Airway Wall Remodeling in Childhood Asthma—A Personalized Perspective from Cell Type-Specific Biology
Abstract
:1. Introduction
2. Early Events Leading to Asthma and Airway Wall Remodeling in Children
3. The Maturation of the Epithelium and the Basement Membrane in Childhood and Its Link to Asthma
4. Sub-Epithelial Mesenchymal Cells and Their Role in Airway Wall Remodeling
5. The Epithelium as the Central Regulator of the Airway Wall
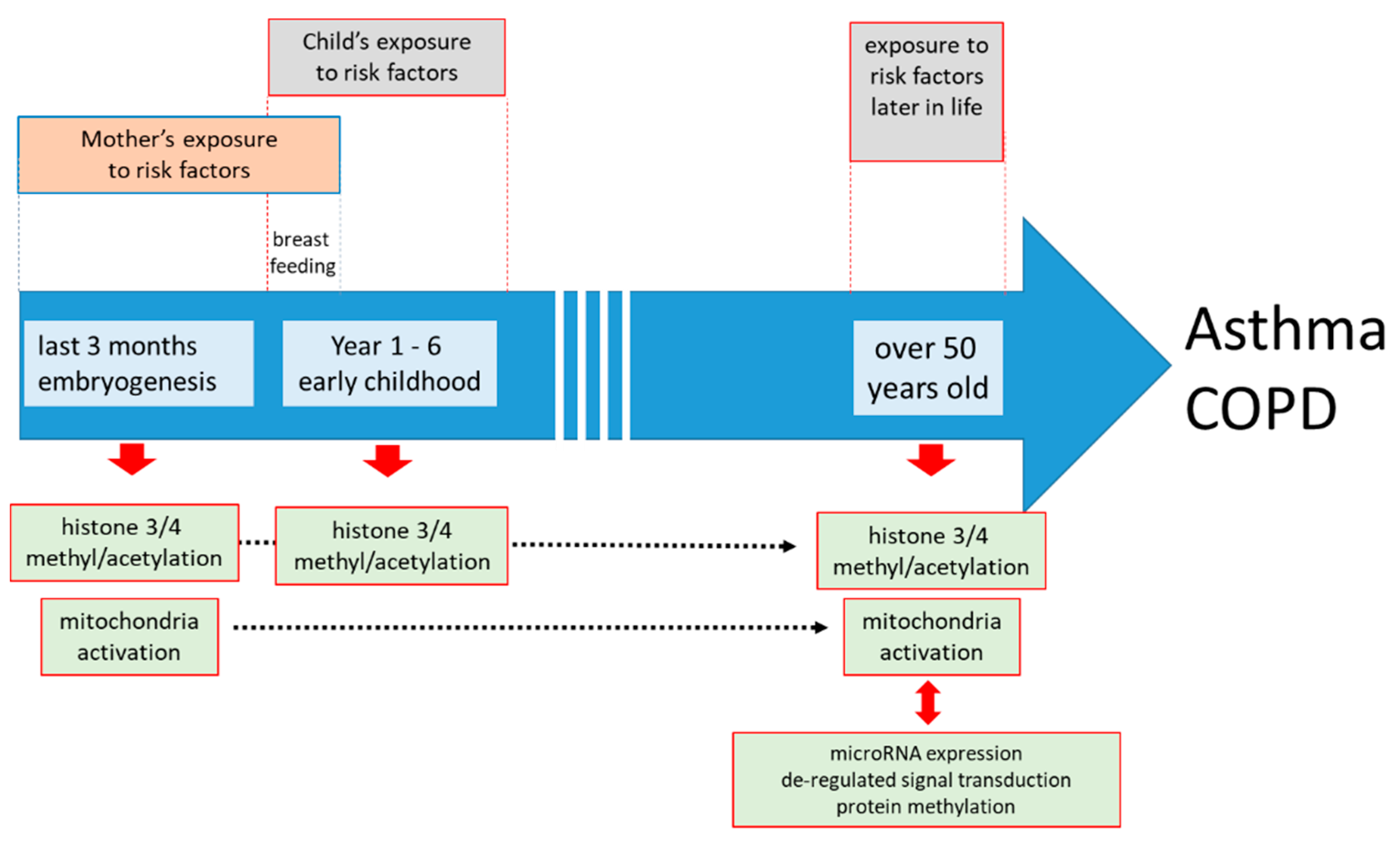
6. Parental Asthma, the, Environment, Epigenetics, and the Epithelium
7. The Difficulty to Study Airway Wall Remodeling, Particularly in Childhood Asthma
8. Conclusions
- (1)
- It must be noted that a large range of environmental asthma risk factors such as cigarette smoke, fine dust, allergens, viruses, bacteria, etc., will initiate a protective response of the airway against these inhaled irritants. The available data suggest that this protective response of the airway wall is very similar, regardless of the nature of the trigger. However, future large cohort studies need to provide more evidence if specific types of tissue structural changes are unique for specific asthma endo- or phenotypes.
- (2)
- There is evidence that pattern recognition receptors such as TLRs could explain how a wide range of different risk factors from the environment initiates airway wall remodeling during embryogenesis and early childhood. In adult asthma, some studies indicated that damage-associated molecular patterns and pathogens-associated molecular patterns play a role in tissue remodeling; however, this was so far only associated with age-related asthma [45]. It has not been investigated if these mechanisms might be active during embryogenesis and early childhood.
- (3)
- Furthermore, it remains unknown why, for some people, this protective response does not shut down after the trigger is gone, and further leads to airway wall remodeling.
- (4)
- Many cellular pathologies of airway wall remodeling in asthma are maintained in isolated cells; hence, indicating that the underlying mechanisms became persistent. Furthermore, these cell type specific pathologies of airway wall remodeling can be initiated by the above-named environmental asthma risk factors and the pattern recognition proteins through epigenetic events, including microRNA expression, DNA, and protein methylation/de-methylation.
- (5)
- The epigenetic events can be passed over at least three generations, but the mechanism underlying this “inheritability” is unknown. Importantly, this “epigenetic inheritance” of the asthma predisposition might mimic real inheritance of susceptibility genes, which needs to be investigated.
