Qualitative Versus Quantitative Mammographic Breast Density Assessment: Applications for the US and Abroad
Abstract
:1. Introduction
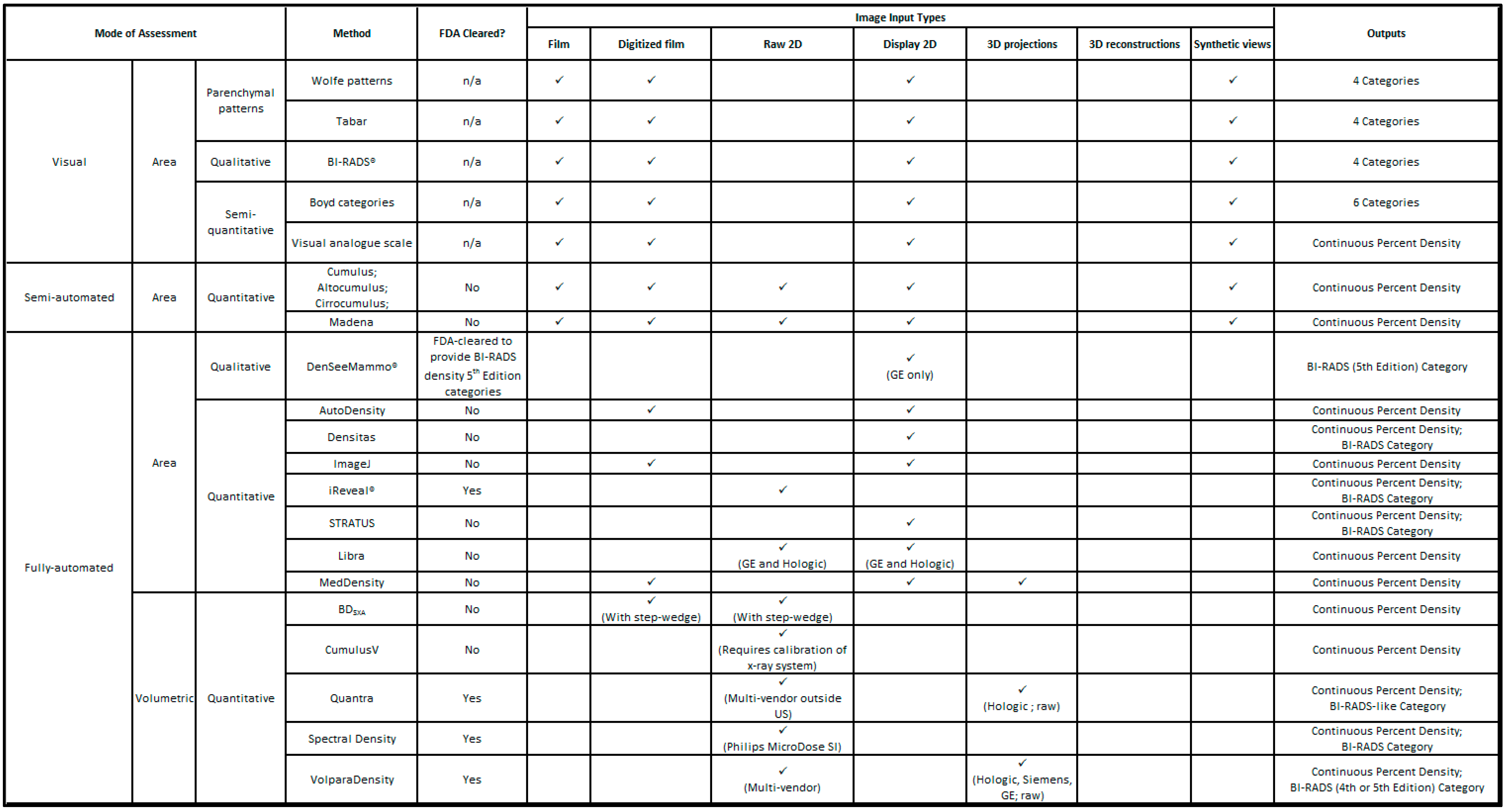
2. Mammographic Density Assessment Methods
3. Visual Methods
3.1. Parenchymal Patterns
3.2. Semi-Quantitative
3.3. BI-RADS
4. Semi-Automated Density Assessment
5. Fully-Automated Density Assessment
5.1. Area Methods
5.2. Volumetric Methods
6. Advantages and Limitations of MBD Assessment Methods
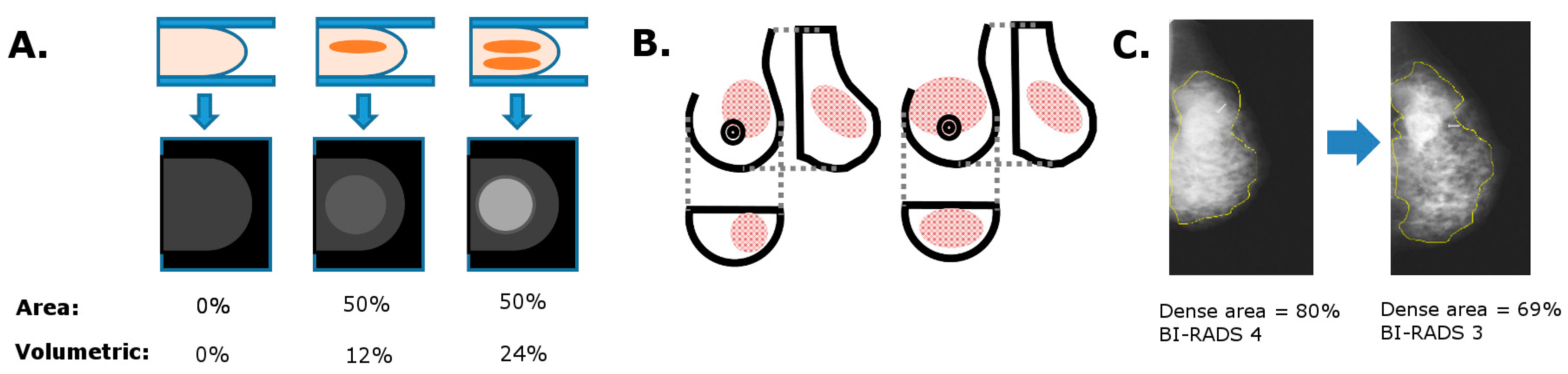
6.1. Area vs. Volumetric MBD
6.2. Consistency in MBD Measurements
6.3. Image Post-Processing Effects
7. The Current Clinical Landscape of MBD
7.1. MBD and Mammographic Sensitivity
7.2. US Density Notification Legislation
7.3. Supplemental Screening
8. MBD and Breast Cancer Risk
8.1. Incorporation of MBD into Risk Prediction Models
8.2. MBD and Breast Cancer Prognosis
8.3. Longitudinal Changes in MBD
8.4. Reducing Breast Density: Reducing Risk?
9. Conclusions
Author Contributions
Conflicts of Interest
References
- Martin, L.J.; Boyd, N.F. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: Hypotheses based on epidemiological evidence. Breast Cancer Res. 2008, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Dite, G.S.; Stone, J.; Gunasekara, A.; English, D.R.; McCredie, M.R.; Giles, G.G.; Tritchler, D.; Chiarelli, A.; Yaffe, M.J.; et al. Heritability of Mammographic Density, a Risk Factor for Breast Cancer. N. Engl. J. Med. 2002, 347, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.S.; Humphreys, K.; Thompson, D.J.; Li, J.; Eriksson, M.; Hall, P.; Czene, K. Volumetric Mammographic Density: Heritability and Association With Breast Cancer Susceptibility Loci. J. Natl. Cancer Inst. 2014, 106, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.; Dite, G.S.; Gunasekara, A.; English, D.R.; McCredie, M.R.E.; Giles, G.G.; Cawson, J.N.; Hegele, R.A.; Chiarelli, A.M.; Yaffe, M.J.; et al. The Heritability of Mammographically Dense and Nondense Breast Tissue. Cancer Edpidemiol. Biomark. Prev. 2006, 15, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Destounis, S.; Johnston, L.; Highnam, R.; Arieno, A.; Morgan, R.; Chan, A. Using Volumetric Breast Density to Quantify the Potential Masking Risk of Mammographic Density. Am. J. Roentgenol. 2017, 208, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Hislop, G.; Chiarelli, A.; Minkin, S.; et al. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Yankaskas, B.C.; Cleveland, R.J.; Schell, M.J.; Kozar, R. Association of Recall Rates with Sensitivity and Positive Predictive Values of Screening Mammography. Am. J. Roentgenol. 2001, 177, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Kerlikowske, K. The mammogram that cried Wolfe. N. Engl. J. Med. 2007, 356, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Mandelson, M.T.; Oestreicher, N.; Porter, P.L.; White, D.; Finder, C.A.; Taplin, S.H.; White, E. Breast density as a predictor of mammographic detection: Comparison of interval- and screen-detected cancers. J. Natl. Cancer Inst. 2000, 92, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Carney, P.A.; Miglioretti, D.L.; Yankaskas, B.C.; Kerlikowske, K.; Rosenberg, R.; Rutter, C.M.; Geller, B.M.; Abraham, L.A.; Taplin, S.H.; Dignan, M.; et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann. Intern. Med. 2003, 138, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Kolb, T.M.; Lichy, J.; Newhouse, J.H. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations. Radiology 2002, 225, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Park, C.C.; Remberg, J.; Chew, K.; Moore, D.; Kerliwkowske, K. High mammographic breast density is independent predictor of local but not distant recurrence after lumpectomy and radiotherapy for invasive breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Czene, K.; Rosenberg, L.U.; Tornberg, S.; Humphreys, K.; Hall, P. Mammographic density and survival in interval breast cancers. Breast Cancer Res. 2013, 15, R48. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.-H.; Lau, S. Vision 20/20: Mammographic breast density and its clinical applications. Med. Phys. 2015, 42, 7059–7077. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Juette, A.; Denton, E.R.E.; Oliver, A.; Martí, R.; Zwiggelaar, R. A review on automatic mammographic density and parenchymal segmentation. Int. J. Breast Cancer 2015, 2015, 276217. [Google Scholar] [CrossRef] [PubMed]
- Ekpo, E.U.; McEntee, M.F. Measurement of breast density with digital breast tomosynthesis—A systematic review. Br. J. Radiol. 2014, 87, 20140460. [Google Scholar] [CrossRef] [PubMed]
- Ekpo, E.U.; Hogg, P.; Highnam, R.; McEntee, M.F. Breast composition: Measurement and clinical use. Radiography 2015, 21, 324–333. [Google Scholar] [CrossRef]
- Wolfe, J.N. Breast patterns as an index of risk for developing breast cancer. Am. J. Roentgenol. 1976, 126, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.N. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer 1976, 37, 2486–2492. [Google Scholar] [CrossRef]
- Gram, I.T.; Funkhouser, E.; Tabar, L. The Tabar classification of mammographic parenchymal patterns. Eur. J. Radiol. 1997, 24, 131–136. [Google Scholar] [CrossRef]
- Egan, R.L.; Mosteller, R.C. Breast cancer mammography patterns. Cancer 1977, 40, 2087–2090. [Google Scholar] [CrossRef]
- Mendell, L.; Rosenbloom, M.; Naimark, A. Are breast patterns a risk index for breast cancer? A reappraisal. Am. J. Roentgenol. 1977, 128, 547. [Google Scholar] [CrossRef] [PubMed]
- Ernster, V.L.; Sacks, S.T.; Peterson, C.A.; Schweitzer, J.J. Mammographic parenchymal patterns and risk factors for breast cancer. Radiology 1980, 134, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.; Gartside, P.; McLaughlin, C. Mammographic patterns as markers for high-risk, benign breast disease and incident cancers. Radiology 1980, 134, 293–295. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, M.B. Breast parenchymal pattern as an indicator of risk for developing breast cancer. J. Med. Assoc. Ga. 1978, 67, 413–414. [Google Scholar] [PubMed]
