Prognostic Value of Circadian Rhythm of Brain Temperature in Traumatic Brain Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Medical Procedure and Assessments
2.3. Population
2.4. Brain Temperature Analysis
2.5. Measurement of Light Intensity in an ICU
2.6. Statistical Analyses
3. Results
3.1. Demographic Data
3.2. Postoperative Outcome
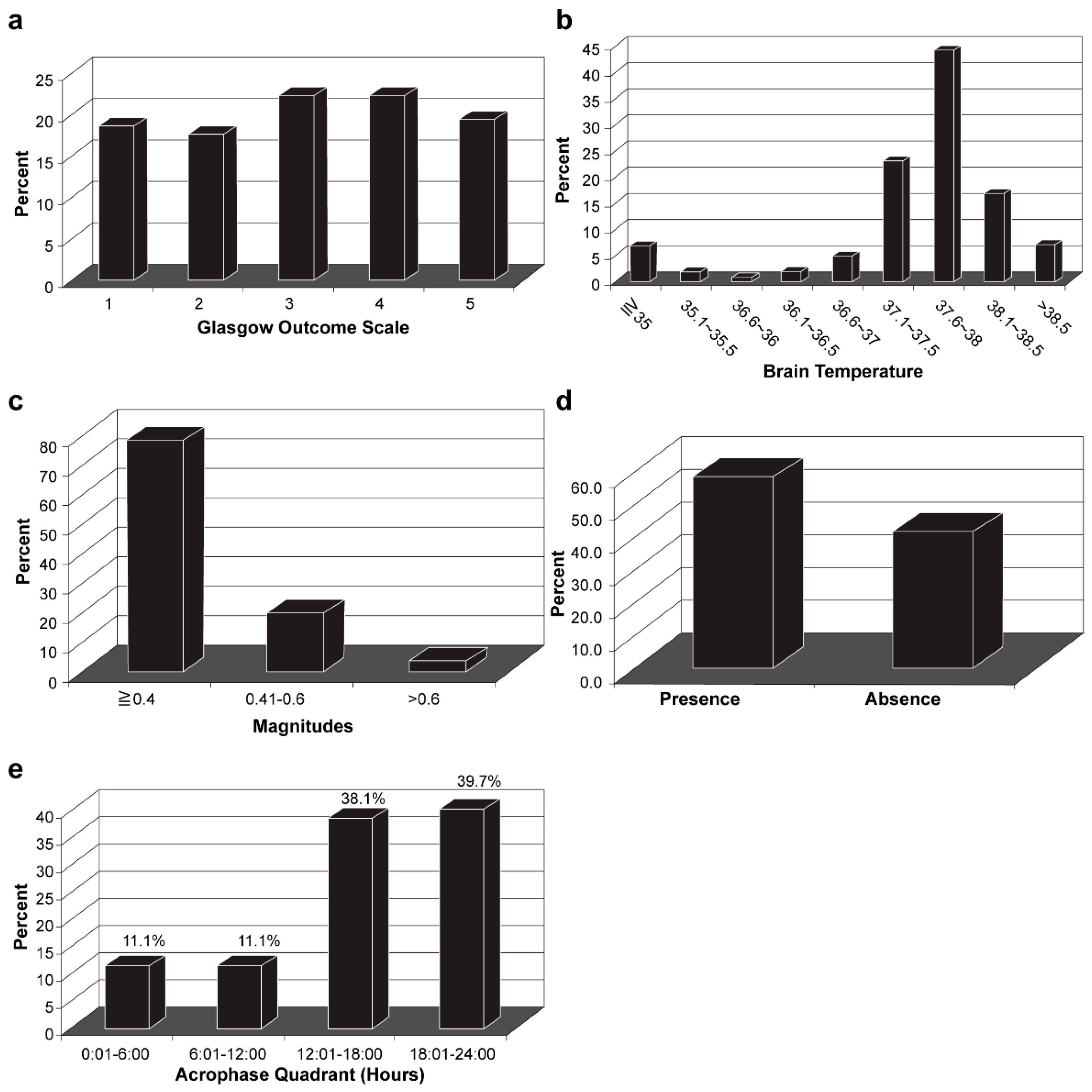
3.3. Characteristics of Brain Temperature Analysis
3.4. Analysis of Predictors for Postoperative Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lingsma, H.F.; Roozenbeek, B.; Steyerberg, E.W.; Murray, G.D.; Maas, A.I. Early prognosis in traumatic brain injury: From prophecies to predictions. Lancet Neurol. 2010, 9, 543–554. [Google Scholar] [CrossRef]
- Rauen, K.; Reichelt, L.; Probst, P.; Schäpers, B.; Müller, F.; Jahn, K.; Plesnila, N. Decompressive craniectomy is associated with good quality of life up to 10 years after rehabilitation from traumatic brain injury. Crit. Care Med. 2020, 48, 1157–1164. [Google Scholar] [CrossRef]
- Haghbayan, H.; Boutin, A.; Laflamme, M.; Lauzier, F.; Shemilt, M.; Moore, L.; Zarychanski, R.; Douville, V.; Fergusson, D.; Turgeon, A.F. The prognostic value of MRI in moderate and severe traumatic brain injury: A systematic review and meta-analysis. Crit. Care Med. 2017, 45, e1280–e1288. [Google Scholar] [CrossRef]
- Shemilt, M.; Boutin, A.; Lauzier, F.; Zarychanski, R.; Moore, L.; McIntyre, L.A.; Nadeau, L.; Fergusson, D.A.; Mercier, E.; Archambault, P.; et al. Prognostic value of glial fibrillary acidic protein in patients with moderate and severe traumatic brain injury: A systematic review and meta-analysis. Crit. Care Med. 2019, 47, e522–e529. [Google Scholar] [CrossRef]
- Lee, H.; Mizrahi, M.A.; Hartings, J.A.; Sharma, S.; Pahren, L.; Ngwenya, L.B.; Moseley, B.D.; Privitera, M.; Tortella, F.C.; Foreman, B. Continuous electroencephalography after moderate to severe traumatic brain injury. Crit. Care Med. 2019, 47, 574–582. [Google Scholar] [CrossRef]
- Attia, J.; Cook, D.J. Prognosis in anoxic and traumatic coma. Crit. Care Clin. 1998, 14, 497–511. [Google Scholar] [CrossRef]
- Nybo, L.; Secher, N.H.; Nielsen, B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J. Physiol. 2002, 545, 697–704. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Normoyle, K.P.; Jackson, K.; Spitler, K.; Sharrock, M.F.; Miller, C.M.; Best, C.; Llano, D.; Du, R. Brain temperature and its fundamental properties: A review for clinical neuroscientists. Front. Neurosci. 2014, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Yablonskiy, D.A.; Ackerman, J.J.; Raichle, M.E. Coupling between changes in human brain temperature and oxidative metabolism during prolonged visual stimulation. Proc. Natl. Acad. Sci. USA 2000, 97, 7603–7608. [Google Scholar] [CrossRef] [Green Version]