- (6)
- A major problem in detecting airway wall remodeling in childhood asthma is the lack of clear markers without obtaining tissue biopsies. This lack of information on the structural changes of the airways at early stages of asthma makes it difficult to correlate asthma pheno- and endo-types with specific aspects of remodeling.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hough, K.P.; Curtiss, M.L.; Blain, T.J.; Liu, R.M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway Remodeling in Asthma. Front. Med. 2020, 7, 191. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, M.; Ou, Y.; Adcock, I.M.; Yao, X. Mechanisms and biomarkers of airway epithelial cell damage in asthma: A review. Clin. Respir. J. 2021, 15, 1027–1045. [Google Scholar] [CrossRef]
- Donovan, G.M.; Langton, D.; Noble, P.B. Phenotype- and patient-specific modelling in asthma: Bronchial thermoplasty and uncertainty quantification. J. Theor. Biol. 2020, 501, 110337. [Google Scholar] [CrossRef] [PubMed]
- Kardas, G.; Kuna, P.; Panek, M. Biological Therapies of Severe Asthma and Their Possible Effects on Airway Remodeling. Front. Immunol. 2020, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.S.; Quirk, J.D.; Goss, C.W.; Lew, D.; Kozlowski, J.; Thomen, R.P.; Woods, J.C.; Tustison, N.J.; Mugler, J.P., 3rd; Gallagher, L.; et al. Single-Session Bronchial Thermoplasty Guided by 129Xe Magnetic Resonance Imaging. A Pilot Randomized Controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2020, 202, 524–534. [Google Scholar] [CrossRef]
- Prakash, Y.S.; Halayko, A.J.; Gosens, R.; Panettieri, R.A., Jr.; Camoretti-Mercado, B.; Penn, R.B. ATS Assembly on Respiratory Structure and Function. An Official American Thoracic Society Research Statement: Current Challenges Facing Research and Therapeutic Advances in Airway Remodeling. Am. J. Respir. Crit. Care Med. 2017, 195, e4–e19. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Eckert, E.; James, A.; Meier-Girard, D.; Kupczyk, M.; Andersson, L.I.; Bossios, A.; Mikus, M.; Ono, J.; Izuhara, K.; Middelveld, R.; et al. Lung function fluctuation patterns unveil asthma and COPD phenotypes unrelated to type 2 inflammation. J. Allergy Clin. Immunol. 2021, 148, 407–419. [Google Scholar] [CrossRef]
- Allen, J.L. Airway function throughout the lifespan: Pediatric origins of adult respiratory disease. Pediatr. Investig. 2019, 3, 236–244. [Google Scholar] [CrossRef]
- Pascoe, C.D.; Green, F.H.Y.; Elliot, J.G.; James, A.L.; Noble, P.B.; Donovan, G.M. Airway remodelling with spatial correlations: Implications for asthma pathogenesis. Respir. Physiol. Neurobiol. 2020, 279, 103469. [Google Scholar] [CrossRef]
- Malmström, K.; Lohi, J.; Malmberg, L.P.; Kotaniemi-Syrjänen, A.; Lindahl, H.; Sarna, S.; Pelkonen, A.S.; Mäkelä, M.J. Airway hyperresponsiveness, remodeling and inflammation in infants with wheeze. Clin. Exp. Allergy 2020, 50, 558–566. [Google Scholar] [CrossRef]
- Castro-Rodriguez, J.A.; Saglani, S.; Rodriguez-Martinez, C.E.; Oyarzun, M.A.; Fleming, L.; Bush, A. The relationship between inflammation and remodeling in childhood asthma: A systematic review. Pediatr. Pulmonol. 2018, 53, 824–835. [Google Scholar] [CrossRef]
- Thurston, G.D.; Balmes, J.R.; Garcia, E.; Gilliland, F.D.; Rice, M.B.; Schikowski, T.; Van Winkle, L.S.; Annesi-Maesano, I.; Burchard, E.G.; Carlsten, C.; et al. Outdoor Air Pollution and New-Onset Airway Disease. An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2020, 17, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Child, F.; Lenney, W.; Clayton, S.; Davies, S.; Jones, P.W.; Strange, R.C.; Fryer, A.A. Correction of bronchial challenge data for age and size may affect the results of genetic association studies in children. Pediatr. Allergy Immunol. 2003, 14, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, I.A.; Wilson, S.J.; Davies, D.E.; Holgate, S.T. Epithelial stress and structural remodelling in childhood asthma. Thorax 2005, 60, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Plopper, C.G.; Smiley-Jewell, S.M.; Miller, L.A.; Fanucchi, M.V.; Evans, M.J.; Buckpitt, A.R.; Avdalovic, M.; Gershwin, L.J.; Joad, J.P.; Kajekar, R.; et al. Asthma/allergic airways disease: Does postnatal exposure to environmental toxicants promote airway pathobiology? Toxicol. Pathol. 2007, 35, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Tsartsali, L.; Hislop, A.A.; McKay, K.; James, A.L.; Elliot, J.; Zhu, J.; Rosenthal, M.; Payne, D.N.; Jeffery, P.K.; Bush, A.; et al. Development of the bronchial epithelial reticular basement membrane: Relationship to epithelial height and age. Thorax 2011, 66, 280–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebold, K.M.; Jacoby, D.B.; Drake, M.G. Inflammatory mechanisms linking maternal and childhood asthma. J. Leukoc. Biol. 2020, 108, 113–121. [Google Scholar] [CrossRef]
- Ntontsi, P.; Photiades, A.; Zervas, E.; Xanthou, G.; Samitas, K. Genetics and Epigenetics in Asthma. Int. J. Mol. Sci. 2021, 22, 2412. [Google Scholar] [CrossRef] [PubMed]
- Veres, T.Z.; Rochlitzer, S.; Braun, A. The role of neuro-immune cross-talk in the regulation of inflammation and remodelling in asthma. Pharmacol. Ther. 2009, 122, 203–214. [Google Scholar] [CrossRef]
- Guerra, S.; Lombardi, E.; Stern, D.A.; Sherrill, D.L.; Gilbertson-Dahdal, D.; Wheatley-Guy, C.M.; Snyder, E.M.; Wright, A.L.; Martinez, F.D.; Morgan, W.J. Fetal Origins of Asthma: A Longitudinal Study from Birth to Age 36 Years. Am. J. Respir. Crit. Care Med. 2020, 202, 1646–1655. [Google Scholar] [CrossRef]
