Glutathione Blood Concentrations: A Biomarker of Oxidative Damage Protection during Cardiopulmonary Bypass in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Anesthetic Technique
2.2. Cardiopulmonary Bypass Management
2.3. GSH Measurement
2.4. Neurological Follow-up
2.5. Statistical Analysis
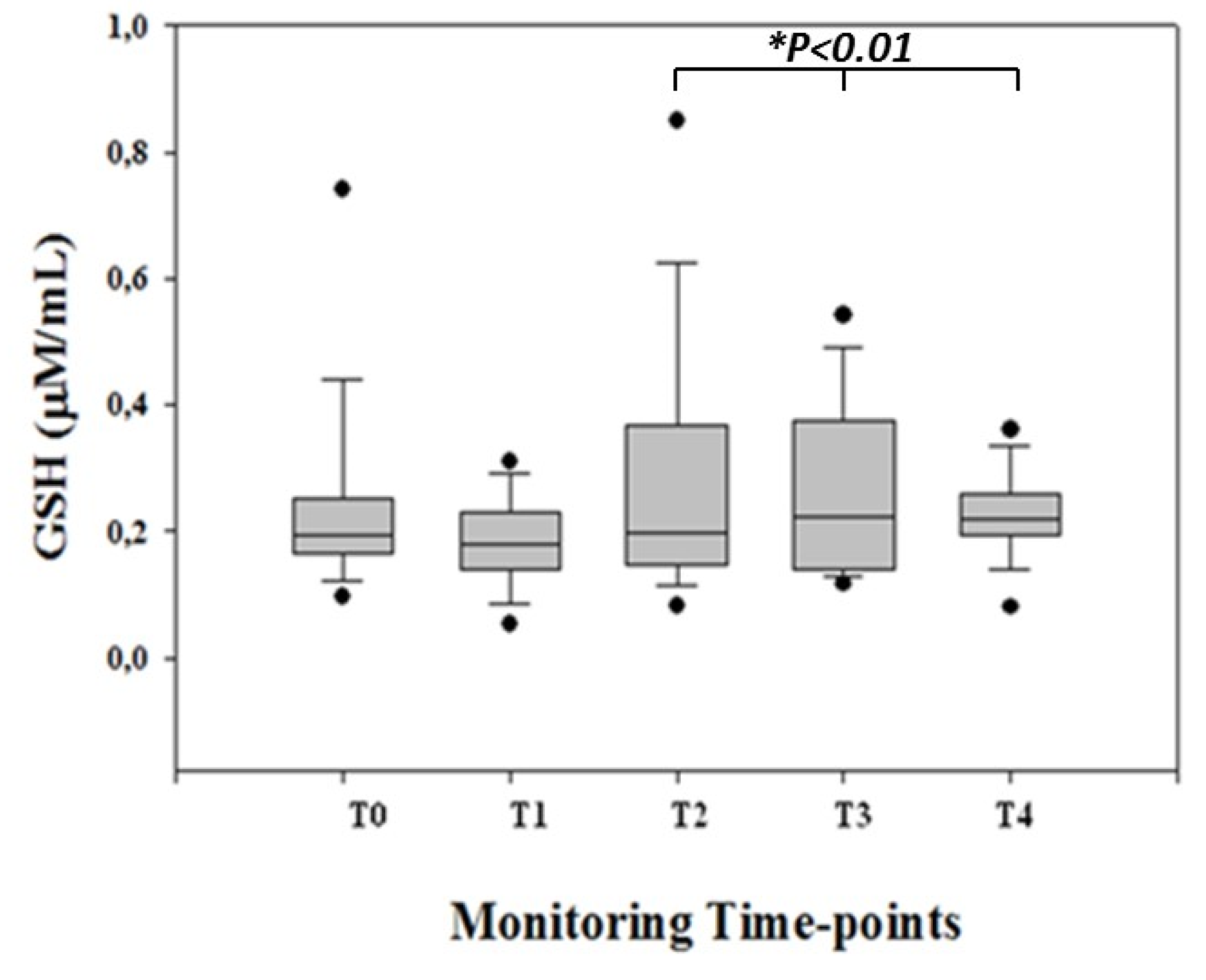
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Employment or Leadership
Honorarium
Competing Interests
Abbreviation List
| BE | base excess |
| BP | blood pressure |
| CHD | congenital heart disease |
| CPB | cardiopulmonary bypass |
| DHCA | deep hypothermic circulatory arrest |
| DTNB | 2,2-dithio-bis-nitrobenzoic acid |
| GSH | glutathione |
| Hb | hemoglobin |
| HCO3 | bicarbonate |
| HR | heart rate |
| Ht | hematocrit rate |
| LA BP | left atrium BP |
| MUF | Modified ultrafiltration |
| PaCO2 | arterial carbon dioxide partial pressure |
| PaO2 | arterial oxygen partial pressure |
| RA BP | right atrium BP |
| RNOS | reactive nitrogen and oxygen species |
| SaO2 | pulsed arterial oxygenation |
| VSD | ventricular septal defect |
References
- Hsia, T.Y.; Gruber, P.J. Factors influencing neurologic out-come after neonatal cardiopulmonary bypass: What we can and cannot control. Ann. Thorac. Surg. 2006, 81, S2381–S2388. [Google Scholar] [CrossRef] [PubMed]
- Gazzolo, D.; Abella, R.; Marinoni, E.; Di Iorio, R.; Livolti, G.; Galvano, F.; Pongiglione, G.; Frigiola, A.; Bertino, E.; Florio, P. Circulating biochemical markers of brain damage in infants complicated by ischemia reperfusion injury. Cardiovasc. Hematol. Agents Med. Chem. 2009, 7, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Hou, W.; Dong, Y.; Yu, Z.; Stites, J.; Weiner, C.P. Brain injury caused by chronic fetal hypoxemia is mediated by inflammatory cascade activation. Reprod. Sci. 2010, 17, 540–548. [Google Scholar] [PubMed]
- Adamy, C.; Mulder, P.; Khouzami, L.; Andrieu-Abadie, N.; Defer, N.; Candiani, G.; Pavoine, C.; Caramelle, P.; Souktani, R.; Le Corvoisier, P.; et al. Neutral sphingomyelinase inhibition participates to the benefits of Nacetylcysteine treatment in post-myocardial infarction failing heart rats. J. Mol. Cell Cardiol. 2007, 43, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Yucel, D.; Aydogdu, S.; Cehreli, S.; Saydam, G.; Canatan, H.; Senes, M.; Ciğdem Topkaya, B.; Nebioğlu, S. Increased oxidative stress in dilated cardiomyopathic heart failure. Clin. Chem. 1998, 44, 148–154. [Google Scholar]
- Haddad, J.J.; Harb, H.L. L-gamma-Glutamyl-L-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: A signaling transcriptional scenario for redox(y) immunologic sensor(s)? Mol. Immunol. 2005, 42, 987–1014. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007, 113, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Bourraindeloup, M.; Adamy, C.; Candiani, G.; Cailleret, M.; Bourin, M.C.; Badoual, T.; Su, J.B.; Adubeiro, S.; Roudot-Thoraval, F.; Dubois-Randé, J.L.; et al. N-acetylcysteine treatment normalizes serum tumor necrosis factor-alpha level and hinders the progression of cardiac injury in hypertensive rats. Circulation 2004, 110, 2003–2009. [Google Scholar] [CrossRef]
