Defining Clinical Response Criteria and Early Response Criteria for Precision Oncology: Current State-of-the-Art and Future Perspectives
Abstract
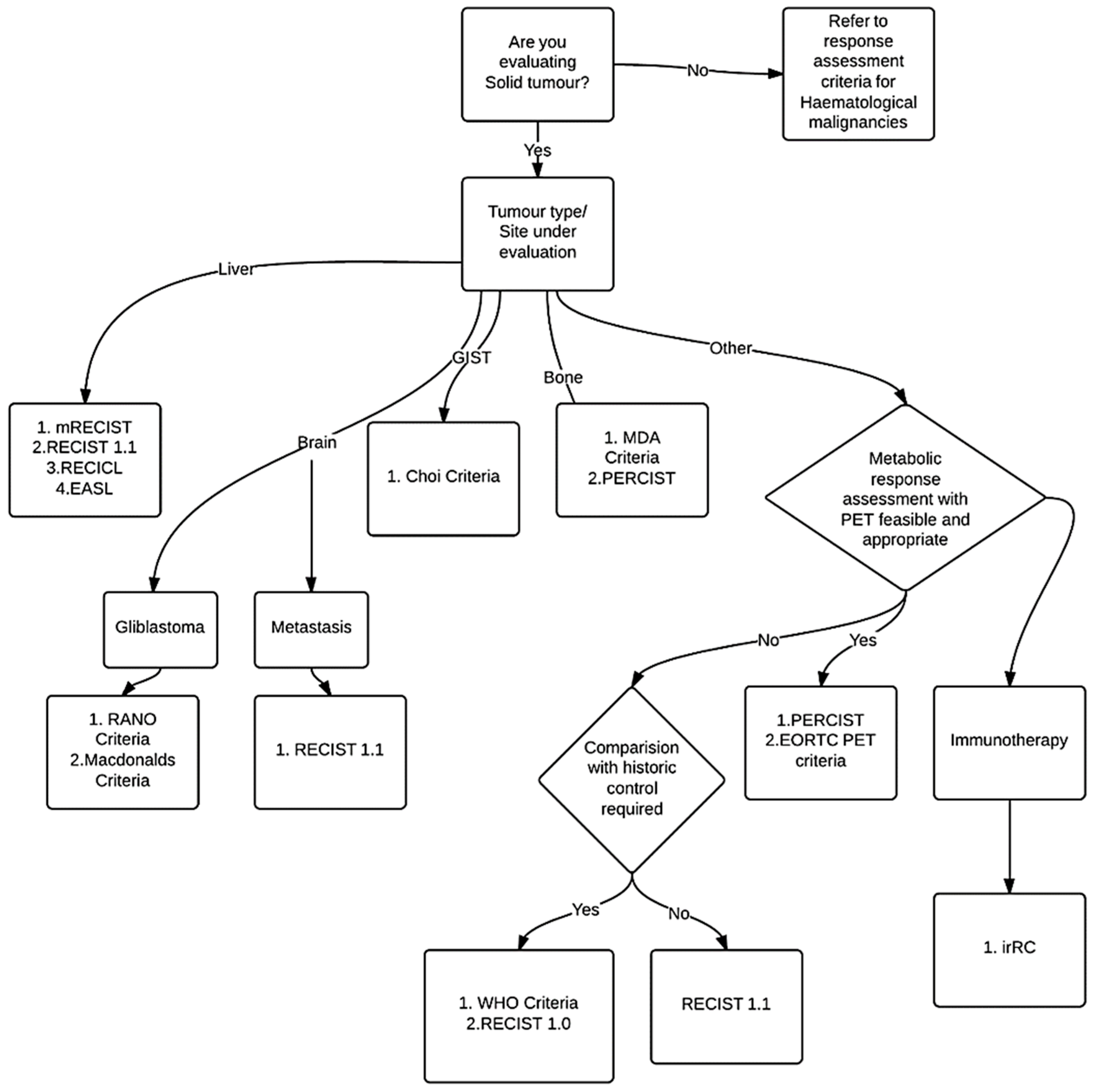
:1. Introduction
2. Methods
- *Key:
- WHO criteria: World Health organization
- RECIST: Response Evaluation Criteria In Solid Tumors
- EASL: European Association for the Study of the Liver
- mRECIST: modified response evaluation criteria in solid tumors
- RECICL: The Response Evaluation Criteria in Cancer of the Liver
- irRC: immune-related response criteria
- PERCIST: PET Response Criteria in Solid Tumors
- EORTC PET response criteria: European Organization for Research and Treatment of Cancer
- MDA criteria: MD Anderson criteria
- RANO criteria: Response Assessment in Neuro-Oncology
3. Results
3.1. History: Development of Response Assessment Criteria
3.2. Response Assessment Criteria
3.2.1. WHO Criteria
3.2.2. RECIST
3.2.3. Organ System Specific Response Criteria
MDA Criteria for Bone Metastasis
Choi Criteria for Gastrointestinal Stromal Tumor (GIST)
RANO Criteria for High-Grade Gliomas
Response Assessment Criteria for Hepatocellular Carcinoma (HCC)—EASL, mRECIST, RECICL
3.3. Functional Assessment Response Criteria
3.3.1. PET Response Criteria in Solid Tumors (PERCIST)