- Brisson, J.; Diorio, C.; Masse, B. Wolfe’s parenchymal pattern and percentage of the breast with mammographic densities: Redundant or complementary classifications? Cancer Epidemiol. Biomark. Prev. 2003, 12, 728–732. [Google Scholar]
- Whitehead, J.; Carlile, T.; Kopecky, K.J.; Thompson, D.J.; Gilbert, F.I.; Present, A.J.; Threatt, B.A.; Krook, P.; Hadaway, E. The Relationship between Wolfe’s Classification of Mammograms, Accepted Breast Cancer Risk Factors, and the Incidence of Breast Cancer. Am. J. Epidemiol. 1985, 122, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Jensen, H.M.; Cooke, G.; Han, H.L. Relationship Between Mammographic and Histological Risk Factors for Breast Cancer. J. Natl. Cancer Inst. 1992, 84, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Byng, J.W.; Jong, R.A.; Fishell, E.K.; Little, L.E.; Miller, A.B.; Lockwood, G.A.; Tritchler, D.L.; Yaffe, M.J. Quantitative classification of mammographic densities and breast cancer risk: Results from the Canadian National Breast Screening Study. J. Natl. Cancer Inst. 1995, 87, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, J.C.; Warwick, J.; Evans, D.G.; Howell, A.; Berks, M.; Stavrinos, P.; Sahin, S.; Wilson, M.; Hufton, A.; Buchan, I.; et al. Volumetric and Area-Based Breast Density Measurement in the Predicting Risk of Cancer at Screening (PROCAS) Study. In IWDM 2012 Breast Imaging; Lecture Notes in Computer Science; Maidment, A.D.A., Bakic, P.R., Gavenonis, S., Eds.; Springer: Berline/Heidelberg, Germany, 2012; Volume 7361. [Google Scholar]
- Sukha, A.; Berks, M.; Morris, J.; Boggis, C.; Wilson, M.; Barr, N.; Astley, S. Visual Assessment of Density in Digital Mammograms. In IWDM 2010 Digital Mammography; Lecture Notes in Computer Science; Marti, J., Oliver, A., Freixenet, J., Marti, R., Eds.; Springer: Berline/Heidelberg, Germany, 2010; Volume 6136. [Google Scholar]
- Sperrin, M.; Bardwell, L.; Sergeant, J.C.; Astley, S.; Buchan, I. Correcting for rater bias in scores on a continuous scale, with application to breast density. Stat. Med. 2013, 32, 4666–4678. [Google Scholar] [CrossRef] [PubMed]
- Beattie, L.; Harkness, E.; Bydder, M.; Sergeant, J.; Maxwell, A.; Barr, N.; Beetles, U.; Coggis, C.; Blundred, S.; Gadde, S.; et al. Factors Affecting Agreement between Breast Density Assessment Using Volumetric Methods and Visual Analogue Scales. In IWDM 2014 Breast Imaging; Lecture Notes in Computer Science; Fujita, H., Hara, T., Muramatsu, C., Eds.; Springer: Cham, Switzerland, 2014; Volume 8539. [Google Scholar]
- Evans, D.G.; Astley, S.; Stavrinos, P.; Harkness, E.; Donnelly, L.S.; Dawe, S.; Jacob, I.; Harvie, M.; Cuzick, J.; Brentnall, A.; et al. Improvement in Risk Prediction, Early Detection and Prevention of Breast Cancer in the NHS Breast Screening Programme and Family History Clinics: A Dual Cohort Study. In Programme Grants for Applied Research; NIHR Journals Library: Southampton, UK, 2016. [Google Scholar]
- American College of Radiology. BI-RADS® Atlas, 4th ed.; American College of Radiology: Reston, VA, USA, 2003. [Google Scholar]
- American College of Radiology. BI-RADS® Atlas, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Byng, J.W.; Boyd, N.F.; Fishell, E.; Jong, R.A.; Yaffe, M.J. The quantitative analysis of mammographic densities. Phys. Med. Biol. 1994, 39, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Bovbjerg, V.E. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology 2004, 230, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Lockwood, G.A.; Byng, J.W.; Tritchler, D.L.; Yaffe, M.J. Mammographic densities and breast cancer risk. Cancer Epidemiol. Biomark. Prev. 1998, 7, 1133–1144. [Google Scholar] [CrossRef]
- Bakic, P.R.; Kontos, D.; Zhang, C.; Yaffe, M.J.; Maidment, A.D.A. Analysis of percent density estimates from digital breast tomosynthesis projection images. In Proceedings of the Medical Imaging 2007: Computer-Aided Diagnosis, San Diego, CA, USA, 17 February 2007; Giger, M.L., Karssemeijer, N., Eds.; Proc SPIE 2007 Volume 6514; Abstract Number 651424. [Google Scholar]
- Ursin, G.; Astrahan, M.A.; Salane, M.; Parisky, Y.R.; Pearce, J.G.; Daniels, J.R.; Pike, M.C.; Spicer, D.V. The detection of changes in mammographic densities. Cancer Epidemiol. Biomark. Prev. 1998, 7, 43–47. [Google Scholar]
- Nguyen, T.L.; Aung, Y.K.; Evans, C.F.; Yoon-Ho, C.; Jenkins, M.A.; Sung, J.; Hopper, J.L.; Song, Y.-M. Mammographic density defined by higher than conventional brightness threshold better predicts breast cancer risk for full-field digital mammograms. Breast Cancer Res. 2015, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Szekely, L.; Eriksson, L.; Heddson, B.; Sundbom, A.; Czene, K.; Hall, P.; Humphreys, K. High-throughput mammographic-density measurement: A tool for risk prediction of breast cancer. Breast Cancer Res. 2012, 14, R114. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.M.; Chen, J.; Daye, D.; Conant, E.F.; Kontos, D. Preliminary evaluation of the publicly available Laboratory for Breast Radiodensity Assessment (LIBRA) software tool: Comparison of fully automated area and volumetric density measures in a case-control study with digital mammography. Breast Cancer Res. 2015, 17, 117. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Czene, K.; Pawitan, Y.; Leifland, K.; Darabi, H.; Hall, P. A clinical model for identifying the short-term risk of breast cancer. Breast Cancer Res. 2017, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.; Tagliafico, G.; Tosto, S.; Chiesa, F.; Martinoli, C.; Derchi, L.E.; Calabrese, M. Mammographic density estimation: Comparison among BI-RADS categories, a semi-automated software and a fully automated one. Breast 2009, 18, 35–40. [Google Scholar] [CrossRef] [PubMed]
- iCad Inc. 2016 iReveal Product Brochure. Available online: http://www.icadmed.com/assets/dmm211_powerlook_density_assessment_reve.pdf (accessed on 29 May 2017).
- FDA, M-VU BREAST DENSITY 510(k) Premarket Notification. 2017. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf13/K132742.pdf (accessed on 28 March 2017).