- Damy, T.; Kirsch, M.; Khouzami, L.; Caramelle, P.; Le Corvoisier, P.; Roudot-Thoraval, F.; Dubois-Randé, J.L.; Hittinger, L.; Pavoine, C.; Pecker, F. Glutathione deficiency in cardiac patients is related to the functional status and structural cardiac abnormalities. PLoS ONE 2009, 4, e4871. [Google Scholar] [CrossRef]
- Hayes, J.D.; McLellan, L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999, 31, 273–300. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Koh, Y.H.; Park, Y.S.; Fujiwara, N.; Sakiyama, H.; Misonou, Y.; Ookawara, T.; Suzuki, K.; Honke, K.; Taniguchi, N. Oxidative stress caused by inactivation of glutathione peroxidase and adaptive responses. Biol. Chem. 2003, 384, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.W.; Parzefall, W. Thiols and the chemoprevention of cancer. Curr. Opin. Pharmacol. 2007, 7, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Ciocoiu, M.; Badescu, M.; Paduraru, I. Protecting antioxidative effects of vitamins E and C in experimental physical stress. J. Physiol. Biochem. 2007, 63, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hadzic, T.; Li, L.; Cheng, N.; Walsh, S.A.; Spitz, D.R.; Knudson, C.M. The role of low molecular weight thiols in T lymphocyte proliferation and IL-2 secretion. J. Immunol. 2005, 175, 7965–7972. [Google Scholar] [CrossRef] [PubMed]
- Calza, G.; Lerzo, F.; Perfumo, F.; Borini, I.; Panizzon, G.; Moretti, R.; Grasso, P.; Virgone, A.; Zannini, L. Clinical evaluation of oxidative stress and myocardial reperfusion injury in pediatric cardiac surgery. J. Cardiovasc. Surg. (Torino) 2002, 43, 441–447. [Google Scholar]
- Abella, R.; Satriano, A.; Frigiola, A.; Varrica, A.; Gavilanes, D.W.A.; Zimmermann, L.J.; Vles, H.J.S.; Florio, P.; Calevo, M.G.; Gazzolo, D. Adrenomedullin alterations related to cardiopulmonary bypass in infants with low cardiac output syndrome. J. Matern. Fetal Neonatal Med. 2012, 25, 2756–2761. [Google Scholar] [CrossRef] [PubMed]
- Ziyaeifard, M.; Alizadehasl, A.; Massoumi, G. Modified ultrafiltration during cardiopulmonary bypass and postoperative course of pediatric cardiac surgery. Res. Cardiovasc. Med. 2014, 3, e17830. [Google Scholar]
- Hu, M. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994, 233, 380–382. [Google Scholar]
- Prechtl, H.F.R. Assessment methods for the newborn infant: A critical evaluation. In Psychobiology of Human Newborn; Stratton, D., Ed.; Wiley Chichester: London, UK, 1982; pp. 21–52. [Google Scholar]