- Lebold, K.M.; Drake, M.G.; Hales-Beck, L.B.; Fryer, A.D.; Jacoby, D.B. IL-5 Exposure in Utero Increases Lung Nerve Density and Airway Reactivity in Adult Offspring. Am. J. Respir. Cell Mol. Biol. 2020, 62, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Mattes, J.; Collison, A. Fetal Eosinophils Get on the Nerves of Airways. Early Origins of Bronchoconstriction. Am. J. Respir. Cell Mol. Biol. 2020, 62, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.N.; Cheng, F.J.; Tsai, M.T.; Tsai, C.M.; Chuang, P.C.; Cheng, C.Y. Fine particulate matter constituents associated with emergency room visits for pediatric asthma: A time-stratified case-crossover study in an urban area. BMC Public Health 2021, 21, 1593. [Google Scholar] [CrossRef]
- Yeh, K.W.; Chen, C.T.; Lee, P.C.; Huang, J.L.; Yan, D.C.; Chen, L.C.; Lin, S.J.; Yao, T.C.; Wu, C.D.; Wan, G.H. Outdoor air pollutants exposure associated with pulmonary function and EBC pH value in atopic asthmatic and non-asthmatic children. J. Asthma 2021, 58, 1278–1284. [Google Scholar] [CrossRef]
- Yen, Y.C.; Yang, C.Y.; Wang, T.N.; Yen, P.C.; Ho, C.K.; Mena, K.D.; Lee, T.C.; Chen, K.S.; Lin, Y.C.; Chen, P.S. Household airborne endotoxin associated with asthma and allergy in elementary school-age children: A case-control study in Kaohsiung, Taiwan. Environ. Sci. Pollut. Res. Int. 2020, 27, 19502–19509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonato, M.; Gallo, E.; Bazzan, E.; Marson, G.; Zagolin, L.; Cosio, M.G.; Barbato, A.; Saetta, M.; Gregori, D.; Baraldo, S. Air Pollution Relates to Airway Pathology in Wheezing Children. Ann. Am. Thorac. Soc. 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/34004126/ (accessed on 31 August 2021). [CrossRef]
- Saif, N.T.; Kleiner, G.I.; Forster, L.Q.; Hershorin, E.R.; Colin, A.A.; Mirsaeidi, M.; Kumar, N. Allergies, Allergic Comorbidities and the Home Environment in Pediatric Asthma in Southern Florida. Int. J. Environ. Res. Public Health 2021, 18, 4142. [Google Scholar] [CrossRef] [PubMed]
- Kwong, K.Y.; Lu, Y.Z.; Jauregui, E.; Scott, L. Persistent airflow obstruction in inner-city children with asthma. Allergy Asthma Proc. 2021, 42, 310–316. [Google Scholar] [CrossRef]
- XuChen, X.; Weinstock, J.; Arroyo, M.; Salka, K.; Chorvinsky, E.; Abutaleb, K.; Aguilar, H.; Kahanowitch, R.; Rodríguez-Martínez, C.E.; Perez, G.F.; et al. Airway Remodeling Factors During Early-Life Rhinovirus Infection and the Effect of Premature Birth. Front. Pediatr. 2021, 9, 610478. [Google Scholar] [CrossRef]
- Spann, K.; Baturcam, E.; Schagen, J.; Jones, C.; Straub, C.P.; Preston, F.M.; Chen, L.; Phipps, S.; Sly, P.D.; Fantino, E. Viral and host factors determine innate immune responses in airway epithelial cells from children with wheeze and atopy. Thorax 2014, 69, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Everman, J.L.; Sajuthi, S.; Saef, B.; Rios, C.; Stoner, A.M.; Numata, M.; Hu, D.; Eng, C.; Oh, S.; Rodriguez-Santana, J.; et al. Functional genomics of CDHR3 confirms its role in HRV-C infection and childhood asthma exacerbations. J. Allergy Clin. Immunol. 2019, 144, 962–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bønnelykke, K.; Sleiman, P.; Nielsen, K.; Kreiner-Møller, E.; Mercader, J.M.; Belgrave, D.; den Dekker, H.T.; Husby, A.; Sevelsted, A.; Faura-Tellez, G.; et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat. Genet. 2014, 46, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Van de Wetering, C.; Elko, E.; Berg, M.; Schiffers, C.H.J.; Stylianidis, V.; van den Berge, M.; Nawijn, M.C.; Wouters, E.F.M.; Janssen-Heininger, Y.M.W.; Reynaert, N.L. Glutathione S-transferases and their implications in the lung diseases asthma and chronic obstructive pulmonary disease: Early life susceptibility? Redox Biol. 2021, 43, 101995. [Google Scholar] [CrossRef]
- Sobkowiak, P.; Narożna, B.; Wojsyk-Banaszak, I.; Bręborowicz, A.; Szczepankiewicz, A. Expression of proteins associated with airway fibrosis differs between children with allergic asthma and allergic rhinitis. Int. J. Immunopathol. Pharmacol. 2021, 35. [Google Scholar] [CrossRef]
- Beaufils, F.; Esteves, P.; Enaud, R.; Germande, O.; Celle, A.; Marthan, R.; Trian, T.; Fayon, M.; Berger, P. Mitochondria are involved in bronchial smooth muscle remodeling in severe preschool wheezers. J. Allergy Clin. Immunol. 2021, 148, 645–651.e11. [Google Scholar] [CrossRef]
- Grainge, C.L.; Lau, L.C.; Ward, J.A.; Dulay, V.; Lahiff, G.; Wilson, S.; Holgate, S.; Davies, D.E.; Howarth, P.H. Effect of bronchoconstriction on airway remodeling in asthma. N. Engl. J. Med. 2011, 364, 2006–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutchen, K.R.; Paré, P.D.; Seow, C.Y. Hyperresponsiveness: Relating the Intact Airway to the Whole Lung. Physiology 2017, 32, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Cokuğraş, H.; Akçakaya, N.; Seçkin Camcioğlu, Y.; Sarimurat, N.; Aksoy, F. Ultrastructural examination of bronchial biopsy specimens from children with moderate asthma. Thorax 2001, 56, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Pohunek, P.; Warner, J.O.; Turzíková, J.; Kudrmann, J.; Roche, W.R. Markers of eosinophilic inflammation and tissue re-modelling in children before clinically diagnosed bronchial asthma. Pediatr. Allergy Immunol. 2005, 16, 43–51. [Google Scholar] [CrossRef]