- Keating, D.M.; D’Alessio, D.D.; Feigin, K.N. BI-RADS 5th Edition Breast Density Classification: Comparison of Radiologist and Automated System. In Proceedings of the RSNA 2015, Radiological Society of North America, Chicago, IL, USA, 29 November–4 December 2015. [Google Scholar]
- Wengert, G.J.; Woitek, R.; Baltzer, P.; Kapetas, P.; Magometschnigg, H.; Bickel, H.; Helbich, K.; Pinker-Domenig, K. Validation of a new fully automated quantitative breast density measuremet system with FFDM-comparison with qualitative ACR BI-RADS assessmet. In Proceedings of the ECR 2015, European Congress of Radiology, Vienna, Austria, 4–8 March 2015. [Google Scholar]
- Abdolell, M.; Tsuruda, K.M.; Lightfoot, C.B.; Payne, J.I.; Caines, J.S.; Iles, S.E. Utility of relative and absolute measures of mammographic density vs clinical risk factors in evaluating breast cancer risk at time of screening mammography. Br. J. Radiol. 2015, 89, 20150522. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.A.; Herve, L.; Landau, J.; Fan, B.; Kerlikowske, K.; Cummings, S.R. Novel use of Single X-ray Absorptiometry for Measuring Breast Density. Technol. Cancer Res. Treat. 2005, 4, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Alonzo-Proulx, O.; Packard, N.; Boone, J.M.; Al-Mayah, A.; Brock, K.K.; Shen, S.Z.; Yaffe, M.J. Validation of a method for measuring the volumetric breast density from digital mammograms. Phys. Med. Biol. 2010, 55, 3027–3044. [Google Scholar] [CrossRef] [PubMed]
- Alonzo-Proulx, O.; Jong, R.A.; Yaffe, M.J. Volumetric breast density characteristics as determined from digital mammograms. Phys. Med. Biol. 2012, 57, 7443–7457. [Google Scholar] [CrossRef] [PubMed]
- Hologic Inc. Understanding Quantra™ 2.0 User Manual; Hologic Inc.: Bedford, MA, USA, 2012; Volume MAN-02004 Rev 004. [Google Scholar]
- Van Engeland, S.; Snoeren, P.R.; Huisman, H.; Boetes, C.; Karssemeijer, N. Volumetric breast density estimation from full-field digital mammograms. IEEE Trans. Med. Imaging 2006, 25, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Highnam, R.; Brady, M.; Yaffe, M.J.; Karssemeijer, N.; Harvey, J. Robust breast composition measurement—Volpara™. In Proceedings of the Digital Mammography: 10th International Workshop, IWDM 2010, Girona, Catalonia, Spain, 16–18 June 2010; Martí, J., Oliver, A., Freixenet, J., Martí, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 342–349. [Google Scholar]
- Lau, S.; Ng, K.-H.; Abdul Aziz, Y.F. Volumetric breast density measurement: Sensitivity analysis of a relative physics approach. Br. J. Radiol. 2016, 89, 20160258. [Google Scholar] [CrossRef] [PubMed]
- Gweon, H.M.; Youk, J.H.; Kim, J.A.; Son, E.J. Radiologist assessment of breast density by BI-RADS categories versus fully automated volumetric assessment. Am. J. Roentgenol. 2013, 201, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.M.; Ko, E.S.; Han, B.K.; Ko, E.Y.; Shin, J.H.; Hahn, S.Y. Automated volumetric breast density estimation: A comparison with visual assessment. Clin. Radiol. 2013, 68, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.R.; Scott, C.G.; Ma, L.; Mahmoudzadeh, A.P.; Jensen, M.R.; Whaley, D.H.; Wu, F.F.; Malkov, S.; Hruska, C.B.; Norman, A.D.; et al. Comparison of clinical and automated breast density measurements: Implications for risk prediction and supplemental screening. Radiology 2016, 279, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Azziz, A.; Fan, B.; Malkov, S.; Klifa, C.; Newitt, D.; Yitta, S.; Hylton, N.; Kerlikowske, K.; Shepherd, J.A. Agreement of mammographic measures of volumetric breast density to MRI. PLoS ONE 2013, 8, e81653. [Google Scholar] [CrossRef] [PubMed]
- Gubern-Merida, A.; Kallenberg, M.; Platel, B.; Mann, R.M.; Marti, R.; Karssemeijer, N. Volumetric breast density estimation from full field digital mammograms: A Validation Study. PLoS ONE 2014, 9, e85952. [Google Scholar] [CrossRef] [PubMed]
- Ducote, J.L.; Molloi, S. Quantification of breast density with dual energy mammography: A simulation study. Med. Phys. 2008, 35, 5411–5418. [Google Scholar] [CrossRef] [PubMed]
- Koninklijke Philips, N.V. Spectral Breast Density Measurement Tool Product Brochure. 2015. Available online: http://incenter.medical.philips.com/doclib/enc/11509347/4522_991_10201_Breast_Density_Tool_Product_Overview_Global_with_US_Final.pdf%3ffunc%3ddoc.Fetch%26nodeid%3d11509347 (accessed on 29 May 2017).
- Kilburn-Toppin, F.; Erhard, K.; Willsher, P.; Buelow, T.; Wieberneit, N.; Fredenberg, E.; Wallis, M.G. Characterisation of Breast Lesions with Spectral Mammography: Results on First Clinical Cases. In Proceedings of the ECR 2015, European Congress on Radiology, Vienna, Austria, 4–8 March 2015. [Google Scholar]
- Machida, Y.; Tozaki, M.; Yoshida, T.; Saita, A.; Yakabe, M.; Nii, K. Feasibility study of a breast density measurement within a direct photon-counting mammography scanner system. Jpn. J. Radiol. 2014, 32, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Molloi, S.; Ding, H.; Feig, S. Breast density evaluation using spectral mammography, radiologist reader assessment, and segmentation techniques: A retrospective study based on left and right breast comparison. Acad. Radiol. 2015, 22, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Ciatto, S.; Houssami, N.; Apruzzese, A.; Bassetti, E.; Brancato, B.; Carozzi, F.; Catarzi, S.; Lamberini, M.P.; Marcelli, G.; Pellizzoni, R.; et al. Categorizing breast mammographic density: Intra- and interobserver reproducibility of BI-RADS density categories. Breast 2005, 14, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Irshad, A.; Leddy, R.; Ackerman, S.; Cluver, A.; Pavic, D.; Abid, A.; Lewis, M.C. Effects of changes in BI-RADS density assessment guidelines (fourth versus fifth edition) on breast density assessment: Intra- and interreader agreements and density distribution. Am. J. Roentgenol. 2016, 207, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Van der Waal, D.; den Heeten, G.J.; Pijnappel, R.M.; Schuur, K.H.; Timmers, J.M.H.; Verbeek, A.L.M.; Broeders, M.J.M. Comparing visually assessed BI-RADS breast density and automated volumetric breast density software: A cross-sectional study in a breast cancer screening setting. PLoS ONE 2015, 10, e0136667. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Estepa, M.; Ruiz-Perales, F.; Miranda, J.; Ascunce, N.; González-Román, I.; Sánchez-Contador, C.; Santamarina, C.; Moreo, P.; Vidal, C.; Peris, M.; et al. Evaluation of mammographic density patterns: Reproducibility and concordance among scales. BMC Cancer 2010, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Sprague, B.L.; Conant, E.F.; Onega, T.; Garcia, M.P.; Beaber, E.F.; Herschorn, S.D.; Lehman, C.D.; Tosteson, A.N.A.; Lacson, R.; Schnall, M.D.; et al. Variation in mammographic breast density assessments among radiologists in clinical practice: A multicenter observational study. Ann. Intern. Med. 2016, 165, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Mackesy, M.M.; Winkler, N.S.; Hurwitz, S.; Birdwell, R.L. Effect of training on qualitative mammographic density assessment. J. Am. Coll. Radiol. 2016, 13, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Youk, J.H.; Gweon, H.M.; Son, E.J.; Kim, J.A. Automated volumetric breast density measurements in the era of the BI-RADS fifth edition: A comparison with visual assessment. Am. J. Roentgenol. 2016, 206, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Conant, E.F.; Li, D.; Gavenonis, S.; Bakic, P.R.; Carton, A.-K.; Zhang, C.; Maidment, A.D.A.; Kontos, D. A Comparative Study of the Inter-Reader Variability of Breast Percent Density Estimation in Digital Mammography: Potential Effect of Reader’s Training and Clinical Experience. In IWDM 2010 Digital Mammography; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6136, pp. 114–120. [Google Scholar]
- Alonzo-Proulx, O.; Mawdsley, G.E.; Patrie, J.T.; Yaffe, M.J.; Harvey, J.A. Reliability of Automated Breast Density Measurements. Radiology 2015, 275, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Zanca, F.; Jacobs, J.; van Ongeval, C.; Claus, F.; Celis, V.; Geniets, C.; Provost, V.; Pauwels, H.; Marchal, G.; Bosmans, H. Evaluation of clinical image processing algorithms used in digital mammography. Med. Phys. 2009, 36, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.; Byrnes, G.; Stone, J.; Tamimi, R.M.; Heine, J.; Vachon, C.; Ozmen, V.; Pereira, A.; Garmendia, M.L.; Scott, C. Mammographic density assessed on paired raw and processed digital images and on paired screen-film and digital images across three mammography systems. Breast Cancer Res. 2016, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Vinnicombe, S.J.; Evans, A.J.; Hart, K.; Whelehan, P.; Dundee, G.B. Visual And Automated Volumetric Assessment of Mammographic Density (MD): Do Measurements Depend on the Digital Mammography Unit? In Proceedings of the ECR 2014, European Congress on Radiology, Vienna, Austria, 6–10 March 2014. [Google Scholar]
- Damases, C.N.; Brennan, P.C.; McEntee, M.F. Mammographic density measurements are not affected by mammography system. J. Med. Imaging 2015, 2, 015501. [Google Scholar] [CrossRef] [PubMed]
- Skaane, P.; Bandos, A.I.; Eben, E.B.; Jebsen, I.N.; Krager, M.; Haakenaasen, U.; Ekseth, U.; Izadi, M.; Hofvind, S.; Gullien, R. Two-view digital breast tomosynthesis screening with synthetically reconstructed projection images: Comparison with digital breast tomosynthesis with full-field digital mammographic images. Radiology 2014, 271, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Svahn, T.M.; Houssami, N.; Sechopoulos, I.; Mattsson, S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 2015, 24, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Miravete, P.; Millor Muruzábal, M.; García-Barquín, P.; Elizalde, A.; Pina, L.; Etxano, J.; Bartolomé, P. Does the Synthesised Digital Mammography (3D-DM) Change the ACR Density Pattern? In Proceedings of the ECR 2015, European Congress of Radiology, Vienna, Austria, 4–8 March 2015. [Google Scholar]
- Conant, E.F.; Keller, B.M.; Pantalone, L.; Gastounioti, A.; McDonald, E.S.; Kontos, D. Agreement between Breast Percentage Density Estimattions from Standard-Dose versus Synthetic Digital Mammograms: Results from a Large Screening Cohort Using Autimated Measures. Radiology 2017, 283, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Pertuz, S.; McDonald, E.S.; Weinstein, S.P.; Conant, E.F.; Kontos, D. Fully automated quantitative estimation of volumetric breast density from digital breast tomosynthesis images: Preliminary results and comparison with digital mammography and MR imaging. Radiology 2016, 279, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Tromans, C.; Highnam, R.; Morrish, O.; Black, R.; Tuckers, L.; Gilbert, F.J. Volumetric Breast Density Estimation on Conventional Mammography Versus Digital Breast Tomosynthesis. In Proceedings of the ECR 2014, European Congress of Radiology, Vienna, Austria, 6–10 March 2014. [Google Scholar]
- Pisano, E.D.; Hendrick, R.E.; Yaffe, M.J.; Baum, J.K.; Acharyya, S.; Cormack, J.B.; Hanna, L.A.; Conant, E.F.; Fajardo, L.L.; Bassett, L.W.; et al. Diagnostic Accuracy of Digital versus Film Mammography: Exploratory Analysis of Selected Population Subgroups in DMIST. Radiology 2008, 246, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Prummel, M.V.; Muradali, D.; Shumak, R.; Majpruz, V.; Brown, P.; Jiang, H.; Done, S.J.; Yaffe, M.J.; Chiarelli, A.M. Digital Compared with Screen-Film Mammography: Measures of Diagnostic Accuracy among Women Screened in the Ontario Breast Screening Program. Radiology 2016, 278, 356–373. [Google Scholar] [CrossRef] [PubMed]
- Wanders, J.O.; Holland, K.; Veldhuis, W.B.; Mann, R.M.; Pijnappel, R.M.; Peeters, P.H.; van Gils, C.H.; Karssemeijer, N. Volumetric breast density affects performance of digital screening mammography. Breast Cancer Res. Treat. 2017, 162, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Maniprize, J.G.; Alonzo-Proulx, O.; Jon, R.A.; Yaffe, M.J. Quantifying masking in clinial mammograms via local detectability of simulated lesions. Med. Phys. 2016, 43, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Holland, K.; van Zelst, J.; den Heeten, G.J.; Imhof-Tas, M.; Mann, R.M.; van Gils, C.H.; Karssemeijer, N. Consistency of breast density categories in serial screening mammograms: A comparison between automated and human assessment. Breast 2016, 29, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Are You Dense Inc. Are You Dense? Exposing the Best-Kept Secret. Available online: https://www.areyoudense.org (accessed on 28 March 2017).