- Strobel, A.M.; Lu le, N. The Critically ill infant with congenital heart disease. Emerg. Med. Clin. North. Am. 2015, 33, 501–518. [Google Scholar] [CrossRef]
- Kansy, A.; Tobota, Z.; Maruszewski, P.; Maruszewski, B. Analysis of 14,843 neonatal congenital heart surgical procedures in the European Association for Cardiothoracic Surgery congenital database. Ann. Thorac. Surg. 2010, 89, 1255–1259. [Google Scholar] [CrossRef]
- Serpero, L.D.; Bellissima, V.; Colivicchi, M.; Sabatini, M.; Frigiola, A.; Ricotti, A.; Ghiglione, V.; Strozzi, M.C.; Livolti, G.; Galvano, F.; et al. Next generation biomarkers for brain injury. J. Matern. Fetal Neonatal Med. 2013, 26, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Abella, R.; Varrica, A.; Satriano, A.; Tettamanti, G.; Pelissero, G.; Gavilanes, A.D.; Zimmermann, L.J.; Vles, H.J.; Strozzi, M.C.; Pluchinotta, F.R.; et al. Biochemical markers for brain injury monitoring in children with or without congenital heart diseases. Cns. Neurol. Disord. Drug Targets. 2015, 14, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Gazzolo, D.; Abella, R.; Marinoni, E.; Di Iorio, R.; Livolti, G.; Galvano, F.; Frigiola, A.; Temporini, F.; Moresco, L.; Colivicchi, M.; et al. New markers of neonatal neurology. J. Matern Fetal Neonatal Med. 2009, 22, 57–61. [Google Scholar] [CrossRef]
- Gazzolo, D.; Michetti, F.; Bruschettini, M.; Marchese, N.; Lituania, M.; Mangraviti, S.; Pedrazzi, E.; Bruschettini, P. Pediatric concentrations of S100B protein in blood: age- and sex-related changes. Clin. Chem. 2003, 49, 967–970. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.I.; Fraser, J.F.; Coombes, J.S.; Fung, Y.L. Oxidative stress during extracorporeal circulation. Eur J. Cardiothorac Surg. 2014, 46, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Türker, F.S.; Doğan, A.; Ozan, G.; Kıbar, K.; Erışır, M. Change in free radical and antioxidant enzyme levels in the patients undergoing open heart surgery with cardiopulmonary bypass. Oxid. Med. Cell Longev. 2016, 2016, 1783728. [Google Scholar] [CrossRef] [PubMed]
- Alva, N.; Azuara, D.; Palomeque, J.; Carbonell, T. Deep hypothermia protects against acute hypoxia in vivo in rats: A mechanism related to the attenuation of oxidative stress. Exp. Physiol. 2013, 98, 1115–1124. [Google Scholar] [CrossRef]
- Turrens, J.F.; Freeman, B.A.; Crapo, J.D. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch. Biochem. Biophys. 1982, 217, 411–421. [Google Scholar] [CrossRef]
- Ben-Yoseph, O.; Boxer, P.A.; Ross, B.D. Assessment of the role of the glutathione and pentose phosphate pathways in the protection of primary cerebrocortical cultures from oxidative stress. J. Neurochem. 1996, 66, 2329–2337. [Google Scholar] [CrossRef]
- Rahman, Q.; Abidi, P.; Afaq, F.; Schiffmann, D.; Mossman, B.T.; Kamp, D.W.; Athar, M. Glutathione redox system in oxidative lung injury. Crit. Rev. Toxicol. 1999, 29, 543–568. [Google Scholar] [CrossRef]
