSTAR database. Ann. Rheum. Dis. 2015, 74, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Clements, P.J.; Hurwitz, E.L.; Wong, W.K.; Seibold, J.R.; Mayes, M.; White, B.; Wigley, F.; Weisman, M.; Barr, W.; Moreland, L.; et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: High-dose versus low-dose penicillamine trial. Arthritis Rheum. 2000, 43, 2445–2454. [Google Scholar] [CrossRef]
- Valentini, G.; Della Rossa, A.; Bombardieri, S.; Bencivelli, W.; Silman, A.J.; D’Angelo, S.; Cerinic, M.M.; Belch, J.F.; Black, C.M.; Bruhlmann, P.; et al. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann. Rheum. Dis. 2001, 60, 592–598. [Google Scholar] [CrossRef]
- Medsger, T.A., Jr.; Bombardieri, S.; Czirjak, L.; Scorza, R.; Della Rossa, A.; Bencivelli, W. Assessment of disease severity and prognosis. Clin. Exp. Rheumatol. 2003, 21, S42–S46. [Google Scholar]
- Cutolo, M.; Sulli, A.; Pizzorni, C.; Accardo, S. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J. Rheumatol. 2000, 27, 155–160. [Google Scholar]
- Winsz-Szczotka, K.; Kuźnik-Trocha, K.; Komosińska-Vassev, K.; Kucharz, E.; Kotulska, A.; Olczyk, K. Relationship between adiponectin, leptin, IGF-1 and total lipid peroxides plasma concentrations in patients with systemic sclerosis: Possible role in disease development. Int. J. Rheum. Dis. 2016, 19, 706–714. [Google Scholar] [CrossRef]
- Kotulska, A.; Kucharz, E.J.; Brzezińska-Wcisło, L.; Wadas, U. A decreased serum leptin level in patients with systemic sclerosis. Clin. Rheumatol. 2001, 20, 300–302. [Google Scholar] [CrossRef]
- Pellicano, C.; Miglionico, M.; Romaggioli, L.; Colalillo, A.; Vantaggio, L.; Napodano, C.; Callà, C.; Gulli, F.; Marino, M.; Basile, U.; et al. Increased Complement Activation in Systemic Sclerosis Patients with Skin and Lung Fibrosis. J. Pers. Med. 2022, 12, 284. [Google Scholar] [CrossRef]
- Gigante, A.; Pellicano, C.; Leodori, G.; Napodano, C.; Vantaggio, L.; Gulli, F.; Marino, M.; Visentini, M.; Rosato, E.; Basile, U. Serum and urine free light chains measurements in patients with systemic sclerosis: Novel biomarkers for disease activity. Clin. Exp. Immunol. 2021, 205, 135–141. [Google Scholar] [CrossRef]
- Pellicano, C.; Colalillo, A.; Basile, V.; Marino, M.; Basile, U.; La Gualana, F.; Mezzaroma, I.; Visentini, M.; Rosato, E. The Effect of SARS-CoV-2 Vaccination on B-Cell Phenotype in Systemic Sclerosis Patients. J. Pers. Med. 2022, 12, 1420. [Google Scholar] [CrossRef]
| Females, n (%) | 66 (100) |
| Age, years, median and IQR | 54 (42–62) |
| dcSSc, n (%) | 38 (58) |
| SSc specific autoantibodies | |
| anti-topoisomerase I, n (%) | 41 (62.1) |
| anti-centromere, n (%) | 22 (33.3) |
| none, n (%) | 3 (4.5) |
| Nailfold capillaroscopic pattern | |
| early, n (%) | 17 (25.8) |
| active, n (%) | 23 (34.8) |
| late, n (%) | 26 (39.4) |
| Adiponectin, ng/mL, median and IQR | 9.8 (5.6–15.6) |
| mRSS, median and IQR | 10 (6–18) |
| mRSS_fu, median and IQR | 12 (7–18) |
| DAI, median and IQR | 2 (1–4.5) |
| DAI_fu, median and IQR | 3 (1–5) |
| DSS, median and IQR | 4 (3–7) |
| DSS_fu, median and IQR | 6 (3–10) |
| BMI, kg/m2, mean ± SD | 22.7 (19.4–26) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leodori, G.; Pellicano, C.; Basile, V.; Colalillo, A.; Navarini, L.; Gigante, A.; Gulli, F.; Marino, M.; Basile, U.; Rosato, E. Serum Adiponectin, a Novel Biomarker Correlates with Skin Thickness in Systemic Sclerosis. J. Pers. Med. 2022, 12, 1737. https://doi.org/10.3390/jpm12101737
Leodori G, Pellicano C, Basile V, Colalillo A, Navarini L, Gigante A, Gulli F, Marino M, Basile U, Rosato E. Serum Adiponectin, a Novel Biomarker Correlates with Skin Thickness in Systemic Sclerosis. Journal of Personalized Medicine. 2022; 12(10):1737. https://doi.org/10.3390/jpm12101737
Chicago/Turabian StyleLeodori, Giorgia, Chiara Pellicano, Valerio Basile, Amalia Colalillo, Luca Navarini, Antonietta Gigante, Francesca Gulli, Mariapaola Marino, Umberto Basile, and Edoardo Rosato. 2022. "Serum Adiponectin, a Novel Biomarker Correlates with Skin Thickness in Systemic Sclerosis" Journal of Personalized Medicine 12, no. 10: 1737. https://doi.org/10.3390/jpm12101737
APA StyleLeodori, G., Pellicano, C., Basile, V., Colalillo, A., Navarini, L., Gigante, A., Gulli, F., Marino, M., Basile, U., & Rosato, E. (2022). Serum Adiponectin, a Novel Biomarker Correlates with Skin Thickness in Systemic Sclerosis. Journal of Personalized Medicine, 12(10), 1737. https://doi.org/10.3390/jpm12101737