- Freer, P.E. Mammographic breast density: Impact on breast cancer risk and implications for screening. Radiographics 2015, 35, 302–315. [Google Scholar] [CrossRef] [PubMed]
- DenseBreast-Info Inc. DenseBreast-Info. Available online: http://densebreast-info.org/ (accessed on 28 March 2017).
- Breast Density and Mammography Reporting Act of 2015, H.R.716. In Proceedings of the 114th Congress (2015), Washington, DC, USA, 3 January 2015–3 January 2017; Available online: https://www.congress.gov/bill/114th-congress/house-bill/716 (accessed on 29 May 2017).
- Schilling, K.; The, J.; Griff, S.; Oliver, L.; Mahal, R.; Saady, M.; Velasquez, V. Impact of quantitative breast density on experienced radiologists’ assessment of mammographic breast density. In Proceedings of the ECR 2015, European Congress of Radiology, Vienna, Austria, 4–8 March 2015. [Google Scholar]
- Berg, W.A.; Blume, J.D.; Cormack, J.B.; Mendelson, E.B.; Lehrer, D.; Bohm-Velez, M.; Pisano, E.D.; Jong, R.A.; Evans, W.P.; Morton, M.J.; et al. Combined Screening With Ultrasound and Mammography vs. Mammography Alone in Women at Elevated Risk of Breast Cancer. JAMA 2008, 299, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Zhang, Z.; Lehrer, D.; Jong, R.A.; Pisano, E.D.; Barr, R.G.; Bohm-Velez, M.; Mahoney, M.C.; Evans, W.P.; Larsen, L.H.; et al. Detection of Breast Cancer With Addition of Annual Screening Ultrasound or a Single Screening MRI to Mammography in Women with Elevated Breast Cancer Risk. JAMA 2012, 307, 1394–1404. [Google Scholar] [PubMed]
- FDA, PMA Notification Letter. Available online: https://google2.fda.gov/search?q=cache:SKN1t-KxTD0J:www.accessdata.fda.gov/cdrh_docs/pdf5/k052355.pdf+U-Systems&client=FDAgov&site=FDAgov&lr=&proxystylesheet=FDAgov&output=xml_no_dtd&ie=UTF-8&access=p&oe=UTF-8 (accessed on 29 May 2017).
- Wilczek, B.; Wilczek, H.E.; Rasouliyan, L.; Leifland, K. Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program. Eur. J. Radiol. 2016, 85, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, S.M.; Rafferty, E.A.; Rose, S.L.; Durand, M.A.; Plecha, D.M.; Greenberg, J.S.; Hayes, M.K.; Copit, D.S.; Carlson, K.L.; Cink, T.M.; et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014, 311, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Ciatto, S.; Houssami, N.; Bernardi, D.; Caumo, F.; Pellegrini, M.; Brunelli, S.; Tuttobene, P.; Bricolo, P.; Fanto, C.; Valentini, M.; et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): A prospective comparison study. Lancet Oncol. 2013, 14, 583–589. [Google Scholar] [CrossRef]
- Destounis, S.V.; Morgan, R.; Arieno, A. Screening for Dense Breasts: Digital Breast Tomosynthesis. Am. J. Roentgenol. 2015, 204, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Calabrese, M.; Mariscotti, G.; Durando, M.; Tosto, S.; Monetti, F.; Airaldi, S.; Bignotti, B.; Nori, J.; Bagni, A.; et al. Adjunct Screening with Tomosynthesis or Ultrasound in Women with Mammography-Negative Dense Breasts: Interim Report of a Prospective Comparative Trial. J. Clin. Oncol. 2016, 34, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Melnikow, J.; Fenton, J.J.; Whitlock, E.P.; Miglioretti, D.L.; Weyrich, M.S.; Thompson, J.H.; Shah, K. Supplemental screening for breast cancer in women with dense breasts: A systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Shermis, R.B.; Wilson, K.D.; Doyle, M.T.; Martin, T.S.; Merryman, D.; Kudrolli, H.; Brenner, R.J. Supplemental breast cancer screening with molecular breast imaging for women with dense breast tissue. Am. J. Roentgenol. 2016, 207, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Emaus, M.J.; Bakker, M.F.; Peeters, P.H.; Loo, C.E.; Mann, R.M.; de Jong, M.D.; Bisschops, R.H.; Veltman, J.; Duvivier, K.M.; Lobbes, M.B. MR Imaging as an Additional Screening Modality for the Detection of Breast Cancer in Women Aged 50–75 Years with Extremely Dense Breasts: The DENSE Trial Study Design. Radiology 2015, 277, 527–537. [Google Scholar] [CrossRef] [PubMed]
- ClincalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02933489 (accessed on 29 May 2017).
- Destounis, S.; Arieno, A.; Morgan, R. Initial Experience With the New York State Breast Density Inform Law at a Community-Based Breast Center. J. Ultrasound. Med. 2015, 34, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Mainiero, M.B.; Lourenco, A.; Mahoney, M.C.; Newell, M.S.; Bailey, L.; Barke, L.D.; D’Orsi, C.; Harvey, J.A.; Hayes, M.K.; Huynh, P.T.; et al. ACR Appropriateness Criteria Breast Cancer Screening. J. Am. Coll. Radiol. 2013, 10, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Sprague, B.L.; Stout, N.K.; Schechter, C.; van Ravesteyn, N.T.; Cevik, M.; Alagoz, O.; Lee, C.I.; van den Broek, J.J.; Miglioretti, D.L.; Mandelblatt, J.S.; et al. Potential impact of legislation mandating breast density notification: Benefits, harms, and cost effectiveness of supplemental utlrasound screening. Ann. Intern. Med. 2015, 162, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Plevritis, S.K.; Kurian, A.W.; Sigal, B.M.; Daniel, B.L.; Ikeda, D.M.; Stockdale, F.E.; Garber, A.M. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA 2006, 295, 2374–2384. [Google Scholar] [CrossRef] [PubMed]
- Eng, A.; Gallant, Z.; Shepherd, J.; McCormack, V.; Li, J.; Dowsett, M.; Vinnicombe, S.; Steve, A.; dos-Santos-Silva, I. Digital mammographic density and breast cancer risk: A case–control study of six alternative density assessment methods. Breast Cancer Res. 2014, 16, 439. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, K.A.; Tamimi, R.M.; Scott, C.G.; Jensen, M.R.; Pankratz, V.S.; Visscher, D.; Norman, A.; Couch, F.; Shepherd, J.; Fan, B.; et al. Mammographic density and risk of breast cancer by age and tumor characteristics. Breast Cancer Res. 2013, 15, R104. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2017; American Cancer Society: Atlanta, GA, USA, 2017. [Google Scholar]
- Sprague, B.L.; Gangnon, R.E.; Burt, V.; Trentham-Dietz, A.; Hampton, J.M.; Wellman, R.D.; Kerlikowske, K.; Miglioretti, D.L. Prevalence of mammographically dense breasts in the United States. J. Natl. Cancer Inst. 2014, 106, dju255. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Schairer, C.; Wolfe, J.; Parekh, N.; Salane, M.; Brinton, L.A.; Hoover, R.; Haile, R. Mammographic features and breast cancer risk: Effects with time, age, and menopause status. J. Natl. Cancer Inst. 1995, 87, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Engmann, N.J.; Golmakani, M.K.; Miglioretti, D.L.; Sprague, B.L.; Kerlikowske, K. Population-Attributable Risk Proportion of Clinical Risk Factors for Breast Cancer. JAMA Oncol. 2017. (online ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Yaffe, M.J. Mammographic density. Measurement of mammographic density. Breast Cancer Res. 2008, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Busana, M.C.; Eng, A.; Denholm, R.; Dowsett, M.; Vinnicombe, S.; Allen, S.; dos-Santos-Silva, I. Impract of type of full-field digital image on mammographic density assessment and breast cancer risk estimation: A case-control study. Breast Cancer Res. 2016, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Astley, S.; Harkness, E.; Sergeant, J.; Stavrinos, P.; Warren, R.; Wilson, M.; Brentnall, A.; Cuzick, J.; Howell, A.; Evans, G. Proffered Paper: A Comparison of four methods of mammogrpahic density measurement in the UK Predicting Risk of Cancer at Screening (PROCAS) study-on behalf of the PROCAS Study team. Eur. J. Cancer 2016, 61, s6. [Google Scholar] [CrossRef]
- Sergeant, J.C.; Wilson, M.; Barr, N.; Beetles, U.; Boggis, C.; Bundred, S.; Bydder, M.; Gadde, S.; Hurley, E.; Jain, A.; et al. PB.17: Inter-observer agreement in visual analogue scale asssessment of percentage breast density. Breast Cancer Res. 2013, 15, P17. [Google Scholar] [CrossRef]