- Saglani, S.; Malmström, K.; Pelkonen, A.S.; Malmberg, L.P.; Lindahl, H.; Kajosaari, M.; Turpeinen, M.; Rogers, A.V.; Payne, D.N.; Bush, A.; et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am. J. Respir. Crit. Care Med. 2005, 171, 722–777. [Google Scholar] [CrossRef]
- Malmström, K.; Pelkonen, A.S.; Malmberg, L.P.; Sarna, S.; Lindahl, H.; Kajosaari, M.; Turpeinen, M.; Saglani, S.; Bush, A.; Haahtela, T.; et al. Lung function, airway remodelling and inflammation in symptomatic infants: Outcome at 3 years. Thorax 2011, 66, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zazara, D.E.; Wegmann, M.; Giannou, A.D.; Hierweger, A.M.; Alawi, M.; Thiele, K.; Huber, S.; Pincus, M.; Muntau, A.C.; Solano, M.E.; et al. A prenatally disrupted airway epithelium orchestrates the fetal origin of asthma in mice. J. Allergy Clin. Immunol. 2020, 145, 1641–1654. [Google Scholar] [CrossRef]
- Andersson, C.K.; Iwasaki, J.; Cook, J.; Robinson, P.; Nagakumar, P.; Mogren, S.; Fleming, L.; Bush, A.; Saglani, S.; Lloyd, C.M. Impaired airway epithelial cell wound-healing capacity is associated with airway remodelling following RSV infection in severe preschool wheeze. Allergy 2020, 75, 3195–3207. [Google Scholar] [CrossRef] [PubMed]
- Schmit, T.; Ghosh, S.; Mathur, R.K.; Barnhardt, T.; Ambigapathy, G.; Wu, M.; Combs, C.; Khan, M.N. IL-6 Deficiency Exacerbates Allergic Asthma and Abrogates the Protective Effect of Allergic Inflammation against Streptococcus pneumoniae Pathogenesis. J. Immunol. 2020, 205, 469–479. [Google Scholar] [CrossRef]
- Schuliga, M.; Read, J.; Knight, D.A. Ageing mechanisms that contribute to tissue remodeling in lung disease. Ageing Res. Rev. 2021, 70, 101405. [Google Scholar] [CrossRef] [PubMed]
- Halayko, A.J.; Pascoe, C.D.; Gereige, J.D.; Peters, M.C.; Cohen, R.T.; Woodruff, P.G. Update in Adult Asthma 2020. Am. J. Respir. Crit. Care Med. 2021, 204, 395–402. [Google Scholar] [CrossRef]
- Osei, E.T.; Booth, S.; Hackett, T.L. What Have In Vitro Co-Culture Models Taught Us about the Contribution of Epithelial-Mesenchymal Interactions to Airway Inflammation and Remodeling in Asthma? Cells 2020, 9, 1694. [Google Scholar] [CrossRef]
- Johnson, P.R.; Roth, M.; Tamm, M.; Hughes, M.; Ge, Q.; King, G.; Burgess, J.K.; Black, J.L. Airway smooth muscle cell proliferation is increased in asthma. Am. J. Respir. Crit. Care Med. 2001, 164, 474–477. [Google Scholar] [CrossRef]
- Yeung, B.H.Y.; Huang, J.; An, S.S.; Solway, J.; Mitzner, W.; Tang, W.Y. Role of Isocitrate Dehydrogenase 2 on DNA Hydroxymethylation in Human Airway Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2020, 63, 36–45. [Google Scholar] [CrossRef]
- Yu, Q.; Yu, X.; Zhao, W.; Zhu, M.; Wang, Z.; Zhang, J.; Huang, M.; Zeng, X. Inhibition of H3K27me3 demethylases attenuates asthma by reversing the shift in airway smooth muscle phenotype. Clin. Exp. Allergy 2018, 48, 1439–1452. [Google Scholar] [CrossRef]
- Roth, M.; Johnson, P.R.; Borger, P.; Bihl, M.P.; Rüdiger, J.J.; King, G.G.; Ge, Q.; Hostettler, K.; Burgess, J.K.; Black, J.L.; et al. Dysfunctional interaction of C/EBPalpha and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. N. Engl. J. Med. 2004, 351, 560–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miglino, N.; Roth, M.; Tamm, M.; Borger, P. House dust mite extract downregulates C/EBPα in asthmatic bronchial smooth muscle cells. Eur. Respir. J. 2011, 38, 50–58. [Google Scholar] [CrossRef]
- Nerlov, C. Transcriptional and translational control of C/EBPs: The case for “deep” genetics to understand physiological function. Bioessays 2010, 32, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, G.; DeMeo, D.L.; Glass, K.; Silverman, E.K.; Napoli, C. Epigenetics and pulmonary diseases in the horizon of precision medicine: A review. Eur. Respir. J. 2021, 57, 2003406. [Google Scholar] [CrossRef]
- Qi, C.; Xu, C.J.; Koppelman, G.H. The role of epigenetics in the development of childhood asthma. Expert Rev. Clin. Immunol. 2019, 15, 1287–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madore, A.M.; Long, A.; Bunning, B.; Sampath, V.; DeKruyff, R.H.; Nadeau, K.C. Epigenetics and the Environment in Airway Disease: Asthma and Allergic Rhinitis. Adv. Exp. Med. Biol. 2020, 1253, 153–181. [Google Scholar] [CrossRef]
- Acevedo, N.; Alashkar Alhamwe, B.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinás-Caballero, K.; et al. Perinatal and Early-Life Nutrition, Epigenetics, and Allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Van Esch, B.C.A.M.; Porbahaie, M.; Abbring, S.; Garssen, J.; Potaczek, D.P.; Savelkoul, H.F.J.; van Neerven, R.J.J. The Impact of Milk and Its Components on Epigenetic Programming of Immune Function in Early Life and Beyond: Implications for Allergy and Asthma. Front. Immunol. 2020, 11, 2141. [Google Scholar] [CrossRef]
- Güngör, D.; Nadaud, P.; LaPergola, C.C.; Dreibelbis, C.; Wong, Y.P.; Terry, N.; Abrams, S.A.; Beker, L.; Jacobovits, T.; Järvinen, K.M.; et al. Infant milk-feeding practices and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span: A systematic review. Am. J. Clin. Nutr. 2019, 109, 772S–799S. [Google Scholar] [CrossRef]
- Oddy, W.H. Breastfeeding, Childhood Asthma, and Allergic Disease. Ann. Nutr. Metab. 2017, 70, 26–36. [Google Scholar] [CrossRef]
- Lee-Sarwar, K.A.; Kelly, R.S.; Lasky-Su, J.; Zeiger, R.S.; O’Connor, G.T.; Sandel, M.T.; Bacharier, L.B.; Beigelman, A.; Laranjo, N.; Gold, D.R.; et al. Integrative analysis of the intestinal metabolome of childhood asthma. J. Allergy Clin. Immunol. 2019, 144, 442–454. [Google Scholar] [CrossRef] [Green Version]