- Childs, C.; Lunn, K.W. Clinical review: Brain-body temperature differences in adults with severe traumatic brain injury. Crit. Care 2013, 17, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, P.J.; Sinclair, H.L.; Rodriguez, A.; Harris, B.A.; Battison, C.G.; Rhodes, J.K.; Murray, G.D.; Eurotherm Trial, C. Hypothermia for Intracranial Hypertension after Traumatic Brain Injury. N. Engl. J. Med. 2015, 373, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Crompton, E.M.; Lubomirova, I.; Cotlarciuc, I.; Han, T.S.; Sharma, S.D.; Sharma, P. Meta-Analysis of Therapeutic Hypothermia for Traumatic Brain Injury in Adult and Pediatric Patients. Crit. Care Med. 2017, 45, 575–583. [Google Scholar] [CrossRef]
- Dietrich, W.D.; Bramlett, H.M. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics 2010, 7, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wu, L.; Wu, X.; Li, B.; Huang, W.; Weng, Z.; Lin, Z.; Song, L.; Guo, Y.; Meng, Z.; et al. Identification of Candidate Growth-Related SNPs and Genes Using GWAS in Brown-Marbled Grouper (Epinephelus fuscoguttatus). Mar. Biotechnol. (NY) 2020, 22, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Ding, Y.; Liu, Y.; Li, Y.; Liu, Y.; Wang, Z. Circadian effects on outcome following surgery for intracerebral hemorrhage in humans? Brain Res. 2009, 1258, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Addis, A.; Gaasch, M.; Schiefecker, A.J.; Kofler, M.; Ianosi, B.; Rass, V.; Lindner, A.; Broessner, G.; Beer, R.; Pfausler, B.; et al. Brain temperature regulation in poor-grade subarachnoid hemorrhage patients-A multimodal neuromonitoring study. J. Cereb. Blood Flow Metab. 2021, 41, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duclos, C.; Dumont, M.; Paquet, J.; Blais, H.; Van der Maren, S.; Menon, D.K.; Bernard, F.; Gosselin, N. Sleep-wake disturbances in hospitalized patients with traumatic brain injury: Association with brain trauma but not with an abnormal melatonin circadian rhythm. Sleep 2020, 43, zsz191. [Google Scholar] [CrossRef]
- Halberg, F. Chronobiology. Annu. Rev. Physiol. 1969, 31, 675–725. [Google Scholar] [CrossRef]