- Jeffers, A.M.; Sieh, W.; Lipson, J.A.; Rothstein, J.H.; McGuire, V.; Whittemore, A.S.; Rubin, D.L. Breast Cancer Risk and Mammographic Density Assessed with Semiautomated and Fully Automated Methods and BI-RADS. Radiology 2016, 282, 348–355. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.A.; dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol Biomark. Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.A.; Kerlikowske, K.; Ma, L.; Duewer, F.; Fan, B.; Wang, J.; Malkov, S.; Vittinghoff, E.; Cummings, S.R. Volume of mammographic density and risk of breast cancer. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Jakes, R.W.; Duffy, S.W.; Ng, F.C.; Gao, F.; Ng, E.H. Mammographic parenchymal patterns and risk of breast cancer at and after a prevalence screen in Singapore women. Int. J. Epidemiol. 2000, 29, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ziv, E.; Tice, J.; Smith-Bindman, R.; Shepherd, J.; Cummings, S.; Kerlikowske, K. Mammographic Density and Estrogen Receptor Status of Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 2090–2095. [Google Scholar]
- Barlow, W.E.; White, E.; Ballard-Barbash, R.; Vacek, P.M.; Titus-Ernstoff, L.; Carney, P.A.; Tice, J.A.; Buist, D.S.; Geller, B.M.; Rosenberg, R.; et al. Prospective Breast Cancer Risk Prediction Model for Women Undergoing Screening Mammography. J. Natl. Cancer Inst. 2006, 98, 1204–1663. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.H.; Bihrmann, K.; Jensen, M.B.; Vejborg, I.; Lynge, E. Breast density and outcome of mammography screening: A cohort study. Br. J. Cancer 2009, 100, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Abdolell, M.; Tsuruda, K.; Payne, J.I.; Iles, S.E.; Lightfoot, C.B.; Caines, J. Breast Density from Full-Field Digital Mammograms and Breast Cancer Risk: A Case-Control Study. In Proceedings of the ECR 2014, European Congress of Radiology, Vienna, Austria, 6–10 March 2014. [Google Scholar]
- Cuzick, J.; Warwick, J.; Pinney, E.; Duffy, S.W.; Cawthorn, S.; Howell, A.; Forbes, J.F.; Warren, R.M. Tamoxifen-Induced Reduction in Mammographic Density and Breast Cancer Risk Reduction: A Nested Case-Control Study. J. Natl. Cancer Inst. 2011, 103, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, A.R.; Harkness, E.F.; Astley, S.M.; Donnelly, L.S.; Stavrinos, P.; Sampson, S.; Fox, L.; Sergeant, J.C.; Harvie, M.N.; Wilson, M.; et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res. 2015, 17, 147. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Martin, L.J.; Sun, L.; Guo, H.; Chiarelli, A.; Hislop, G.; Yaffe, M.; Minkin, S. Body size, mammographic density, and breast cancer risk. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Aiken, Z.; McCormack, V.A.; Highnam, R.P.; Martin, L.; Gunasekara, A.; Melnichouk, O.; Mawdsley, G.; Peressotti, C.; Yaffe, M.; Boyd, N.F.; et al. Screen-film mammographic density and breast cancer risk: A comparison of the volumetric standard mammogram form and the interactive threshold measurement methods. Cancer Epidemiol. Biomark. Prev. 2010, 19, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Lokate, M.; Peeters, P.H.; Peelen, L.M.; Haars, G.; Veldhuis, W.B.; Veldhuis, W.B.; van Gils, C.H. Mammographic density and breast cancer risk: The role of the fat surrounding the fibroglandular tissue. Breast Cancer Res. 2011, 13, R103. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Hankinson, S.E.; Willett, W.C.; Lagiou, P.; Trichopoulos, D.; Tamimi, R.M. Nondense mammographic area and risk of breast cancer. Breast Cancer Res. 2011, 13, R100. [Google Scholar] [CrossRef] [PubMed]
- Nickson, C.; Arzhaeva, Y.; Aitken, Z.; Elgindy, T.; Buckley, M.; Li, M.; English, D.R.; Kavanagh, A.M. AutoDensity: An automated method to measure mammographic breast density that predicts breast cancer risk and screening outcomes. Breast Cancer Res. 2013, 15, R80. [Google Scholar] [CrossRef] [PubMed]
- Ursin, G.; Ma, H.; Wu, A.H.; Bernstein, L.; Salane, M.; Parisky, Y.R.; Astrahan, M.; Siozon, C.C.; Pike, M.C. Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol. Biomark. Prev. 2003, 12, 332–338. [Google Scholar]
- Rauh, C.; Hack, C.C.; Haberle, L.; Hein, A.; Engel, A.; Schrauder, M.G.; Fasching, P.A.; Jud, S.M.; Ekici, A.B.; Loehberg, C.R.; et al. Percent Mammographic Density and Dense Area as Risk Factors for Breast Cancer. Geburtshilife Frauenheilkd. 2012, 72, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.R.; Hsieh, M.-K.; Scott, C.G.; Pantalone, L.; Jensen, M.; Winham, S.; Whaley, D.H.; Hruska, C.B.; Wu, F.F.; Norman, A.; et al. Validation Study of the Publically-Available Fully-Automated “LIBRA” Software for Mammographic Density Estimation: Results from a Case-Control Study of Breast Cancer. In Proceedings of the RSNA 2016, Radiological Society of North America 2016 Scientific Assembly and Annual Meeting, Chicago, IL, USA, 27 November–2 December 2016. [Google Scholar]
- Yaffe & Alonzo-Proulx. Volumetric Breast Density and Breast Cancer Risk from Digital Mammograms—Preliminary Results. In Proceedings of the 5th International Workshop on Breast Densitometry and Breast Cancer Risk Assessment, San Francisco, CA, USA, 9–10 June 2011. [Google Scholar]
- Kallenberg, M.; van Gils, C.; Mann, R.; Karssemeijer, N. Association between Automated, Volumetric Measures of Breast Density and Diagnostic Outcome of Mammography Screening Examinations. In Proceedings of the RSNA 2012, Radiological Society of North America 2012 Scientific Assembly and Annual Meeting, Chicago, IL, USA, 25–30 November 2012. [Google Scholar]
- Park, I.H.; Ko, K.; Joo, J.; Park, B.; Jung, S.Y.; Lee, S.; Kwon, Y.; Kang, H.S.; Lee, E.S.; Lee, K.S.; et al. High volumetric breast density predicts risk for breast cancer in postmenopausal, but not premenopausal, Korean Women. Ann. Surg. Oncol. 2014, 21, 4124–4132. [Google Scholar] [CrossRef] [PubMed]
- Battle, B.; Malak, S.F.; Dhakal, I.; Lee, J.; Keith, N.; Fuhrman, B. Associations of Volumetric Mammographic Density Measures with Breast Cancer Risk in 5746 Women. In Proceedings of the RSNA 2016, Radiological Society of North America 2016 Scientific Assembly and Annual Meeting, Chicago, IL, USA, 27 November–2 December 2016. [Google Scholar]
- Tice, J.A.; Cummings, S.R.; Ziv, E.; Kerlikowske, K. Mammographic breast density and the Gail model for breast cancer risk prediction in a screening population. Breast Cancer Res. Treat. 2005, 94, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Tice, J.A.; Cummings, S.R.; Smith-Bindman, R.; Ichikawa, L.; Barlow, W.E.; Kerlikowske, K. Using clinical factors and mammographic breast density to estimate breast cancer risk: Development and validation of a new predictive model. Ann. Intern. Med. 2008, 148, 337–347. [Google Scholar] [CrossRef] [PubMed]
- NCCN, Breast Cancer Risk Reduction. 2016. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf (accessed on 28 March 2017).
- Smith, R.A.; Andrews, K.; Brooks, D.; DeSantis, C.E.; Fedewa, S.A.; Lortet-Tieulent, J.; Manassaram-Baptiste, D.; Brawley, O.W.; Wender, R.C. Cancer screening in the United States, 2016: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2016, 66, 96–114. [Google Scholar] [CrossRef] [PubMed]
- USPSTF, Medications for Risk Reduction of Primary Breast Cancer in Women: U.S. Preventive Services Task Force Recommendation Statement. 2016. Available online: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-medications-for-risk-reduction (accessed on 28 March 2017).
- Cuzick, J. IBIS Breast Cancer Risk Evaluation Tool. Available online: http://www.ems-trials.org/riskevaluator/ (accessed on 28 March 2017).