- Hansen, R.; Gerasimidis, K.; Turner, S. Asthma causation and the gastrointestinal microbiome and metabolome: Might there be a signal, or is it just noise? J. Allergy Clin. Immunol. 2019, 144, 401–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallisetty, Y.; Mukherjee, N.; Jiang, Y.; Chen, S.; Ewart, S.; Arshad, S.H.; Holloway, J.W.; Zhang, H.; Karmaus, W. Epigenome-Wide Association of Infant Feeding and Changes in DNA Methylation from Birth to 10 Years. Nutrients 2020, 13, 99. [Google Scholar] [CrossRef]
- Obbagy, J.E.; English, L.K.; Wong, Y.P.; Butte, N.F.; Dewey, K.G.; Fleischer, D.M.; Fox, M.K.; Greer, F.R.; Krebs, N.F.; Scanlon, K.S.; et al. Complementary feeding and food allergy, atopic dermatitis/eczema, asthma, and allergic rhinitis: A systematic review. Am. J. Clin. Nutr. 2019, 109, 890S–934S. [Google Scholar] [CrossRef]
- Alashkar Alhamwe, B.; Miethe, S.; Pogge von Strandmann, E.; Potaczek, D.P.; Garn, H. Epigenetic Regulation of Airway Epithelium Immune Functions in Asthma. Front. Immunol. 2020, 11, 1747. [Google Scholar] [CrossRef]
- Huber, H.L.; Koessler, K.K. The pathology of bronchial asthma. Arch. Intern. Med. 1922, 30, 689–760. [Google Scholar] [CrossRef] [Green Version]
- Bossley, C.J.; Fleming, L.; Gupta, A.; Regamey, N.; Frith, J.; Oates, T.; Tsartsali, L.; Lloyd, C.M.; Bush, A.; Saglani, S. Pediatric severe asthma is characterized by eosinophilia and remodeling without T(H)2 cytokines. J. Allergy Clin. Immunol. 2012, 129, 974–982.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Reilly, R.; Ullmann, N.; Irving, S.; Bossley, C.J.; Sonnappa, S.; Zhu, J.; Oates, T.; Banya, W.; Jeffery, P.K.; Bush, A.; et al. Increased airway smooth muscle in preschool wheezers who have asthma at school age. J. Allergy Clin. Immunol. 2013, 131, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Baraldo, S.; Turato, G.; Bazzan, E.; Ballarin, A.; Damin, M.; Balestro, E.; Lokar Oliani, K.; Calabrese, F.; Maestrelli, P.; Snijders, D.; et al. Noneosinophilic asthma in children: Relation with airway remodelling. Eur. Respir. J. 2011, 38, 575–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lezmi, G.; Deschildre, A.; Abou Taam, R.; Fayon, M.; Blanchon, S.; Troussier, F.; Mallinger, P.; Mahut, B.; Gosset, P.; de Blic, J. Remodelling and inflammation in preschoolers with severe recurrent wheeze and asthma outcome at school age. Clin. Exp. Allergy 2018, 48, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Préfontaine, D.; Lajoie-Kadoch, S.; Foley, S.; Audusseau, S.; Olivenstein, R.; Halayko, A.J.; Lemière, C.; Martin, J.G.; Hamid, Q. Increased expression of IL-33 in severe asthma: Evidence of expression by airway muscle. J. Immunol. 2009, 183, 5094–5103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, C.D.; Gonzalez, E.M.; Ferrando, R.E.; Solon, M.; Baca, M.; Mesh, K.; Bradding, P.; Gauvreau, G.M.; Sumino, K.; FitzGerald, J.M.; et al. A randomized, placebo-controlled trial evaluating effects of lebrikizumab on airway eosinophilic inflammation and remodelling in uncontrolled asthma (CLAVIER). Clin. Exp. Allergy 2020, 50, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, B.; Xia, C.; Movassagh, H.; Koziol-White, C.; Chang, Y.; Al-Alwan, L.; Bourke, J.E.; Oliver, B.G. Emerging mediators of airway smooth muscle dysfunction in asthma. Pulm. Pharmacol. Ther. 2013, 26, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.Y.; Prakash, Y.S. Contributions of IL-33 in Non-hematopoietic Lung Cells to Obstructive Lung Disease. Front. Immunol. 2020, 11, 1798. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Gomez, E.; Doe, C.; Berair, R.; Woodman, L.; Saunders, R.; Hollins, F.; Rose, F.R.; Amrani, Y.; May, R.; et al. IL-33 drives airway hyper-responsiveness through IL-13-mediated mast cell: Airway smooth muscle crosstalk. Allergy 2015, 70, 556–567. [Google Scholar] [CrossRef] [Green Version]
- McCuaig, S.; Martin, J.G. How the airway smooth muscle in cystic fibrosis reacts in proinflammatory conditions: Implications for airway hyper-responsiveness and asthma in cystic fibrosis. Lancet Respir. Med. 2013, 1, 137–147. [Google Scholar] [CrossRef]
- Lu, S.; Li, H.; Gao, R.; Gao, X.; Xu, F.; Wang, Q.; Lu, G.; Xia, D.; Zhou, J. IL-17A, but not IL-17F, is indispensable for airway vascular remodeling induced by exaggerated Th17 cell responses in prolonged ovalbumin-challenged mice. J. Immunol. 2015, 194, 3557–3566. [Google Scholar] [CrossRef] [Green Version]
- Movassagh, H.; Tatari, N.; Shan, L.; Koussih, L.; Alsubait, D.; Khattabi, M.; Redhu, N.S.; Roth, M.; Tamm, M.; Chakir, J.; et al. Human airway smooth muscle cell proliferation from asthmatics is negatively regulated by semaphorin3A. Oncotarget 2016, 7, 80238–80251. [Google Scholar] [CrossRef] [Green Version]
- Plé, C.; Fan, Y.; Ait Yahia, S.; Vorng, H.; Everaere, L.; Chenivesse, C.; Balsamelli, J.; Azzaoui, I.; de Nadai, P.; Wallaert, B.; et al. Polycyclic aromatic hydrocarbons reciprocally regulate IL-22 and IL-17 cytokines in peripheral blood mononuclear cells from both healthy and asthmatic subjects. PLoS ONE 2015, 10, e0122372. [Google Scholar] [CrossRef]
- Redhu, N.S.; Shan, L.; Movassagh, H.; Gounni, A.S. Thymic stromal lymphopoietin induces migration in human airway smooth muscle cells. Sci. Rep. 2013, 3, 2301. [Google Scholar] [CrossRef] [Green Version]
