Mobile Health, Disease Knowledge, and Self-Care Behavior in Chronic Kidney Disease: A Prospective Cohort Study
Abstract
:1. Introduction
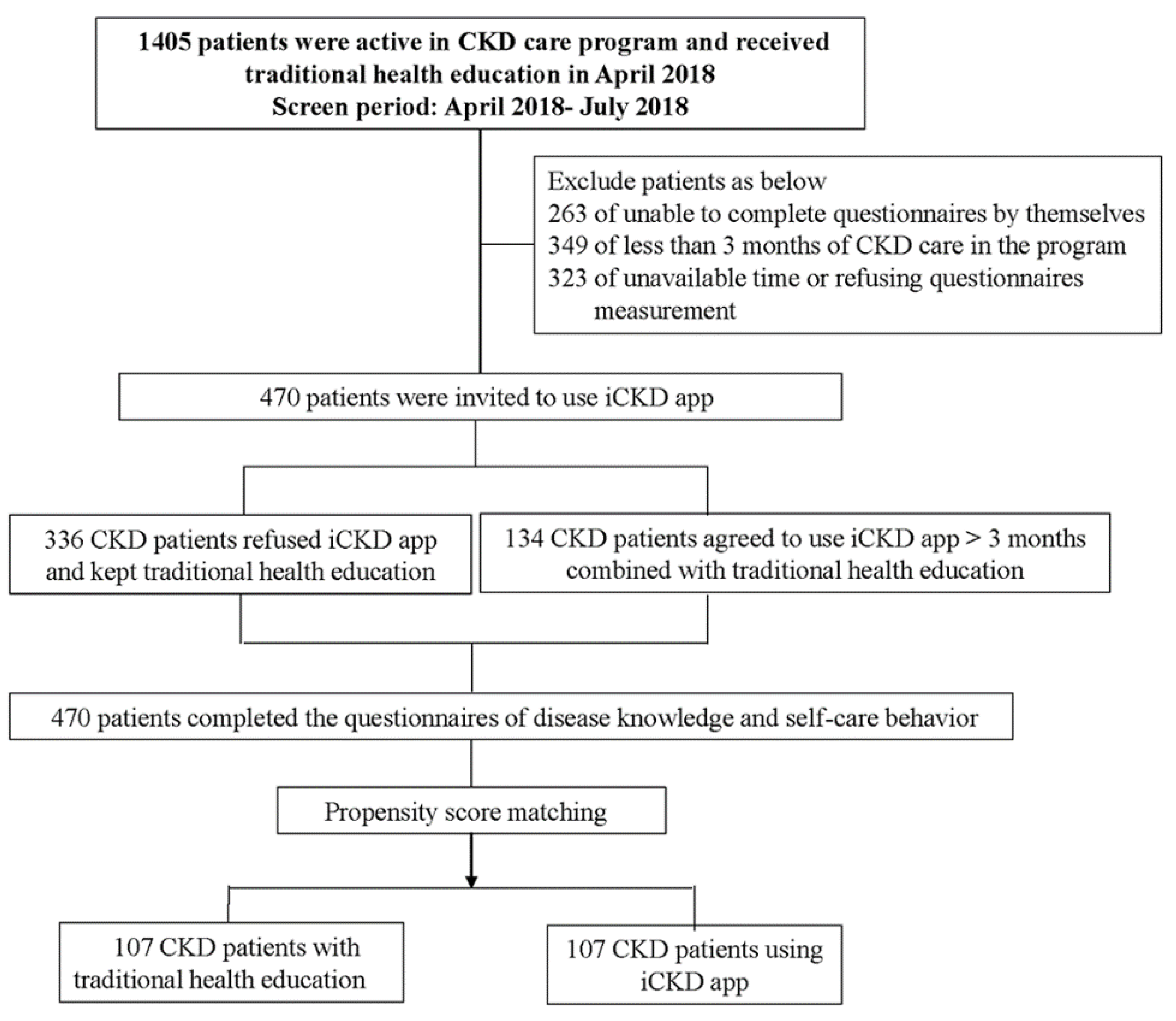
2. Materials and Methods
2.1. Study Participants
2.2. iCKD Development
2.3. Clinical Measurements
2.4. Disease Knowledge and Self-Care Behavior Measurement
2.5. Propensity Score Matching
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Entire Cohort
3.2. mHealth, Disease Knowledge and Self-Behavior
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddique, A.B.; Krebs, M.; Alvarez, S.; Greenspan, I.; Patel, A.; Kinsolving, J.; Koizumi, N. Mobile Apps for the Care Management of Chronic Kidney and End-Stage Renal Diseases: Systematic Search in App Stores and Evaluation. JMIR Mhealth Uhealth 2019, 7, e12604. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.P.; Cheng, T.Y.; Tsai, M.K.; Chang, Y.C.; Chan, H.T.; Tsai, S.P.; Chiang, P.H.; Hsu, C.C.; Sung, P.K.; Hsu, Y.H.; et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008, 371, 2173–2182. [Google Scholar] [CrossRef]
- Chen, S.H.; Tsai, Y.F.; Sun, C.Y.; Wu, I.W.; Lee, C.C.; Wu, M.S. The impact of self-management support on the progression of chronic kidney disease--a prospective randomized controlled trial. Nephrol. Dial. Transplant. 2011, 26, 3560–3566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocco, M.V. Disease management programs for CKD patients: The potential and pitfalls. Am. J. Kidney Dis. 2009, 53, S56–S63. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, H.; Qazi, H.; Morita, P.P. Intervention and Evaluation of Mobile Health Technologies in Management of Patients Undergoing Chronic Dialysis: Scoping Review. JMIR Mhealth Uhealth 2020, 8, e15549. [Google Scholar] [CrossRef]
- Free, C.; Phillips, G.; Watson, L.; Galli, L.; Felix, L.; Edwards, P.; Patel, V.; Haines, A. The effectiveness of mobile-health technologies to improve health care service delivery processes: A systematic review and meta-analysis. PLoS Med. 2013, 10, e1001363. [Google Scholar] [CrossRef]
- Hsu, C.C.; Hwang, S.J.; Wen, C.P.; Chang, H.Y.; Chen, T.; Shiu, R.S.; Horng, S.S.; Chang, Y.K.; Yang, W.C. High prevalence and low awareness of CKD in Taiwan: A study on the relationship between serum creatinine and awareness from a nationally representative survey. Am. J. Kidney Dis. 2006, 48, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Curtin, R.B.; Sitter, D.C.; Schatell, D.; Chewning, B.A. Self-management, knowledge, and functioning and well-being of patients on hemodialysis. Nephrol. Nurs. J. 2004, 31, 378–386, 396. [Google Scholar]