- Ekpo, E.U.; Brennan, P.C.; Mello-Thoms, C.; McEntee, M.F. Relationship between breast density and selective estrogen-receptor modulators, aromatase inhibitors, physical activity, and diet: A systematic review. Integr. Cancer Ther. 2016, 15, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Habel, L.A.; Capra, A.M.; Achacoso, N.S.; Janga, A.; Acton, L.; Puligandla, B.; Quesenberrgy, C.P., Jr. Mammographic Density and Risk of Second Breast Cancer After Ductal Carcinoma in situ. J. Natl. Cancer Inst. 2004, 96, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Cil, T.; Fishell, E.; Hanna, W.; Sun, P.; Rawlinson, E.; Narod, S.A.; McCready, D.R. Mammographic Density and the Risk of Breast Cancer Recurrence After Breast-Conserving Surgery. Cancer 2009, 115, 5780–5787. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.Y.; Duffy, S.; Yen, A.M.; Tabar, L.; Smith, R.A.; Chen, H.H. Effect of baseline breast density on breast cancer incidence, stage, mortality, and screening parameters: 25-Year follow-up of a Swedish mammographic screening. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.J.R.; Evans, A.J.; Cornford, E.J.; Burrell, H.C.; James, J.J.; Lee, A.H.S.; Charkrabarti, J. Influence of Mammographic Parenchymal Pattern in Screening-Detected and Interval Invasive Breast Cancers on Pathologic Features, Mammographic Features, and Patient Survival. Am. J. Roentgenol. 2007, 188, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Gierach, G.L.; Ichikawa, L.; Kerlikowske, K.; Brinton, L.A.; Farhat, G.N.; Vacek, P.M.; Weaver, D.L.; Schairer, C.; Taplin, S.H.; Sherman, M.E. Relationship Between Mammographic Density and Breast Cancer Death in the Breast Cancer Surveillance Consortium. J. Natl. Cancer Inst. 2012, 104, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.; Humphreys, K.; Li, J.; Ploner, A.; Cheddad, A.; Eriksson, M.; Tornberg, S.; Hall, P.; Czene, K. Risk factors and tumor characteristics of interval cancers by mammographic density. J. Clin. Oncol. 2015, 33, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Pagano, I.S.; Little, M.A.; Conroy, S.M.; Park, S.-Y.; Kolonel, L.N. Mammographic density as a predictor of breast cancer survival: The Multiethnic Cohort. Breast Cancer Res. 2013, 15, R7. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Hartmann, L.C.; Reynolds, C.; Visscher, D.W.; Brandt, K.R.; Vierkant, R.A.; Scott, C.G.; Radisky, D.C.; Sellers, T.A.; Pankratz, V.S.; et al. Association Between Mammographic Density and Age-Related Lobular Involution of the Breast. J. Clin. Oncol. 2010, 28, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.A.; Perry, N.M.; Vinnicombe, S.J.; dos Santos Silva, I. Changes and tracking of mammographic density in relation to Pike’s model of breast tissue aging: A UK longitudinal study. Int. J. Cancer 2010, 127, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, L.E.; Pankratz, V.S.; Sellers, T.A.; Brandt, K.R.; Wang, A.; Janney, C.; Fredericksen, Z.S.; Cerhan, J.R.; Vachon, C.M. Age-specific trends in mammographic density: The Minnesota Breast Cancer Family Study. Am. J. Epidemiol. 2008, 167, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Reboussin, B.A.; Slone, S.; Wasilauskas, C.; Pike, M.C.; Ursin, G. Postmenopausal hormone therapy and change in mammographic density. J. Natl. Cancer Inst. 2003, 95, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Pagano, I.; Lurie, G.; Kolonel, L.N. A longitudinal investigation of mammographic density: The multiethnic cohort. Cancer Epidemiol. Biomark. Prev. 2006, 15, 732–739. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Martin, C.F.; Peck, J.D.; Aragaki, A.K.; Chlebowski, R.T.; Pisano, E.D.; Wang, C.Y.; Brunner, R.L.; Johnson, K.C.; Manson, J.E.; et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women’s Health Initiative randomized trial. J. Natl. Cancer Inst. 2005, 97, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Ursin, G.; Martin, C.F.; Peck, J.D.; Cole, E.B.; Zeng, D.; Kim, E.; Yaffe, M.D.; Boyd, N.F.; Heiss, G.; et al. Mammographic density change with estrogen and progestin therapy and breast cancer risk. J. Natl. Cancer Inst. 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Humphreys, K.; Eriksson, L.; Edgren, G.; Czene, K.; Hall, P. Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy in postmenopausal patients with breast cancer. J. Clin. Oncol. 2013, 31, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Busana, M.C.; De Stavola, B.L.; Sovio, U.; Li, J.; Moss, S.; Humphreys, K.; dos-Santos-Silva, I. Assessing within-woman changes in mammographic density: A comparison of fully versus semi-automated area-based approaches. Cancer Causes Control 2016, 27, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Hammann-Kloss, J.S.; Bick, U.; Fallenberg, E.; Engelken, F. Volumetric quantification of the effect of aging and hormone replacement therapy on breast composition from digital mammograms. Eur. J. Radiol. 2014, 83, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Lokate, M.; Stellato, R.K.; Veldhuis, W.B.; Peeters, P.H.; van Gils, C.H. Age-related changes in mammographic density and breast cancer risk. Am. J. Epidemiol. 2013, 178, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.M.; Pankratz, V.S.; Scott, C.G.; Maloney, S.D.; Ghosh, K.; Brandt, K.R.; Milanese, T.; Carston, M.J.; Sellers, T.A. Longitudinal trends in mammographic percent density and breast cancer risk. Cancer Epidemiol. Biomark. Prev. 2007, 16, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.M.; Suman, V.J.; Brandt, K.R.; Kosel, M.L.; Buzdar, A.U.; Olson, J.E.; Wu, F.F.; Flickinger, L.M.; Ursin, G.; Elliott, C.R.; et al. Mammographic breast density response to aromatase inhibition. Clin. Cancer Res. 2013, 19, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Meggiorini, M.L.; Labi, L.; Vestri, A.R.; Porfiri, L.M.; Savelli, S.; DeFelice, C. Tamoxifen in women with breast cancer and mammographic density. Eur. J. Gynaecol. Oncol. 2008, 29, 598–601. [Google Scholar] [PubMed]
- Nyante, S.J.; Sherman, M.E.; Pfeiffer, R.M.; de Gonzalez, A.B.; Brinton, L.A.; Bowles, E.J.A.; Hoover, R.N.; Glass, A.; Gierach, G.L. Longitudinal Change in Mammographic Density among ER-Positive Breast Cancer Patient Using Tamoxifen. Cancer Epidemiol. Biomark. Prev. 2016, 25, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Borgquist, S.; Eriksson, M.; Czene, K.; Hall, P. Abstract OT3-06-01: KARISMA, The karma intervention study—A tamoxifen dose determination trial. In Proceedings of the 2016 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 6–10 December 2016. [Google Scholar]
- Nyante, S.J.; Sherman, M.E.; Pfeiffer, R.M.; Berrington de Gonzalez, A.; Brinton, L.A.; Aiello Bowles, E.J.; Hoover, R.N.; Glass, A.; Gierach, G.L. Prognostic Significance of Mammographic Density Change after Initiation of Tamoxifen for ER-Positive Breast Cancer. J. Natl. Cancer Inst. 2015, 107, dju425. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Shin, I.; You, J.; Jung, S.-Y.; Ro, J.; Lee, E.S. Adjuvant tamoxifen-induced mammographic breast density reduction as a predictor for recurrence in estrogen receptor-positive premenopausal breast cancer patients. Breast Cancer Res. Treat. 2013, 142, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wonshik, H.; Hyeong-Gon, M.; Ahn, S.K.; Shin, H.-C.; You, J.-M.; Han, S.-W.; Im, S-A.; Kim, T.-Y.; Koo, H.R.; et al. Breast density change as a predictive surrogate for response to adjuvant endocrine therapy in hormone receptor positive breast cancer. Breast Cancer Res. 2012, 14, R102. [Google Scholar] [CrossRef] [PubMed]
- Mourits, M.J.; de Vries, E.G.; Willemse, P.H.; Ten Hoor, K.A.; Hollema, H.; van der Zee, A.G. Tamoxifen treatment and gynecologic side effects: A review. Obstet. Gynecol. 2001, 97, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Moyer, V.A. Medications to decrease the risk for breast cancer in women: Recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2013, 159, 698–708. [Google Scholar] [PubMed]
- Jordan, V.C. New insights into the metabolism of tamoxifen and its role in the treatment and prevention of breast cancer. Steroids 2007, 72, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Engmann, N.J.; Scott, C.; Jensen, M.R.; Ma, L.; Brandt, K.R.; Mahmoudzadeh, A.; Maikov, S.; Whaley, D.H.; Hruska, C.; Wu, F.F.; et al. Longitudinal changes in volumetric breast density with tamoxifen and aromatase inhibitors. Cancer Epidemiol. Biomark. Prev. 2017. (online ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Shawky, M.S.; Martin, H.; Hugo, H.J.; Lloyd, T.; Britt, K.L.; Redfern, A.; Thompson, E.W. Mammographic density: A potential monitoring biomarker for adjuvant and preventative breast cancer endocrine therapies. Oncotarget 2017, 8, 5578–5591. [Google Scholar] [CrossRef] [PubMed]