- Melhem, S.; Shutter, L.; Kaynar, A. A trial of intracranial pressure monitoring in traumatic brain injury. Crit. Care 2014, 18, 302. [Google Scholar] [CrossRef] [Green Version]
- Forsyth, R.J.; Raper, J.; Todhunter, E. Routine intracranial pressure monitoring in acute coma. Cochrane Database Syst. Rev. 2015, 11, CD002043. [Google Scholar] [CrossRef]
- Chesnut, R.M.; Temkin, N.; Carney, N.; Dikmen, S.; Rondina, C.; Videtta, W.; Petroni, G.; Lujan, S.; Pridgeon, J.; Barber, J.; et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N. Engl. J. Med. 2012, 367, 2471–2481. [Google Scholar] [CrossRef] [Green Version]
- Childs, C.; Vail, A.; Leach, P.; Rainey, T.; Protheroe, R.; King, A. Brain temperature and outcome after severe traumatic brain injury. Neurocrit. Care 2006, 5, 10–14. [Google Scholar] [CrossRef]
- Soukup, J.; Zauner, A.; Doppenberg, E.M.; Menzel, M.; Gilman, C.; Young, H.F.; Bullock, R. The importance of brain temperature in patients after severe head injury: Relationship to intracranial pressure, cerebral perfusion pressure, cerebral blood flow, and outcome. J. Neurotrauma 2002, 19, 559–571. [Google Scholar] [CrossRef]
- Mrozek, S.; Vardon, F.; Geeraerts, T. Brain temperature: Physiology and pathophysiology after brain injury. Anesthesiol. Res. Pract. 2012, 2012, 989487. [Google Scholar] [CrossRef]
- Goodman, J.C.; Valadka, A.B.; Gopinath, S.P.; Uzura, M.; Robertson, C.S. Extracellular lactate and glucose alterations in the brain after head injury measured by microdialysis. Crit. Care Med. 1999, 27, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Marion, D.W.; Darby, J.; Yonas, H. Acute regional cerebral blood flow changes caused by severe head injuries. J. Neurosurg. 1991, 74, 407–414. [Google Scholar] [CrossRef]
- Goss, J.R.; Styren, S.D.; Miller, P.D.; Kochanek, P.M.; Palmer, A.M.; Marion, D.W.; DeKosky, S.T. Hypothermia attenuates the normal increase in interleukin 1β RNA and nerve growth factor following traumatic brain injury in the rat. J. Neurotrauma 1995, 12, 159–167. [Google Scholar] [CrossRef]
- Rossi, S.; Zanier, E.R.; Mauri, I.; Columbo, A.; Stocchetti, N. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J. Neurol. Neurosurg. Psychiatry 2001, 71, 448–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fountas, K.N.; Kapsalaki, E.Z.; Feltes, C.H.; Smisson, H.F., 3rd; Johnston, K.W.; Grigorian, A.; Robinson, J.S., Jr. Disassociation between intracranial and systemic temperatures as an early sign of brain death. J. Neurosurg. Anesthesiol. 2003, 15, 87–89. [Google Scholar] [CrossRef]