- Ouyang, C.M.; Dwyer, J.T.; Jacques, P.F.; Chuang, L.M.; Haas, C.F.; Weinger, K. Diabetes self-care behaviours and clinical outcomes among Taiwanese patients with type 2 diabetes. Asia Pac. J. Clin. Nutr. 2015, 24, 438–443. [Google Scholar] [PubMed]
- Kennedy, A.; Rogers, A.; Bower, P. Support for self care for patients with chronic disease. BMJ 2007, 335, 968–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditewig, J.B.; Blok, H.; Havers, J.; van Veenendaal, H. Effectiveness of self-management interventions on mortality, hospital readmissions, chronic heart failure hospitalization rate and quality of life in patients with chronic heart failure: A systematic review. Patient Educ. Couns. 2010, 78, 297–315. [Google Scholar] [CrossRef]
- Gray, N.A.; Kapojos, J.J.; Burke, M.T.; Sammartino, C.; Clark, C.J. Patient kidney disease knowledge remains inadequate with standard nephrology outpatient care. Clin. Kidney J. 2016, 9, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, M.; Huang, J.J.; Teng, H.L. Education for patients with chronic kidney disease in Taiwan: A prospective repeated measures study. J. Clin. Nurs. 2008, 17, 2927–2934. [Google Scholar] [CrossRef]
- National Kidney, F. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Wright Nunes, J.A.; Wallston, K.A.; Eden, S.K.; Shintani, A.K.; Ikizler, T.A.; Cavanaugh, K.L. Associations among perceived and objective disease knowledge and satisfaction with physician communication in patients with chronic kidney disease. Kidney Int. 2011, 80, 1344–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.L.; Chiu, Y.W.; Kung, L.F.; Chen, T.H.; Hsiao, S.M.; Hsiao, P.N.; Hwang, S.J.; Hsieh, H.M. Patient assessment of chronic kidney disease self-care using the chronic kidney disease self-care scale in Taiwan. Nephrology (Carlton) 2019, 24, 615–621. [Google Scholar] [CrossRef]
- Ali, M.S.; Groenwold, R.H.; Belitser, S.V.; Pestman, W.R.; Hoes, A.W.; Roes, K.C.; Boer, A.; Klungel, O.H. Reporting of covariate selection and balance assessment in propensity score analysis is suboptimal: A systematic review. J. Clin. Epidemiol. 2015, 68, 112–121. [Google Scholar] [CrossRef]
- Narva, A.S.; Briggs, M. The National Kidney Disease Education Program: Improving understanding, detection, and management of CKD. Am. J. Kidney Dis. 2009, 53, S115–S120. [Google Scholar] [CrossRef]