- Sestak, I.; Cuzick, J. Breast cancer chemoprevention. Oncol. Rev. 2008, 2, 223–228. [Google Scholar] [CrossRef]
- Guvakova, M.A.; Surmacz, E. Tamoxifen interferes with the insulin-like growth factor I receptor (IGF-IR) signaling pathway in breast cancer cells. Cancer Res. 1997, 57, 2606–2610. [Google Scholar] [PubMed]
- Pollak, M.N.; Huynh, H.T.; Lefebvre, S.P. Tamoxifen reduces serum insulin-like growth factor I (IGF-I). Breast Cancer Res. Treat. 1992, 22, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Diorio, C.; Pollak, M.; Byrne, C.; Masse, B.; Hebert-Croteau, N.; Yaffe, M.; Cote, G.; Berube, S.; Morin, C.; Brisson, J. Insulin-like growth factor-I, IGF-binding protein-3, and mammographic breast density. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Silva, I.; Johnson, N.; de Stavola, B.; Torres-Mejia, G.; Fletcher, O.; Allen, D.S.; Allen, N.E.; Key, T.J.; Fentiman, I.S.; Holly, J.M.P.; et al. The insulin-like growth factor system and mammographic features in premenopausal and postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2006, 15, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.W.; Chew, G.L.; Britt, K.L.; Ingman, W.V.; Henderson, M.A.; Hopper, J.L.; Thompson, E.W. Mammographic density—A review on the current understanding of its association with breast cancer. Breast Cancer Res. Treat. 2014, 144, 479–502. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Glynn, D.J.; Hodson, L.J.; Huo, C.; Britt, K.; Thompson, E.W.; Woolford, L.; Evdokiou, A.; Pollard, J.W.; Robertson, S.A.; et al. CCL2-driven inflammation increases mammary gland stromal density and cancer susceptibility in a transgenic mouse model. Breast Cancer Res. 2017, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Kerlikowske, K.; Zhu, W.; Tosteson, A.N.; Sprague, B.L.; Tice, J.A.; Lehman, C.D.; Miglioretti, D.L. Identifying women with dense breats at high risk for interval cancer: A cohort study. Ann. Intern. Med. 2015, 162, 673–681. [Google Scholar] [CrossRef] [PubMed]
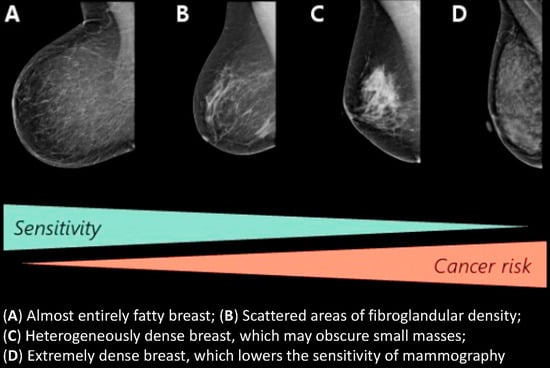
| BI-RADS 4th Edition | BI-RADS 5th Edition | ||
|---|---|---|---|
| 1 | The breast is almost entirely fat (<25% glandular) | a | The breasts are almost entirely fatty |
| 2 | There are scattered densities (approximately 25–50% glandular) | b | There are scattered areas of fibroglandular density |
| 3 | The breast tissue is heterogeneously dense, which could obscure detection of small masses (approximately 51–75% glandular) | c | The breasts are heterogeneously dense, which may obscure detection of small masses |
| 4 | The breast tissue is extremely dense. This may lower the sensitivity of mammography (>75% glandular) | d | The breasts are extremely dense, which lowers the sensitivity of mammography |
| Study | Population | Period | Number of Women | MBD Classification | Sensitivity | SF vs. FFDM | |
|---|---|---|---|---|---|---|---|
| Mandelson et al., 2000 [9] | US Breast Cancer Screening Program | 1988–1993 | 149 women with interval cancer; 388 women with screen-detected | BI-RADS 3rd ed. | BI-RADS 1 + 2 | 80.3% | SF |
| BI-RADS 3 | 58.8% | ||||||
| BI-RADS 4 | 30.4% | ||||||
| Kolb, Lichy, and Newhouse, 2002 [11] | US DMIST Trial | 1995–2000 | 27,825 screening sessions; 246 cancer diagnoses in 221 women | BI-RADS 3rd ed. | BI-RADS1 | 98% | SF |
| BI-RADS 2 | 82.9% | ||||||
| BI-RADS 3 | 64.4% | ||||||
| BI-RADS 4 | 47.8% | ||||||
| Carney et al., 2003 [10] | US Breast Cancer Surveillance Consortium | 1996–1999 | 329,495 women; 2223 breast cancer diagnoses | BI-RADS 3rd ed. | BI-RADS1 | 88.2% | SF |
| BI-RADS 2 | 82.1% | ||||||
| BI-RADS 3 | 68.9% | ||||||
| BI-RADS 4 | 62.2% | ||||||
| Boyd et al., 2007 [6] | National Breast Screening study (Canada) | 1981–1990 | 45,000 women | SCC | <10% | 75.2% | SF |
| 10–25% | 62.9% | ||||||
| Screening Mammography Program of British Colombia | 1993–1999 | 254,082 women | 25–50% | 65.2% | |||
| 50–75% | 57.3% | ||||||
| Ontario Breast Screening Program | 1996–2003 | 166,254 women | ≥75% | 54.2% | |||
| Kerlikowske et al., 2007 [8] | US Breast Cancer Surveillance Consortium | 1996–2003 | 1,714,351 women | BI-RADS | BI-RADS1 | 89% | SF |
| BI-RADS 2 | 84% | ||||||
| BI-RADS 3 | 77% | ||||||
| BI-RADS 4 | 64% | ||||||
| Method | Risk Association | Reference Group | Adjustment | Population (n) | Country | Postmenopausal % | Image Type | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual | Area-based | Parenchymal patterns | Wolfe patterns | RR 3.98 (95% CI 2.54, 3.66) incidence studies; RR 2.42 (95% CI 1.98, 2.97) prevalence studies | DY vs. N1 | Meta-analysis | Film | McCormack 2006 [125] | |||
| Tabar | 2.42-fold risk increase | Pattern IV vs. pattern I (pattern V—no increase) | None | 174 | Singapore | 89% | Film | Jakes 2000 [127] | |||
| Qualitative | BI-RADS® | HR 2.09 (95% CI 1.59, 2.75) | BI-RADS 4 vs. 2 (3rd ed.) | A, BMI, FH, HRT, M, P, R | 44,811 | USA | 58.1% post- or perimenopausal | Film | Ziv 2004 [128] | ||
| OR 3.93 (95% CI 2.46, 6.28) premenopausal; OR 3.15 (95% 2.72, 3.66) postmenopausal | BI-RADS 4 vs.1 (4th ed.) | A, FH (1st degree), MBD, prior breast procedure; if postmenopausal, also—BMI, FB, Hispanic ethnicity, HRT, previous mammographic outcome, R, surgical menopause | 1,007,600 | USA | 74.3% | Film | Barlow 2006 [129] | ||||
| RR 4.08 (95% CI 2.96, 5.63) | BI-RADS 4 vs. 1 (3rd ed.) | Meta-analysis | Film | McCormack 2006 [125] | |||||||
| Incidence rate ratio 2.45 (95% CI 2.14, 2.81) | BI-RADS 3 and 4 vs. 1 and 2 (4th ed.) | A | 48,052 | Denmark | Not reported | Film | Olsen 2009 [130] | ||||
| OR 2.96 (95% CI 0.50, 17.49) | BI-RADS 4 vs. 1 (4th ed.) | A, BMI, M, P | 1099 | UK | 86.4% | FFDM | Eng 2014 [114] | ||||
| OR 1.19 (95% CI 0.33, 4.33) | BI-RADS 4 vs. 1 (4th ed.) | A, BMI, FH, Men, PrevBiop, R | 424 | USA | Not reported | FFDM | Keller 2015 [44] | ||||
| OR 2.29 (95% CI 1.87, 2.81) | BI-RADS 4 vs. 2 (4th ed.) | A, BMI | 6081 | USA | Both, breakdown not reported | FFDM | Brandt 2016 [61] | ||||
| OR 2.03 (95% CI 0.85, 4.97) | BI-RADS D vs. B (ed. not reported) | BMI, M, P | 399 | USA | 67.2% | FFDM | Jeffers 2016 [124] | ||||
| OR 1.81 (95% CI 1.65–1.99) premenopausal; OR 1.58 (95% CI 1.46, 1.71) postmenopausal | BI-RADS D vs. B (ed. not reported) | BMI, FB, FH, history of benign breast biopsy | 202,746 | USA | 71.3% | Not reported | Engmann 2017 [119] | ||||
| Semi-quantitative | Boyd categories | RR 6.05 (95% CI 2.82, 12.97) | ≥75% vs. 0% density | FB, FH, height, Men, P, weight | 310 | Canada | Not reported | Film | Boyd 1995 [29] | ||
| OR 4.7 (95% CI 3.0, 7.4) | ≥75% vs. <10% density | A, age at menopause, BMI, FB, FH (1st degree), HRT M, Men, observation time P, study | 2224 | Canada | 75.4% | Not reported | Boyd 2007 [6] | ||||
| OR 3.5 (95% CI 2.0, 6.2) screen-detected cancers only | ≥75% vs. <10% density | A, age at menopause, BMI, FB, FH (1st degree), HRT M, Men, observation time P, study | 1434 | Canada | 75.4% | Not reported | Boyd 2007 [6] | ||||
| OR 3.55 (95% CI 0.78, 16.09) | ≥75% vs. ≤5% (MODIFIED SCC) | A, FH (1st degree), HRT M, P | 1287 | Canada | 75.3% | FFDM | Abdolell 2014 [131] | ||||
| Visual analogue scale | OR 3.43 (95% CI 1.43, 8.19) | 76–100% vs. 0% | A, atypical hyperplasia or LCIS, BMI, HRT | 1065 | UK, Finland | 46.5% | Film | Cuzick 2011 [132] | |||
| OR 1.48 (95% CI 1.34, 1.63) | Density residual 75th vs. 25th percentile | A, BMI, mammography type | 50,628 | UK | 72% | ~20% film, remainder FFDM | Brentnall 2015 [133] | ||||
| OR 4.64 (95% CI 2.84–7.56); screen-detected cancers | Quintile 5 vs. 1 | None specified | 1464 | UK | Not reported | FFDM | Astley 2016 [122] | ||||
| OR 4.85 (95% CI 3.00–7.83); future development of cancer | Quintile 5 vs. 1 | None specified | 1352 | UK | Not reported | FFDM | Astley 2016 [122] | ||||
| OR 2.12 (95% CI 1.59, 2.84) univariate analysis; OR 2.75 (95% CI 1.99, 3.81) multivariate analysis; screen-detected cancers | Quartile 4 vs. 1 | None | 1296 | UK | Not reported | FFDM | Evans 2016 [34] | ||||
| OR 3.59 (95% CI 2.37, 5.43); future development of cancer | Quartile 4 vs. 1 | When adjusted: A, BMI, M | 33,142 | UK | Not reported | FFDM | Evans 2016 [34] | ||||
| Method | Risk Association | Reference Group | Adjustment | Population (n) | Country | Postmenopausal % | Image Type | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Semi-automated | Area-based | Quantitative | Cumulus | RR 4.04 (95% CI 2.12, 7.69) | >75% vs. 0% density | FB, FH, height, Men, P, weight | 310 | Canada | Not reported | Film | Boyd 1995 [29] |