- Ko, Y.E.; Lee, I.H.; So, H.M.; Kim, H.V.; Kim, Y.H. Mechanism of glutathione depletion during simulated ischemia-reperfusion of H9c2 cardiac myocytes. Free Radic. Res. 2011, 45, 1074–1082. [Google Scholar] [CrossRef]
- Rosa, S.; Bristor, M.; Topanotti, M.; Tomasi, C.; Felisberto, F.; Vuolo, F.; Petronilho, F.; Pizzol, F.D.; Ritter, C. Effect of red cell transfusion on parameters of inflammation and oxidative stress in critically ill patients. Rev. Bras. Ter. Intensiva. 2011, 23, 30–35. [Google Scholar] [CrossRef]
- Krotz, F.; Sohn, H.Y.; Pohl, U. Reactive oxygen species: Players in the platelet game. Arter. Thromb Vasc Biol. 2004, 24, 1988–1996. [Google Scholar] [CrossRef]
- Buckberg, G.D. Studies of hypoxemic/reoxygenation injury: I. Linkage between cardiac function and oxidant damage. J. Thorac. Cardiovasc. Surg. 1995, 110, 1164–1170. [Google Scholar] [CrossRef]
- Kirklin, J.W.; Barratt-Boyes, B.G. Cardiac surgery: Morphology, Diagnostic Criteria, Natural History, Techniques, Results and Indication; Churchill Livingston Inc.: New York, NY, USA, 1993; p. 77. [Google Scholar]
- Morita, K.; Ihnken, K.; Buckberg, G.D. Studies of hypoxemic/reoxygenation injury: With aortic clamping. XII. Delay of cardiac reoxygenation damage in the presence of cyanosis: A new concept of controlled cardiac reoxygenation. J. Thorac. Cardiovasc. Surg. 1995, 110, 1265–1273. [Google Scholar] [CrossRef]
- Del Nido, P.J.; Mickle, D.A.; Wilson, G.J.; Benson, M.L.; Coles, J.G.; Trusler, G.A.; Williams, W.G. Evidence of myocardial free radical injury during elective repair of tetralogy of Fallot. Circulation 1987, 76, V174–V179. [Google Scholar]
- Schurr, A. Lactate, glucose and energy metabolism in the ischemic brain. Int J. Mol Med. 2002, 10, 131–136. [Google Scholar] [CrossRef]
- Arrica, M.; Bissonnette, B. Therapeutic hypothermia. Semin. Cardiothorac. Vasc. Anesth. 2007, 11, 6–15. [Google Scholar] [CrossRef]
- Hong, S.Y.; Gil, H.W.; Yang, J.O.; Lee, E.Y.; Kim, H.K.; Kim, S.H.; Chung, Y.H.; Hwang, S.K.; Lee, Z.W. Pharmacokinetics of glutathione and its metabolites in normal subjects. J. Korean Med. Sci. 2005, 20, 721–726. [Google Scholar] [CrossRef][Green Version]



| CHD (n = 35) | |
|---|---|
| CHD characteristics | |
| Tetralogy of Fallot | 15 |
| Transposition of great arteries | 6 |
| Tricuspid atresia | 8 |
| Total anomalous pulmonary venous return | 6 |
| Age (months) | 30 ± 8 |
| Weight (kg) | 11 ± 2 |
| Gender (F/M) | 10/25 |
| Laboratory parameters | |
| Hemoglobin (g/dL) | 12.3 ± 1.2 |
| Hematocrit (%) | 35.5 ± 2.9 |
| Platelet count (103/mmc) | 325 ± 102 |
| Creatinine (mg/dL) | 0.43 ± 0.25 |
| Urea (mg/dL) | 29 ± 14 |
| LDH (UI/L) | 565 ± 206 |
| CK (UI/L) | 168 ± 114 |
| Glycaemia (mg/dL) | 103 ± 12 |