- Brown, W.W.; Peters, R.M.; Ohmit, S.E.; Keane, W.F.; Collins, A.; Chen, S.C.; King, K.; Klag, M.J.; Molony, D.A.; Flack, J.M. Early detection of kidney disease in community settings: The Kidney Early Evaluation Program (KEEP). Am. J. Kidney Dis. 2003, 42, 22–35. [Google Scholar] [CrossRef]
- Tuot, D.S.; Boulware, L.E. Telehealth Applications to Enhance CKD Knowledge and Awareness among Patients and Providers. Adv. Chronic Kidney Dis. 2017, 24, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Shneerson, C.; Windle, R.; Cox, K. Innovating information-delivery for potential clinical trials participants. What do patients want from multi-media resources? Patient Educ. Couns. 2013, 90, 111–117. [Google Scholar] [CrossRef]
- Sarkar, U.; Karter, A.J.; Liu, J.Y.; Adler, N.E.; Nguyen, R.; Lopez, A.; Schillinger, D. Social disparities in internet patient portal use in diabetes: Evidence that the digital divide extends beyond access. J. Am. Med. Inform. Assoc. 2011, 18, 318–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quittschalle, J.; Stein, J.; Luppa, M.; Pabst, A.; Lobner, M.; Koenig, H.H.; Riedel-Heller, S.G. Internet Use in Old Age: Results of a German Population-Representative Survey. J. Med. Internet Res. 2020, 22, e15543. [Google Scholar] [CrossRef]
- Manafo, E.; Wong, S. Promoting eHealth literacy in older adults: Key informant perspectives. Can. J. Diet. Pract. Res. 2013, 74, 37–41. [Google Scholar] [CrossRef] [PubMed]
- McManus, R.J.; Mant, J.; Haque, M.S.; Bray, E.P.; Bryan, S.; Greenfield, S.M.; Jones, M.I.; Jowett, S.; Little, P.; Penaloza, C.; et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: The TASMIN-SR randomized clinical trial. JAMA 2014, 312, 799–808. [Google Scholar] [CrossRef]
- Ong, S.W.; Jassal, S.V.; Miller, J.A.; Porter, E.C.; Cafazzo, J.A.; Seto, E.; Thorpe, K.E.; Logan, A.G. Integrating a Smartphone-Based Self-Management System into Usual Care of Advanced CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- McGrane, N.; Galvin, R.; Cusack, T.; Stokes, E. Addition of motivational interventions to exercise and traditional physiotherapy: A review and meta-analysis. Physiotherapy 2015, 101, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Muchiri, J.W.; Gericke, G.J.; Rheeder, P. Adapting a diabetes nutrition education programme for adults with type 2 diabetes from a primary to tertiary. S. Afr. J. Clin. Nutr. 2019, 34, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Hamine, S.; Gerth-Guyette, E.; Faulx, D.; Green, B.B.; Ginsburg, A.S. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: A systematic review. J. Med. Internet Res. 2015, 17, e52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vehi, J.; Regincos Isern, J.; Parcerisas, A.; Calm, R.; Contreras, I. Impact of Use Frequency of a Mobile Diabetes Management App on Blood Glucose Control: Evaluation Study. JMIR Mhealth Uhealth 2019, 7, e11933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaghefi, I.; Tulu, B. The Continued Use of Mobile Health Apps: Insights from a Longitudinal Study. JMIR Mhealth Uhealth 2019, 7, e12983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuvesson, H.; Eriksen, S.; Fagerstrom, C. mHealth and Engagement Concerning Persons with Chronic Somatic Health Conditions: Integrative Literature Review. JMIR Mhealth Uhealth 2020, 8, e14315. [Google Scholar] [PubMed]
- Borrelli, B.; Bartlett, Y.K.; Tooley, E.; Armitage, C.J.; Wearden, A. Prevalence and Frequency of mHealth and eHealth Use Among US and UK Smokers and Differences by Motivation to Quit. J. Med. Internet Res. 2015, 17, e164. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Clinical characteristics | Entire Cohort N = 214 | iCKD N = 107 | Non-iCKD N = 107 | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 63.9 ± 11.5 | 63.5 ± 11.1 | 64.2 ± 11.9 | 0.67 |
| Sex (male, %) | 59.3 | 62.6 | 56.1 | 0.33 |
| Smoke (yes, %) | 25.7 | 26.2 | 25.2 | 0.87 |
| Alcohol (yes, %) | 10.7 | 12.1 | 9.3 | 0.50 |
| Marital status (married, %) | 79.0 | 83.2 | 74.8 | 0.13 |
| Currently working (yes, %) | 37.9 | 38.3 | 37.4 | 0.88 |
| Education (senior high school or above, %) | 66.8 | 70.1 | 63.6 | 0.31 |
| Hypertension (yes, %) | 83.6 | 84.1 | 83.2 | 0.85 |
| Diabetes mellitus (yes, %) | 33.6 | 35.5 | 31.8 | 0.56 |
| Heart disease (yes, %) | 12.6 | 13.1 | 12.1 | 0.83 |
| Body mass index (kg/m2) | 24.5 ± 4.4 | 24.3 ± 4.4 | 24.5 ± 3.8 | 0.78 |
| Traditional health education (session) | 19.7 ± 11.1 | 17.0 ± 10.7 | 22.5 ± 10.9 | < 0.001 |
| The duration of CKD (year) | 11.3 ± 8.2 | 10.4. ± 8.9 | 12.1 ± 7.5 | 0.12 |
| Systolic blood pressure (mmHg) | 136 ± 17 | 137. ± 15 | 135 ± 18 | 0.25 |
| Diastolic blood pressure (mmHg) | 77 ± 10 | 77. ± 10 | 76 ± 10 | 0.46 |
| Questionnaires | ||||
| Self-care score | 64.3 ± 10.1 | 64.4 ± 9.4 | 64.1 ± 10.7 | 0.84 |
| Disease knowledge score | 23.6 ± 6.0 | 24.6 ± 6.6 | 22.6 ± 5.3 | 0.02 |
| Laboratory parameters | ||||