| OR 5.86 (95% CI 2.2, 15.6) | >75% vs. 0% density | A, age at menopause, BMI, FB, FH, HRT, M, Men, observation time, P, study | 2228 | Canada | 65.3% | Film | Boyd 2006 [134] | ||||
| OR 2.19 (95% CI 1.28, 3.72) | Quintile 5 vs. 1 | Age, BMI, FB, FH, HRT, M, Men, P | 1028 | Canada | 69.8% | Film | Aitken 2010 [135] | ||||
| OR 1.8 (95% CI 1.0, 2.9) | Quintile 5 vs. 1 | A, age menopause, BMI, FB, FH, height, HRT, Men, OC, P | 1217 | Netherlands | 100% | Film | Lokate 2011 [136] | ||||
| OR 2.72 (95% CI 1.93, 3.83) premenopausal | Tertile 3 vs. 1 | A, age at menopause (if postmenopausal), alcohol use, BMI, FB, FH, Men, P, study | 4084 | USA | 64.2% | Film | Pettersen 2011 [137] | ||||
| OR 3.28 (95% CI 2.41, 4.45) postmenopausal | Quintile 5 vs. 1 | ||||||||||
| OR 2.5 (95% CI 1.5, 4.3) | Quintile 5 vs. 1 | A, BMI, FB, FH, history of benign breast biopsy, mammography system, R | 1100 | USA | 67.2% | Film | Shepherd 2011 [126] | ||||
| OR 2.47 | >25% vs. 0–5% density | None specified | 1512 | Sweden | 100% | Film | Li 2012 [43] | ||||
| OR 2.4 (95% CI 1.9, 3.1) | Decile 10 vs. quintile 1 (dense AREA) | A, FH, HRT, screening round, symptoms | 6327 | Australia | Not reported | Film | Nickson 2013 [138] | ||||
| OR 3.38 (95% CI 2.00, 5.72) | Quintile 5 vs. 1 | A, BMI, M, P | 1099 | UK | 86.4% | FFDM | Eng 2014 [114] | ||||
| OR 1.58 (95% CI 1.33, 1.88) | per SD | ||||||||||
| OR 1.98 (95% CI 1.14, 3.44) raw images; OR 2.90 (95% CI 1.66, 5.06) processed images; OR 3.02 (95% CI 1.77, 5.16) analogue-like images | Quintile 5 vs. 1 | A, BMI, HRT, M, Men, OC, P | 1098 | UK | 86.3% | FFDM | Busana 2016 [121] | ||||
| OR 1.93 (95% CI 1.12, 3.34) univariate analysis; screen-detected cancer | Quartile 4 vs. 1 | None | 720 | UK | Not reported | FFDM | Evans 2016 [34] | ||||
| OR 2.00 (95% CI 1.19, 2.19) | Quartile 4 vs. 2 | BMI, M, P | 399 | USA | 67.2% | FFDM | Jeffers 2016 [124] | ||||
| Madena | OR 5.23 (95% CI 1.40, 16.13) | ≥75% vs. <1% density | A, BMI, FB, FH, HRT, M, Men, P | 1065 | USA | 55.5% | Film | Ursin 2003 [139] | |||
| OR 2.12 (95% CI 1.25, 3.62) | Quartile 4 vs. 1 | A, BMI, HRT, M, P | 937 | Germany | 78.2% | Both (proportions not specified) | Rauh 2012 [140] | ||||
| Fully automated | Quantitative | AutoDensity | OR 3.2 (95% CI 2.5, 4.1) | Decile 10 vs. quintile 1 (dense AREA) | A, FH, HRT, screening round, symptoms | 6327 | Australia | Not reported | Film | Nickson 2013 [138] | |
| ImageJ | OR 2.37 | >25% vs. 0–5% density | None specified | 1512 | Sweden | 100% | Film | Li 2012 [43] | |||
| OR 2.25 (95% CI 1.46, 4.43) | Quintile 5 vs. 1 | A, BMI, M, P | 1099 | UK | 86.4% | FFDM | Eng 2014 [114] | ||||
| OR 1.45 (95% CI 1.21, 1.74) | per SD | ||||||||||
| Libra | OR 6.68 (95% CI 2.85, 15.58) | 90th vs. 10th percentile | A, BMI, FH, Men, PrevBiop, R | 424 | USA | Not reported | FFDM | Keller 2015 [44] | |||
| OR 2.24 (95% CI 1.56, 3.21) | per SD increase | ||||||||||
| OR 1.3 (95% CI 1.1, 1.5) processed images; OR 1.1 (95% CI 1.0, 1.3) raw images | per SD increase | A, BMI | 1662 | USA | Not reported | FFDM | Brandt 2016 [141] | ||||
| OR 1.94 (95% CI 1.16, 3.22) raw images; OR 2.07 (95% CI 1.12, 3.83) processed images | Quintile 5 vs. 1 | A, BMI, HRT, M, Men, OC, P | 1098 | UK | 86.3% | FFDM | Busana 2016 [121] | ||||
| STRATUS | HR 1.6 (95% CI 1.4, 1.8) | per SD increase | A, BMI, FH, HRT, M, masses, microcalcifications | 2165 | Sweden | 65% | FFDM | Eriksson 2017 [45] | |||
| HR 4.8 (95% CI 2.6, 8.8) | BI-RADS-like category (4 vs. 1) | ||||||||||
| Method | Risk Association | Reference Group | Adjustment | Population (n) | Country | % Postmenopausal | Image Type | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fully automated | Volumetric | Quantitative | BDSXA | OR 4.1 (95% CI 2.3, 7.2) | Quintile 5 vs. 1 | A, BMI, FB, FH, history of benign breast biopsy, mammography system, R | 1100 | USA | 67.2% | Film | Shepherd 2011 [126] |
| OR 2.99 (95% CI 1.76, 5.09) | Quintile 5 vs. 1 | A, BMI, M, P | 1099 | UK | 86.4% | FFDM | Eng 2014 [114] | ||||
| OR 1.37 (95% CI 1.16, 1.63) | per SD increase | ||||||||||
| CumulusV | RR 2.8 | Octile 8 vs. 1 | None specified | 1158 | Canada | Not reported | FFDM | Yaffe 2011 [142] | |||
| Quantra | OR 3.94 (95% CI 2.26, 6.86) | Quintile 5 vs. 1 | A, BMI, M, P | 1099 | UK | 86.4% | FFDM | Eng 2014 [114] | |||
| OR 1.40 (95% CI 1.19, 1.66) | per SD increase | ||||||||||
| OR 10.88 (95% CI 4.18, 28.21) | 90th vs. 10th percentile | A, BMI, FH, Men, PrevBiop, R | 424 | USA | Not reported | FFDM | Keller 2015 [44] | ||||
| OR 2.64 (95% CI 1.79, 3.89) | per SD increase | ||||||||||
| No association; screen-detected cancer | Quintile 5 vs. 1 | None specified | 1464 | UK | Not reported | FFDM | Astley 2016 [122] | ||||
| OR 1.52 (95% CI 1.04, 2.23); future cancer | Quintile 5 vs. 1 | Breast volume, P | 1352 | UK | Not reported | FFDM | Astley 2016 [122] | ||||
| OR 1.78 (95% CI 1.46, 2.17) | Quintile 5 vs. 1 | A, BMI | 6081 | USA | Both, breakdown not reported | FFDM | Brandt 2016 [61] | ||||
| OR 1.94 (1.48, 2.54) | BI-RADS-like category (4 vs. 2) | ||||||||||
| OR 1.3 (95% CI 1.1, 1.4) | per SD increase | A, BMI | 1662 | USA | Not reported | FFDM | Brandt 2016 [141] | ||||
| OR 1.51 (95% CI 1.12, 2.02) univariate analysis; OR 1.67 (95% CI 1.12, 2.27) multivariate analysis; screen-detected cancer | Quartile 4 vs. 1 | None | 1296 | UK | Not reported | FFDM | Evans 2016 [34] | ||||
| OR 0.91 (95% CI 0.62, 1.33); future cancer | Quartile 4 vs. 1 | When adjusted: A, BMI, M | 33,142 | UK | Not reported | FFDM | Evans 2016 [34] | ||||
| Volpara | RR 2.7 | Octile 8 vs. 1 | None specified | 1158 | Canada | Not reported | FFDM | Yaffe 2011 [143] | |||
| OR 1.53 (95% CI 0.91, 2.68) | BI-RADS-like category (4 vs. 1) | A | 33,029 | Netherlands | Not reported | FFDM | Kallenberg 2012 [143] | ||||
| OR 8.26 (95% CI 4.28, 15.96) | Quintile 5 vs. 1 | A, BMI, M, P | 1099 | UK | 86.4% | FFDM | Eng 2014 [114] | ||||
| OR 1.83 (95% CI 1.51, 2.21) | per SD increase | ||||||||||
| OR 2.05 (95% CI 0.99,4.23) premenopausal; OR 3.07 (95% CI 1.89, 4.99) postmenopausal | BI-RADS-like category (4 vs. 2 and 1) | A, BMI, HRT, P | 1984 | South Korea | 58.3% | FFDM | Park 2014 [144] | ||||
| OR 2.96 (95% CI 1.78, 4.93); screen-detected cancer | Quintile 5 vs. 1 | None specified | 1464 | UK | Not reported | FFDM | Astley 2016 [122] | ||||
| OR 4.04 (95% CI 2.33, 7.01); future cancer | Quintile 5 vs. 1 | None specified | 1352 | UK | Not reported | FFDM | Astley 2016 [122] | ||||
| HR 2.2 (95% CI 1.2, 4.1) | Quartile 4 vs. 1 | None specified | 5746 | USA | Not reported | FFDM | Battle 2016 [145] | ||||
| OR 2.03 (95% CI 1.64, 2.51) | Quintile 5 vs. 2 | A, BMI | 6081 | USA | Both, breakdown not reported | FFDM | Brandt 2016 [61] | ||||
| OR 1.82 (1.49, 2.21) | BI-RADS-like category (4 vs. 2) | ||||||||||
| OR 1.4 (95% CI 1.2, 1.6) | per SD increase | A, BMI | 1662 | USA | Not reported | FFDM | Brandt 2016 [141] | ||||
| OR 6.91 (95% CI 3.67, 13.04) raw images | Quintile 5 vs. 1 | A, BMI, HRT, M, Men, OC, P | 1098 | UK | 86.3% | FFDM | Busana 2016 [121] | ||||
| OR 1.20 (95% CI 0.92, 1.58) univariate analysis; OR 1.60 (95% CI 1.15, 2.23) multivariate analysis; screen-detected cancer | Quartile 4 vs. 1 | None | 1296 | UK | Not reported | FFDM | Evans 2016 [34] | ||||
| OR 2.33 (95% CI 1.46, 3.72); future cancer | Quartile 4 vs. 1 | When adjusted: A, BMI, M | 33,142 | UK | Not reported | FFDM | Evans 2016 [34] | ||||
| OR 1.71 (95% CI 0.83, 3.53) | Quartile 4 vs. 2 | BMI, M, P | 399 | USA | 67.2% | FFDM | Jeffers 2016 [124] | ||||
| OR 2.05 (95% CI 0.90, 6.64) | BI-RADS-like category (4 vs. 2) | ||||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Destounis, S.; Arieno, A.; Morgan, R.; Roberts, C.; Chan, A. Qualitative Versus Quantitative Mammographic Breast Density Assessment: Applications for the US and Abroad. Diagnostics 2017, 7, 30. https://doi.org/10.3390/diagnostics7020030
Destounis S, Arieno A, Morgan R, Roberts C, Chan A. Qualitative Versus Quantitative Mammographic Breast Density Assessment: Applications for the US and Abroad. Diagnostics. 2017; 7(2):30. https://doi.org/10.3390/diagnostics7020030
Chicago/Turabian StyleDestounis, Stamatia, Andrea Arieno, Renee Morgan, Christina Roberts, and Ariane Chan. 2017. "Qualitative Versus Quantitative Mammographic Breast Density Assessment: Applications for the US and Abroad" Diagnostics 7, no. 2: 30. https://doi.org/10.3390/diagnostics7020030