- Mostaço-Guidolin, L.B.; Yang, C.X.; Hackett, T.L. Pulmonary Vascular Remodeling Is an Early Feature of Fatal and Nonfatal Asthma. Am. J. Respir. Cell Mol. Biol. 2021, 65, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Köksal, B.T.; Ozbek, O.Y.; Bayraktar, N.; Yazici, A.C. Evaluation of angiopoietin 1 and 2, vascular endothelial growth factor, and tumor necrosis factor alpha levels in asthmatic children. Allergy Asthma Proc. 2014, 35, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.; Roth, M.; Tamm, M.; Eickelberg, O.; Wieland, H.; Stulz, P.; Perruchoud, A.P. Induction of vascular endothelial growth factor by platelet-activating factor and platelet-derived growth factor is downregulated by corticosteroids. Am. J. Respir. Cell Mol. Biol. 1997, 16, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Lunding, L.P.; Ehlers, J.C.; Weckmann, M.; Zissler, U.M.; Wegmann, M. More Than Just a Barrier: The Immune Functions of the Airway Epithelium in Asthma Pathogenesis. Front. Immunol. 2020, 11, 761. [Google Scholar] [CrossRef]
- Olafsdottir, T.A.; Theodors, F.; Bjarnadottir, K.; Bjornsdottir, U.S.; Agustsdottir, A.B.; Stefansson, O.A.; Ivarsdottir, E.V.; Sigurdsson, J.K.; Benonisdottir, S.; Eyjolfsson, G.I.; et al. Eighty-eight variants highlight the role of T cell regulation and airway remodeling in asthma pathogenesis. Nat. Commun. 2020, 11, 393. [Google Scholar] [CrossRef] [Green Version]
- Kicic, A.; Hallstrand, T.S.; Sutanto, E.N.; Stevens, P.T.; Kobor, M.S.; Taplin, C.; Paré, P.D.; Beyer, R.P.; Stick, S.M.; Knight, D.A. Decreased fibronectin production significantly contributes to dysregulated repair of asthmatic epithelium. Am. J. Respir. Crit. Care Med. 2010, 181, 889–898. [Google Scholar] [CrossRef] [Green Version]
- Baschal, E.E.; Larson, E.D.; Bootpetch Roberts, T.C.; Pathak, S.; Frank, G.; Handley, E.; Dinwiddie, J.; Moloney, M.; Yoon, P.J.; Gubbels, S.P.; et al. Identification of Novel Genes and Biological Pathways That Overlap in Infectious and Nonallergic Diseases of the Upper and Lower Airways Using Network Analyses. Front. Genet. 2020, 10, 1352. [Google Scholar] [CrossRef]
- LaVerda, D.; Kalayoglu, M.V.; Byrne, G.I. Chlamydial heat shock proteins and disease pathology: New paradigms for old problems? Infect. Dis. Obstet. Gynecol. 1999, 7, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Huittinen, T.; Hahn, D.; Anttila, T.; Wahlström, E.; Saikku, P.; Leinonen, M. Host immune response to Chlamydia pneumoniae heat shock protein 60 is associated with asthma. Eur. Respir. J. 2001, 17, 1078–1082. [Google Scholar] [CrossRef] [Green Version]
- Hahn, D.L.; Peeling, R.W. Airflow limitation, asthma, and Chlamydia pneumoniae—Specific heat shock protein 60. Ann. Allergy Asthma Immunol. 2008, 101, 614–618. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, F.; Lu, Z.; Ying, H.; Zhang, H.; Ding, W.; Wang, C.; Shi, L. MAPK kinase 3 potentiates Chlamydia HSP60-induced inflammatory response through distinct activation of NF-κB. J. Immunol. 2013, 191, 386–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Wu, T.; Cheng, L.; Wang, F.; Wei, Q.; Tanguay, R.M. Plasma antibodies against heat shock protein 70 correlate with the incidence and severity of asthma in a Chinese population. Respir. Res. 2005, 6, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Victora, G.D.; Bilate, A.M.; Socorro-Silva, A.; Caldas, C.; Lima, R.C.; Kalil, J.; Coelho, V.; Victora, C.G. Mother-child immunological interactions in early life affect long-term humoral autoreactivity to heat shock protein 60 at age 18 years. J. Autoimmun. 2007, 29, 38–43. [Google Scholar] [CrossRef]
- Gruzieva, O.; Xu, C.J.; Yousefi, P.; Relton, C.; Merid, S.K.; Breton, C.V.; Gao, L.; Volk, H.E.; Feinberg, J.I.; Ladd-Acosta, C.; et al. Prenatal Particulate Air Pollution and DNA Methylation in Newborns: An Epigenome-Wide Meta-Analysis. Environ. Health Perspect. 2019, 127, 57012. [Google Scholar] [CrossRef]
- Sun, Q.; Fang, L.; Roth, M.; Tang, X.; Papakonstantinou, E.; Zhai, W.; Louis, R.; Heinen, V.; Schleich, F.N.; Lu, S.; et al. Bronchial thermoplasty decreases airway remodelling by blocking epithelium-derived heat shock protein-60 secretion and protein arginine methyltransferase-1 in fibroblasts. Eur. Respir. J. 2019, 54, 1900300. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, L.; Wang, H.; Mandal, J.; Khan, P.; Hostettler, K.E.; Stolz, D.; Tamm, M.; Molino, A.; Lardinois, D.; et al. Constitutive high expression of protein arginine methyltransferase 1 in asthmatic airway smooth muscle cells is caused by reduced microRNA-19a expression and leads to enhanced remodeling. J. Allergy Clin. Immunol. 2017, 140, 510–524.e3. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Li, J.; Papakonstantinou, E.; Karakioulaki, M.; Sun, Q.; Schumann, D.; Tamm, M.; Stolz, D.; Roth, M. Secreted heat shock proteins control airway remodeling: Evidence from bronchial thermoplasty. J. Allergy Clin. Immunol. 2021, 148, 1249–1261.e8. [Google Scholar] [CrossRef]
- Sun, Q.; Fang, L.; Tang, X.; Lu, S.; Tamm, M.; Stolz, D.; Roth, M. TGF-β Upregulated Mitochondria Mass through the SMAD2/3→C/EBPβ→PRMT1 Signal Pathway in Primary Human Lung Fibroblasts. J. Immunol. 2019, 202, 37–47. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Koletsa, T.; Zhou, L.; Fang, L.; Roth, M.; Karakioulaki, M.; Savic, S.; Grize, L.; Tamm, M.; Stolz, D. Bronchial thermoplasty in asthma: An exploratory histopathological evaluation in distinct asthma endotypes/phenotypes. Respir. Res. 2021, 22, 186. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, L.; Mandal, J.; Molino, A.; Stolz, D.; Tamm, M.; Lu, S.; Roth, M. PDGF-BB induces PRMT1 expression through ERK1/2 dependent STAT1 activation and regulates remodeling in primary human lung fibroblasts. Cell. Signal. 2016, 28, 307–315. [Google Scholar] [CrossRef]