| Blood urea nitrogen (mg/dL) | 27.6 (19.5, 42.5) | 26.4 (19.8, 43.4) | 27.6 (18.1, 41.8) | 0.39 |
| eGFR (ml/min/1.73 m2) | 36.0 ± 24.9 | 35.0 ± 23.5 | 37.0 ± 26.3 | 0.55 |
| Hemoglobin (g/dL) | 12.1 ± 2.2 | 12.2 ± 2.2 | 12.1 ± 2.1 | 0.67 |
| Albumin (g/dL) | 4.3 ± 0.4 | 4.3 ± 0.3 | 4.3 ± 0.4 | 0.34 |
| Uric acid (mg/dL) | 6.5 ± 1.6 | 6.5 ± 1.6 | 6.6 ± 1.5 | 0.88 |
| Cholesterol (mg/dL) | 174 ± 38 | 172 ± 38 | 177 ± 38 | 0.34 |
| Triglyceride (mg/dL) | 105 (78, 147) | 107 (80, 146) | 99 (76, 152) | 0.29 |
| Urine protein/creatinine ratio (mg/mg) | 0.6 (0.2, 1.5) | 0.7 (0.2, 1.6) | 0.5 (0.2, 1.4) | 0.35 |
| Glycated hemoglobin (%) | 5.8 (5.5, 6.3) | 5.8 (5.5, 6.3) | 5.9 (5.5, 6.3) | 0.52 |
| Questionnaires | Entire cohort N = 214 | iCKD N = 107 | Non-iCKD N = 107 | p-Value |
|---|---|---|---|---|
| Disease knowledge | ||||
| Knowledge of medications that help the kidney | 2.3 ± 1.0 | 2.4 ± 1.0 | 2.2 ± 0.9 | 0.26 |
| Knowledge of medications that can hurt the kidney | 2.5 ± 0.9 | 2.6 ± 0.9 | 2.4 ± 0.9 | 0.18 |
| Knowledge of foods to avoid if kidney function is low | 2.9 ± 0.8 | 2.9 ± 0.8 | 2.8 ± 0.8 | 0.60 |
| Knowledge of blood pressure goal | 2.9 ± 0.8 | 3.1 ± 0.8 | 2.7 ± 0.7 | 0.001 |
| Knowledge of treatment options if kidney function gets worse | 2.4 ± 1.0 | 2.5 ± 1.0 | 2.3 ± 0.9 | 0.04 |
| Knowledge of symptoms of chronic kidney disease | 2.3 ± 0.9 | 2.6 ± 0.9 | 2.1 ± 0.9 | 0.001 |
| Knowledge of how kidney function is checked | 2.7 ± 0.9 | 2.8 ± 1.0 | 2.6 ± 0.8 | 0.31 |
| Knowledge of functions of the kidney | 2.6 ± 0.9 | 2.7 ± 0.9 | 2.5 ± 0.8 | 0.01 |
| Knowledge of why patient was sent to a kidney doctor | 3.0 ± 0.8 | 3.1 ± 0.9 | 2.9 ± 0.7 | 0.25 |
| Self-care behavior | ||||
| Diet | 14.8 ± 3.6 | 14.7 ± 3.5 | 14.9 ± 3.8 | 0.76 |
| Exercise | 10.2 ± 4.1 | 10.2 ± 3.9 | 10.1 ± 4.3 | 0.95 |
| Blood pressure monitoring | 6.9 ± 2.6 | 7.4 ± 2.4 | 6.4 ± 2.7 | 0.005 |
| Smoking habits | 9.0 ± 2.2 | 8.8 ± 2.4 | 9.2 ± 2.0 | 0.21 |
| Medication adherence | 23.4 ± 2.7 | 23.2 ± 2.6 | 2.3.5 ± 2.9 | 0.44 |
| Disease Knowledge Score | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| β(95% Cl) | p-Value | β(95% Cl) | p-Value | |
| Clinical characteristics | ||||
| Age (per year) | −0.15(−0.22, −0.08) | <0.001 | −0.09(−0.17, −0.01) | 0.03 |
| Sex (female vs. male) | −0.81(−2.47, 0.85) | 0.34 | −0.00(−1.68, 1.68) | 0.99 |
| Smoke (yes vs. no) | −1.72(−3.57, 0.14) | 0.07 | -- | -- |
| Alcohol (yes vs. no) | 0.32(−2.32, 2.96) | 0.81 | -- | -- |