- Thorley, R.R.; Wertsch, J.J.; Klingbeil, G.E. Acute hypothalamic instability in traumatic brain injury: A case report. Arch. Phys. Med. Rehabil. 2001, 82, 246–249. [Google Scholar] [CrossRef] [PubMed]
- De Tanti, A.; Gasperini, G.; Rossini, M. Paroxysmal episodic hypothalamic instability with hypothermia after traumatic brain injury. Brain Inj. 2005, 19, 1277–1283. [Google Scholar] [CrossRef]
- Pizza, F.; Vetrugno, R.; Antelmi, E.; Pierangeli, G.; Montagna, P.; Cortelli, P. Narcoleptic-like hypersomnia and inverted circadian rhythm of body core temperature after traumatic brain injury involving the hypothalamus. Sleep Med. 2011, 12, 1044–1045. [Google Scholar] [CrossRef]
- Ayalon, L.; Borodkin, K.; Dishon, L.; Kanety, H.; Dagan, Y. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology 2007, 68, 1136–1140. [Google Scholar] [CrossRef]
- Tan, H.; Yang, W.; Wu, C.; Liu, B.; Lu, H.; Wang, H.; Yan, H. Assessment of the role of intracranial hypertension and stress on hippocampal cell apoptosis and hypothalamic-pituitary dysfunction after TBI. Sci. Rep. 2017, 7, 3805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamouzis, I.; Pagano, L.; Prodam, F.; Mele, C.; Zavattaro, M.; Busti, A.; Marzullo, P.; Aimaretti, G. Clinical and diagnostic approach to patients with hypopituitarism due to traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), and ischemic stroke (IS). Endocrine 2016, 52, 441–450. [Google Scholar] [CrossRef]
- Sav, A.; Rotondo, F.; Syro, L.V.; Serna, C.A.; Kovacs, K. Pituitary pathology in traumatic brain injury: A review. Pituitary 2019, 22, 201–211. [Google Scholar] [CrossRef]
- Crompton, M.R. Hypothalamic lesions following closed head injury. Brain 1971, 94, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleason, J.D.; Oishi, M.M.K.; Wen, J.T.; Julius, A.; Pappu, S.; Yonas, H. Assessing circadian rhythms and entrainment via intracranial temperature after severe head trauma. Biomed. Signal Process. Control 2019, 54. [Google Scholar] [CrossRef]
- Rzechorzek, N.M.; Thrippleton, M.J.; Chappell, F.M.; Mair, G.; Ercole, A.; Cabeleira, M.; Rhodes, J.; Marshall, I.; O’Neill, J.S. Diurnal brain temperature rhythms and mortality after brain injury: A prospective and retrospective cohort study. medRxiv 2021. [Google Scholar] [CrossRef]
- Killgore, W.D.S.; Vanuk, J.R.; Shane, B.R.; Weber, M.; Bajaj, S. A randomized, double-blind, placebo-controlled trial of blue wavelength light exposure on sleep and recovery of brain structure, function, and cognition following mild traumatic brain injury. Neurobiol. Dis. 2020, 134, 104679. [Google Scholar] [CrossRef]