- Zhai, W.; Sun, H.; Li, Z.; Li, L.; Jin, A.; Li, Y.; Chen, J.; Yang, X.; Sun, Q.; Lu, S.; et al. PRMT1 Modulates Processing of Asthma-Related Primary MicroRNAs (Pri-miRNAs) into Mature miRNAs in Lung Epithelial Cells. J. Immunol. 2021, 206, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, M.; Servuli, E.; Albakova, Z.; Kanevskiy, L.; Sapozhnikov, A. The Role of Heat Shock Protein 70 kDa in Asthma. J. Asthma Allergy 2021, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Sheikhpour, M.; Maleki, M.; Ebrahimi Vargoorani, M.; Amiri, V. A review of epigenetic changes in asthma: Methylation and acetylation. Clin. Epigenet. 2021, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, D. Are we what our mothers made us? Lessons from epigenetics. J. Allergy Clin. Immunol. 2018, 141, 525–526. [Google Scholar] [CrossRef]
- Sharp, G.C.; Salas, L.A.; Monnereau, C.; Allard, C.; Yousefi, P.; Everson, T.M.; Bohlin, J.; Xu, Z.; Huang, R.C.; Reese, S.E.; et al. Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: Findings from the pregnancy and childhood epigenetics (PACE) consortium. Hum. Mol. Genet. 2017, 26, 4067–4085. [Google Scholar] [CrossRef] [Green Version]
- Pulczinski, J.C.; Shang, Y.; Dao, T.; Limjunyawong, N.; Sun, Q.; Mitzner, W.; Cheng, R.Y.; Tang, W.Y. Multigenerational Epigenetic Regulation of Allergic Diseases: Utilizing an Experimental Dust Mite-Induced Asthma Model. Front. Genet. 2021, 12, 624561. [Google Scholar] [CrossRef]
- Suen, J.L.; Wu, T.T.; Li, Y.H.; Lee, C.L.; Kuo, F.C.; Yan, P.S.; Wu, C.F.; Tran, M.; Wang, C.J.; Hung, C.H.; et al. Environmental Factor-Mediated Transgenerational Inheritance of Igf2r Hypomethylation and Pulmonary Allergic Response via Targeting Dendritic Cells. Front. Immunol. 2020, 11, 603831. [Google Scholar] [CrossRef]
- Arshad, S.H.; Karmaus, W.; Zhang, H.; Holloway, J.W. Multigenerational cohorts in patients with asthma and allergy. J. Allergy Clin. Immunol. 2017, 139, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Paaso, E.M.; Jaakkola, M.S.; Rantala, A.K.; Hugg, T.T.; Jaakkola, J.J. Allergic diseases and asthma in the family predict the persistence and onset-age of asthma: A prospective cohort study. Respir. Res. 2014, 15, 152. [Google Scholar] [CrossRef] [Green Version]
- Lim, R.H.; Kobzik, L.; Dahl, M. Risk for asthma in offspring of asthmatic mothers versus fathers: A meta-analysis. PLoS ONE 2010, 5, e10134. [Google Scholar] [CrossRef]
- Liu, X.; Agerbo, E.; Schlünssen, V.; Wright, R.J.; Li, J.; Munk-Olsen, T. Maternal asthma severity and control during pregnancy and risk of offspring asthma. J. Allergy Clin. Immunol. 2018, 141, 886–892.e3. [Google Scholar] [CrossRef] [Green Version]
- Young, S.; Le Souëf, P.N.; Geelhoed, G.C.; Stick, S.M.; Turner, K.J.; Landau, L.I. The influence of a family history of asthma and parental smoking on airway responsiveness in early infancy. N. Engl. J. Med. 1991, 324, 1168–1173. [Google Scholar] [CrossRef]
- Martel, M.J.; Rey, E.; Beauchesne, M.F.; Malo, J.L.; Perreault, S.; Forget, A.; Blais, L. Control and severity of asthma during pregnancy are associated with asthma incidence in offspring: Two-stage case-control study. Eur. Respir. J. 2009, 34, 579–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, M.; Tang, Y.; Liu, K.; Huang, K.; Yan, S.; Ding, P.; Zhang, J.; Wang, B.; Wang, S.; Li, S.; et al. Prenatal low-dose antibiotic exposure and children allergic diseases at 4 years of age: A prospective birth cohort study. Ecotoxicol. Environ. Saf. 2021, 225, 112736. [Google Scholar] [CrossRef]
- Belvisi, M.G. Overview of the innervation of the lung. Curr. Opin. Pharmacol. 2002, 2, 211–215. [Google Scholar] [CrossRef]
- Drake, M.G.; Scott, G.D.; Blum, E.D.; Lebold, K.M.; Nie, Z.; Lee, J.J.; Fryer, A.D.; Costello, R.W.; Jacoby, D.B. Eosinophils increase airway sensory nerve density in mice and in human asthma. Sci. Transl. Med. 2018, 10, eaar8477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Xu, J.; Chen, Y.E.; Li, J.S.; Cui, Y.; Shen, L.; Li, J.J.; Li, W. The concurrence of DNA methylation and demethylation is associated with transcription regulation. Nat. Commun. 2021, 12, 5285. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.E.; Xu, C.J.; den Dekker, H.T.; Lee, M.K.; Sikdar, S.; Ruiz-Arenas, C.; Merid, S.K.; Rezwan, F.I.; Page, C.M.; Ullemar, V.; et al. Epigenome-wide meta-analysis of DNA methylation and childhood asthma. J. Allergy Clin. Immunol. 2019, 143, 2062–2074. [Google Scholar] [CrossRef] [Green Version]
- Solazzo, G.; Ferrante, G.; La Grutta, S. DNA Methylation in Nasal Epithelium: Strengths and Limitations of an Emergent Biomarker for Childhood Asthma. Front. Pediatr. 2020, 8, 256. [Google Scholar] [CrossRef]
- Yang, I.V.; Pedersen, B.S.; Liu, A.H.; O’Connor, G.T.; Pillai, D.; Kattan, M.; Misiak, R.T.; Gruchalla, R.; Szefler, S.J.; Khurana Hershey, G.K.; et al. The nasal methylome and childhood atopic asthma. J. Allergy Clin. Immunol. 2017, 139, 1478–1488. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Biagini Myers, J.M.; Burleson, J.D.; Ulm, A.; Bryan, K.S.; Chen, X.; Weirauch, M.T.; Baker, T.A.; Butsch Kovacic, M.S.; Ji, H. Nasal DNA methylation is associated with childhood asthma. Epigenomics 2018, 10, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Forno, E.; Wang, T.; Qi, C.; Yan, Q.; Xu, C.J.; Boutaoui, N.; Han, Y.Y.; Weeks, D.E.; Jiang, Y.; Rosser, F.; et al. DNA methylation in nasal epithelium, atopy, and atopic asthma in children: A genome-wide study. Lancet Respir. Med. 2019, 7, 336–346. [Google Scholar] [CrossRef]