| Married status (yes vs. no) | 3.02(1.06, 4.98) | 0.003 | 1.71(−0.24, 3.67) | 0.09 |
| Current working (yes vs. no) | 2.12(0.46, 3.78) | 0.01 | 0.18(−1.65, 2.01) | 0.85 |
| Education (senior high school or above vs. below senior high school) | 4.69(3.08, 6.30) | <0.001 | 3.22(1.46, 4.98) | <0.001 |
| Hypertension (yes vs. no) | −0.43(−2.64, 1.78) | 0.70 | -- | -- |
| Diabetes mellitus (yes vs. no) | −1.61(−3.33, 0.10) | 0.07 | -- | -- |
| Heart disease (yes vs. no) | 0.48(−1.98, 2.93) | 0.70 | -- | -- |
| Body mass index (per kg/m2) | −0.12(−0.32, 0.08) | 0.23 | -- | -- |
| Health education time (per session) | 0.06(−0.02, 0.13) | 0.13 | -- | -- |
| CKD duration (per year) | 0.11(0.01, 0.21) | 0.03 | 0.11(0.02, 0.20) | 0.02 |
| iCKD usage (yes vs. no) | 1.95(0.34, 3.56) | 0.02 | 1.78(0.30, 3.27) | 0.02 |
| Blood urea nitrogen (per mg/dL) | 0.03(−0.01, 0.07) | 0.15 | -- | -- |
| eGFR (per ml/min/1.73 m2) | −0.01(−0.05, 0.02) | 0.44 | -- | -- |
| Log-formed glycated hemoglobin | −1.05(−1.89, −0.22) | 0.01 | −0.90(−1.66, −0.13) | 0.02 |
| Hemoglobin (per g/dL) | −0.08(−0.46, 0.29) | 0.66 | -- | -- |
| Albumin (per g/dL) | 1.25(−0.99, 3.49) | 0.27 | -- | -- |
| Uric acid (per mg/dL) | −0.25(−0.78, 0.28) | 0.36 | -- | -- |
| Cholesterol (per mg/dL) | 0.01(−0.01, 0.03) | 0.27 | -- | -- |
| Log-formed triglyceride | −3.22(−6.78, 0.34) | 0.08 | -- | -- |
| Log-formed urine protein/creatinine ratio | −0.21(−1.64, 1.21) | 0.77 | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-C.; Hsiao, P.-N.; Kuo, M.-C.; Wang, S.-L.; Chen, T.-H.; Kung, L.-F.; Hsiao, S.-M.; Lin, M.-Y.; Hwang, S.-J.; Chen, H.-C.; et al. Mobile Health, Disease Knowledge, and Self-Care Behavior in Chronic Kidney Disease: A Prospective Cohort Study. J. Pers. Med. 2021, 11, 845. https://doi.org/10.3390/jpm11090845
Tsai Y-C, Hsiao P-N, Kuo M-C, Wang S-L, Chen T-H, Kung L-F, Hsiao S-M, Lin M-Y, Hwang S-J, Chen H-C, et al. Mobile Health, Disease Knowledge, and Self-Care Behavior in Chronic Kidney Disease: A Prospective Cohort Study. Journal of Personalized Medicine. 2021; 11(9):845. https://doi.org/10.3390/jpm11090845
Chicago/Turabian StyleTsai, Yi-Chun, Pei-Ni Hsiao, Mei-Chuan Kuo, Shu-Li Wang, Tzu-Hui Chen, Lan-Fang Kung, Shih-Ming Hsiao, Ming-Yen Lin, Shang-Jyh Hwang, Hung-Chun Chen, and et al. 2021. "Mobile Health, Disease Knowledge, and Self-Care Behavior in Chronic Kidney Disease: A Prospective Cohort Study" Journal of Personalized Medicine 11, no. 9: 845. https://doi.org/10.3390/jpm11090845