- Zalai, D.M.; Girard, T.A.; Cusimano, M.D.; Shapiro, C.M. Circadian rhythm in the assessment of postconcussion insomnia: A cross-sectional observational study. CMAJ Open 2020, 8, E142–E147. [Google Scholar] [CrossRef] [Green Version]
- Gubin, D.G.; Weinert, D.; Bolotnova, T.V. Age-dependent changes of the temporal order--Causes and treatment. Curr. Aging Sci. 2016, 9, 14–25. [Google Scholar] [CrossRef]
- Weinert, D. Ontogenetic development of the mammalian circadian system. Chronobiol. Int. 2005, 22, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Dijkland, S.A.; Foks, K.A.; Polinder, S.; Dippel, D.W.J.; Maas, A.I.R.; Lingsma, H.F.; Steyerberg, E.W. Prognosis in Moderate and Severe Traumatic Brain Injury: A Systematic Review of Contemporary Models and Validation Studies. J. Neurotrauma 2020, 37, 1–13. [Google Scholar] [CrossRef]
- Kehoe, A.; Rennie, S.; Smith, J.E. Glasgow Coma Scale is unreliable for the prediction of severe head injury in elderly trauma patients. Emerg. Med. J. 2015, 32, 613–615. [Google Scholar] [CrossRef]
- Livingston, D.H.; Mosenthal, A.C. Withdrawing life-sustaining therapy for patients with severe traumatic brain injury. CMAJ 2011, 183, 1570–1571. [Google Scholar] [CrossRef] [Green Version]

| Parameters | Results (n = 108) |
|---|---|
| Male, n (%) | 74 (68.5) |
| Age, years | |
| Mean (SD) | 52.3 (20.7) |
| Median | 52 |
| Range | 18–80 |
| Diabetes mellitus, n (%) | 17 (15.7) |
| Hypertension, n (%) | 26 (24.1) |
| Diagnosis, n (%) | |
| Subdural hematoma | 57 (52.8) |
| Epidural hematoma | 15 (13.9) |
| Contusion | 15 (13.9) |
| Others, including two or more | 21 (19.4) |
| Initial Glasgow Coma Scale score | |
| Mean (SD) | 6.9 (2.8) |
| Median | 7 |
| Range | 3–10 |
| ICP (24 h) | |
| Mean (SD) | 16.4 (14.8) |
| CCP (24 h) | |
| Mean (SD) | 78.5 (15.0) |
| 12-month Glasgow Outcome Scale (%) | |
| 1 | 20 (18.5) |
| 2 | 19 (17.6) |
| 3 | 24 (22.2) |
| 4 | 23 (21.3) |
| 5 | 22 (20.4) |
| Mean (SD) | 3.1 (1.4) |
| Unfavorable 1 n = 63 | Favorable 1 n = 45 | p a | Dead 2 n = 20 | Alive 2 n = 88 | p a | |
|---|---|---|---|---|---|---|
| Male, n (%) | 37 (58.7) | 37 (82.2) | <0.01 | 13 (65.0) | 61 (69.3) | 0.71 |
| Age (SD) | 60.0 (17.9) | 41.5 (19.4) | <0.01 | 65.2 (15.8) | 49.4 (20.5) | <0.01 |
| DM, n (%) | 14 (22.2) | 3 (6.7) | <0.05 | 4 (20.0) | 13 (14.8) | 0.56 |
| HTN, n (%) | 20 (31.7) | 6 (13.3) | <0.05 | 5 (25.0) | 21 (23.9) | 0.92 |