| Neurological examination | |
| Preoperative (normal/suspect/abnormal) | 35/0/0 |
| Postoperative (normal/suspect/abnormal) | 35/0/0 |
| Main interventions | |
| CPB (min) | 90 ± 66 |
| Filtration (n/total) | 19/25 |
| Clamping (min) | 46 ± 34 |
| Circulatory arrest (n/total) | 2/25 |
| Cooling (°C) | 31.9 ± 3.1 |
| Parameters | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 11.7 ± 2.2 | 11.5 ± 2.4 | 11 ± 2.9 | 11.1 ± 1.4 | 11.8 ± 1.5 |
| Hematocrit rate (%) | 35.9 ± 3.8 | 33.8 ± 5.4 | 32.9 ± 6.3 | 33.4 ± 4.2 | 34.5 ± 4.7 |
| pH | 7.36 ± 0.10 | 7.36 ± 0.10 | 7.38 ± 0.08 | 7.39 ± 0.08 | 7.42 ± 0.07 |
| PaCO2 (mmHg) | 36.9 ± 6.6 | 35.4 ± 3.9 | 34.4 ± 5.5 | 35.5 ± 5.1 | 36.2 ± 5.9 |
| PaO2 (mmHg) | 101 ± 37 | 144 ± 78 * | 211 ± 101 * | 163 ± 96 * | 155 ± 86 * |
| HCO3 (mmol/L) | 22.1 ± 3.1 | 22.2 ± 3.9 | 21.1 ± 3.2 | 21.2 ± 1.9 | 22.2 ± 1.8 |
| BE (mmol/L) | 0.2 ± 2.5 | −1.8 ± 3.6 | −3.0 ± 2.1 | −0.5 ± 0.7 | 1.5 ± 1.5 |
| SaO2 (mmHg) | 94.9 ± 8.8 | 93.8 ± 7.2 | 97.3 ± 1.8 | 92.7 ± 2.6 | 95.3 ± 5.5 |
| Heart rate (bpm) | 104 ± 11 | 113 ± 14 | 121 ± 14 | 122 ± 12 | 125 ± 19 |
| LA BP (mmHg) | 8.0 ± 3.9 | 7.7 ± 4.0 | 9.2 ± 3.5 | 9.5 ± 4.2 | 9.3 ± 4.0 |
| RA BP (mmHg) | 9.1 ± 2.2 | 8.7 ± 1.6 | 8.8 ± 2.1 | 9.8 ± 2.3 | 10 ± 2.8 |
| Systolic BP (mmHg) | 88 ± 12 | 87 ± 17 | 86 ± 14 | 95 ± 15 | 96 ± 13 |
| Diastolic BP (mmHg) | 42 ± 11 | 53 ± 10 | 54 ± 10 | 56 ± 10 | 57 ± 11 |
| Glycaemia (mg/dl) | 103 ± 12 | 118 ± 11 | 130 ± 19 | 125 ± 15 | 119 ± 14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satriano, A.; Franchini, S.; Lapergola, G.; Pluchinotta, F.; Anastasia, L.; Baryshnikova, E.; Livolti, G.; Gazzolo, D. Glutathione Blood Concentrations: A Biomarker of Oxidative Damage Protection during Cardiopulmonary Bypass in Children. Diagnostics 2019, 9, 118. https://doi.org/10.3390/diagnostics9030118
Satriano A, Franchini S, Lapergola G, Pluchinotta F, Anastasia L, Baryshnikova E, Livolti G, Gazzolo D. Glutathione Blood Concentrations: A Biomarker of Oxidative Damage Protection during Cardiopulmonary Bypass in Children. Diagnostics. 2019; 9(3):118. https://doi.org/10.3390/diagnostics9030118
Chicago/Turabian StyleSatriano, Angela, Simone Franchini, Giuseppe Lapergola, Francesca Pluchinotta, Luigi Anastasia, Ekaterina Baryshnikova, Giovanni Livolti, and Diego Gazzolo. 2019. "Glutathione Blood Concentrations: A Biomarker of Oxidative Damage Protection during Cardiopulmonary Bypass in Children" Diagnostics 9, no. 3: 118. https://doi.org/10.3390/diagnostics9030118
APA StyleSatriano, A., Franchini, S., Lapergola, G., Pluchinotta, F., Anastasia, L., Baryshnikova, E., Livolti, G., & Gazzolo, D. (2019). Glutathione Blood Concentrations: A Biomarker of Oxidative Damage Protection during Cardiopulmonary Bypass in Children. Diagnostics, 9(3), 118. https://doi.org/10.3390/diagnostics9030118