- Cardenas, A.; Sordillo, J.E.; Rifas-Shiman, S.L.; Chung, W.; Liang, L.; Coull, B.A.; Hivert, M.F.; Lai, P.S.; Forno, E.; Celedón, J.C.; et al. The nasal methylome as a biomarker of asthma and airway inflammation in children. Nat. Commun. 2019, 10, 3095. [Google Scholar] [CrossRef] [Green Version]
- Uwaezuoke, S.N.; Ayuk, A.C.; Eze, J.N. Severe bronchial asthma in children: A review of novel biomarkers used as predictors of the disease. J. Asthma Allergy 2018, 11, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, J.L. Epigenetics in Asthma. Curr. Allergy Asthma Rep. 2019, 19, 56. [Google Scholar] [CrossRef]
- Akar-Ghibril, N.; Casale, T.; Custovic, A.; Phipatanakul, W. Allergic Endotypes and Phenotypes of Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 429–440. [Google Scholar] [CrossRef]
- Tsang, Y.P.; Marchant, J.M.; Li, A.M.; Chang, A.B. Stability of sputum inflammatory phenotypes in childhood asthma during stable and exacerbation phases. Pediatr. Pulmonol. 2021, 56, 1484–1489. [Google Scholar] [CrossRef]
- Fainardi, V.; Esposito, S.; Chetta, A.; Pisi, G. Asthma phenotypes and endotypes in childhood. Minerva Med. 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/33576199/ (accessed on 31 August 2021). [CrossRef]
- Banić, I.; Lovrić, M.; Cuder, G.; Kern, R.; Rijavec, M.; Korošec, P.; Turkalj, M. Treatment outcome clustering patterns correspond to discrete asthma phenotypes in children. Asthma Res. Pract. 2021, 7, 11. [Google Scholar] [CrossRef]
- Salvermoser, M.; Zeber, K.; Boeck, A.; Klucker, E.; Schaub, B. Childhood asthma: Novel endotyping by cytokines, validated through sensitization profiles and clinical characteristics. Clin. Exp. Allergy 2021, 51, 654–665. [Google Scholar] [CrossRef]
- Altman, M.C.; Flynn, K.; Rosasco, M.G.; Dapas, M.; Kattan, M.; Lovinsky-Desir, S.; O’Connor, G.T.; Gill, M.A.; Gruchalla, R.S.; Liu, A.H.; et al. Inducible expression quantitative trait locus analysis of the MUC5AC gene in asthma in urban populations of children. J. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol. Rev. 2011, 242, 205–219. [Google Scholar] [CrossRef]
- Mørkve Knudsen, T.; Rezwan, F.I.; Jiang, Y.; Karmaus, W.; Svanes, C.; Holloway, J.W. Transgenerational and intergenerational epigenetic inheritance in allergic diseases. J. Allergy Clin. Immunol. 2018, 142, 765–772. [Google Scholar] [CrossRef]
- Gutierrez, M.J.; Perez, G.F.; Gomez, J.L.; Rodriguez-Martinez, C.E.; Castro-Rodriguez, J.A.; Nino, G. Genes, environment, and developmental timing: New insights from translational approaches to understand early origins of respiratory diseases. Pediatr. Pulmonol. 2021, 56, 3157–3165. [Google Scholar] [CrossRef] [PubMed]
- Murphy, V.E.; Karmaus, W.; Mattes, J.; Brew, B.K.; Collison, A.; Holliday, E.; Jensen, M.E.; Morgan, G.G.; Zosky, G.R.; McDonald, V.M.; et al. Exposure to Stress and Air Pollution from Bushfires during Pregnancy: Could Epigenetic Changes Explain Effects on the Offspring? Int. J. Environ. Res. Public Health 2021, 18, 7465. [Google Scholar] [CrossRef]
- Yan, Q.; Forno, E.; Celedón, J.C.; Chen, W. A region-based method for causal mediation analysis of DNA methylation data. Epigenetics 2021, 1–11. [Google Scholar] [CrossRef]
- Accordini, S.; Calciano, L.; Johannessen, A.; Portas, L.; Benediktsdóttir, B.; Bertelsen, R.J.; Bråbäck, L.; Carsin, A.E.; Dharmage, S.C.; Dratva, J.; et al. Ageing Lungs in European Cohorts (ALEC) Study. A three-generation study on the association of tobacco smoking with asthma. Int. J. Epidemiol. 2018, 47, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Lodge, C.J.; Bråbäck, L.; Lowe, A.J.; Dharmage, S.C.; Olsson, D.; Forsberg, B. Grandmaternal smoking increases asthma risk in grandchildren: A nationwide Swedish cohort. Clin. Exp. Allergy 2018, 48, 167–174. [Google Scholar] [CrossRef]
- Zakarya, R.; Adcock, I.; Oliver, B.G. Epigenetic impacts of maternal tobacco and e-vapour exposure on the offspring lung. Clin. Epigenet. 2019, 11, 32. [Google Scholar] [CrossRef]
- Sarnowski, C.; Laprise, C.; Malerba, G.; Moffatt, M.F.; Dizier, M.H.; Morin, A.; Vincent, Q.B.; Rohde, K.; Esparza-Gordillo, J.; Margaritte-Jeannin, P.; et al. DNA methylation within melatonin receptor 1A (MTNR1A) mediates paternally transmitted genetic variant effect on asthma plus rhinitis. J. Allergy Clin. Immunol. 2016, 138, 748–753. [Google Scholar] [CrossRef] [Green Version]
- Altıntaş, A.; Liu, J.; Fabre, O.; Chuang, T.D.; Wang, Y.; Sakurai, R.; Chehabi, G.N.; Barrès, R.; Rehan, V.K. Perinatal exposure to nicotine alters spermatozoal DNA methylation near genes controlling nicotine action. FASEB J. 2021, 35, e21702. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, L.; Roth, M. Airway Wall Remodeling in Childhood Asthma—A Personalized Perspective from Cell Type-Specific Biology. J. Pers. Med. 2021, 11, 1229. https://doi.org/10.3390/jpm11111229
Fang L, Roth M. Airway Wall Remodeling in Childhood Asthma—A Personalized Perspective from Cell Type-Specific Biology. Journal of Personalized Medicine. 2021; 11(11):1229. https://doi.org/10.3390/jpm11111229
Chicago/Turabian StyleFang, Lei, and Michael Roth. 2021. "Airway Wall Remodeling in Childhood Asthma—A Personalized Perspective from Cell Type-Specific Biology" Journal of Personalized Medicine 11, no. 11: 1229. https://doi.org/10.3390/jpm11111229