| GCS | 6.6 (2.5) | 8.7 (2.7) | <0.01 | 6.2 (2.8) | 7.7 (2.8) | <0.05 |
| WBC > 10,000/μL, n (%) | 42 (66.7) | 36 (80.0) | 0.13 | 15 (75.0) | 63 (71.6) | 0.76 |
| Glucose > 120 mg/dL, n (%) | 53 (84.1) | 25 (55.6) | <0.01 | 16 (80.0) | 62 (70.5) | 0.39 |
| ICP (24 h) > 20 mmHg, n (%) | 20 (31.7) | 11 (24.4) | 0.41 | 12 (60.0) | 19 (21.6) | <0.01 |
| CPP (24 h), (SD) | 77.1 (17.2) | 80.5 (11.3) | 0.24 | 63.0 (19.0) | 82.1 (11.4) | <0.01 |
| Mesor (SD) | 37.2 (1.5) | 37.7 (0.5) | <0.05 | 36.0 (2.2) | 37.7 (0.5) | <0.01 |
| Mesor 50%, n (%) | 26 (41.3) | 28 (62.2) | <0.05 | 4 (20.0) | 50 (56.8) | <0.01 |
| Intact rhythm of brain Temperature, n (%) | 30 (47.6) | 33 (73.3) | <0.01 | 8 (40.0) | 55 (62.5) | 0.07 |
| Unfavorable 1 | Favorable 1 | Alive 2 | ||||
|---|---|---|---|---|---|---|
| Male n = 37 | Female n = 26 | Male n = 37 | Female n = 8 | Male n = 61 | Female n = 27 | |
| Age (SD) | - | - | - | - | 45.7 (20.2) | 57.7 (19.0) ** |
| DM, n (%) | 5 (13.5) | 9 (34.6) * | - | - | 6 (9.8) | 7 (25.9) * |
| Glucose > 120 mg/dL, n (%) | - | - | 18 (48.6) | 7 (87.5) * | 38 (62.3) | 24 (88.9) * |
| ICP (24 h) > 20 mmHg, n (%) | - | - | - | - | 18 (29.5) | 1 (3.7) ** |
| Mesor mid 50%, n (%) | - | - | 20 (54.1) | 8 (100.0) * | - | - |
| Functional Outcome 1 (Favorable/Unfavorable) | Survival Rate 2 (Alive/Dead) | |||
|---|---|---|---|---|
| Coefficient | OR (95% CI) | Coefficient | OR (95% CI) | |
| Age | −0.7 ** | 0.94 (0.90–0.97) | −0.04* | 0.96 (0.93–1.00) |
| GCS | 0.43 ** | 1.53 (1.21–1.93) | ||
| Glucose > 120 mg/dL | −1.57 * | 0.21 (0.06–0.76) | ||
| ICP (24 h) > 20 mmHg | −0.98 | 0.38 (0.07–2.10) | ||
| CPP (24 h) | 0.03 | 1.21 (0.97–1.09) | ||
| Mesor 50% | 1.56 ** | 4.77(1.40–16.21) | ||
| Rhythm of Brain temperature | 1.67 ** | 5.28 (1.61–17.64) | 0.73 | 2.07 (0.70–6.13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, L.-T.; Lu, H.-Y.; Huang, A.P.-H. Prognostic Value of Circadian Rhythm of Brain Temperature in Traumatic Brain Injury. J. Pers. Med. 2021, 11, 620. https://doi.org/10.3390/jpm11070620
Kuo L-T, Lu H-Y, Huang AP-H. Prognostic Value of Circadian Rhythm of Brain Temperature in Traumatic Brain Injury. Journal of Personalized Medicine. 2021; 11(7):620. https://doi.org/10.3390/jpm11070620
Chicago/Turabian StyleKuo, Lu-Ting, Hsueh-Yi Lu, and Abel Po-Hao Huang. 2021. "Prognostic Value of Circadian Rhythm of Brain Temperature in Traumatic Brain Injury" Journal of Personalized Medicine 11, no. 7: 620. https://doi.org/10.3390/jpm11070620