3.3.2. EORTC
4. Mechanism of Action Dependent Response Assessment Criteria
Immune Related Response Criteria (irRC)
5. Response Criteria: Standardization, Challenges and Pitfalls
6. Early Response Assessments and Imaging Biomarkers
7. Case Studies of Early Response Assessments
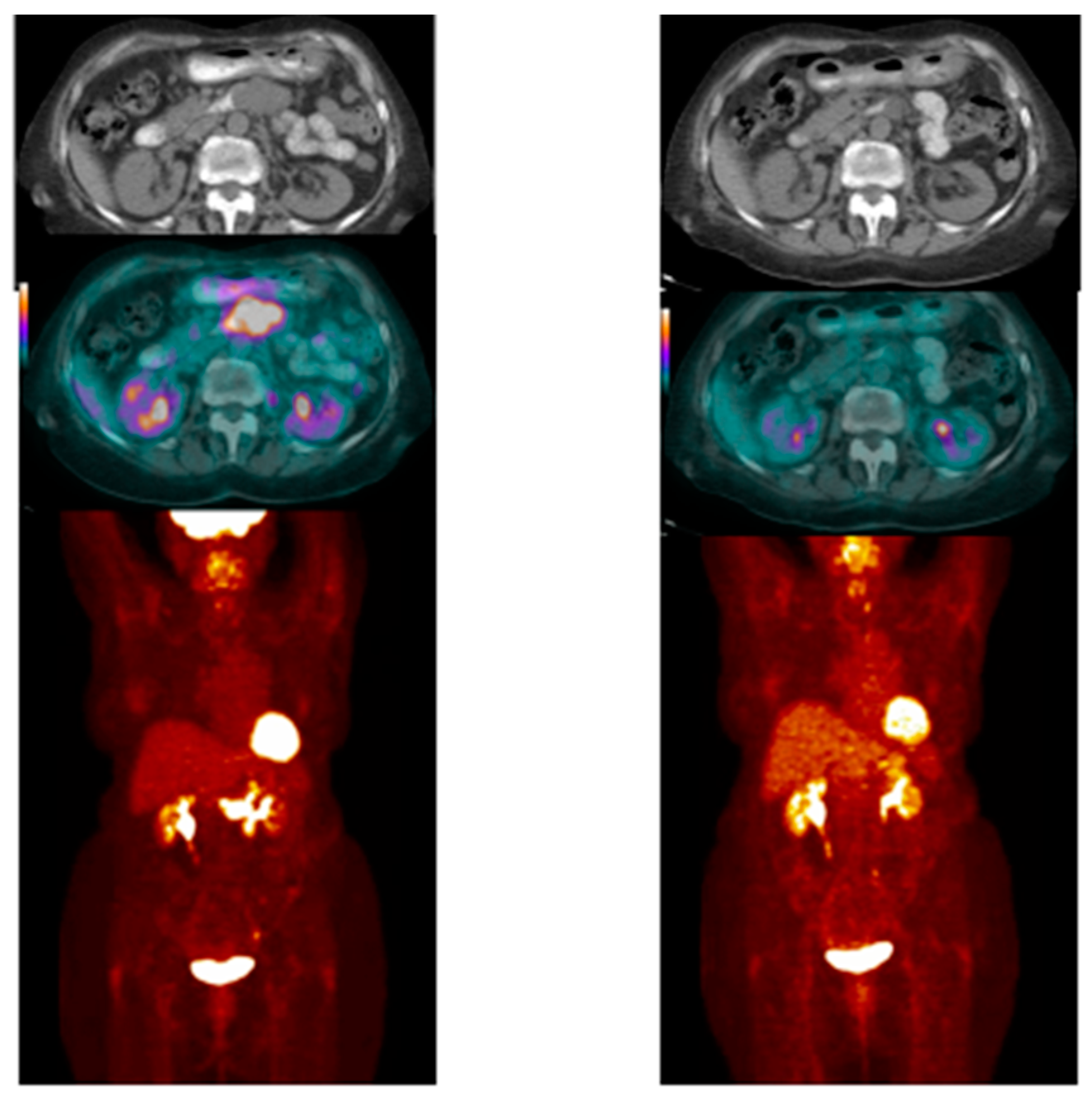
- Case # 1:
- FDG PET/CT was performed in a 47 y/o female with metastatic breast cancer. Patient had multiple osseous metastases. The baseline study showed focal sites of activity in the bone marrow space, without discernible anatomic abnormality (Figure 2). About 6 weeks after starting therapy, there was diffuse marrow activation; however, there was relative loss of normal marrow activity where tumor was previously seen, and there was new sclerotic change at those sites. About 4 months after starting therapy, there was still diffuse marrow activation with loss of normal marrow activity where tumor was, and increased sclerosis on CT images. Unfortunately, about 10 months after starting therapy, she relapsed, with new focal sites of activity, without anatomic abnormality (similar to baseline study), whereas previously tumor sites became densely sclerotic and remained without activity to suggest active tumor.
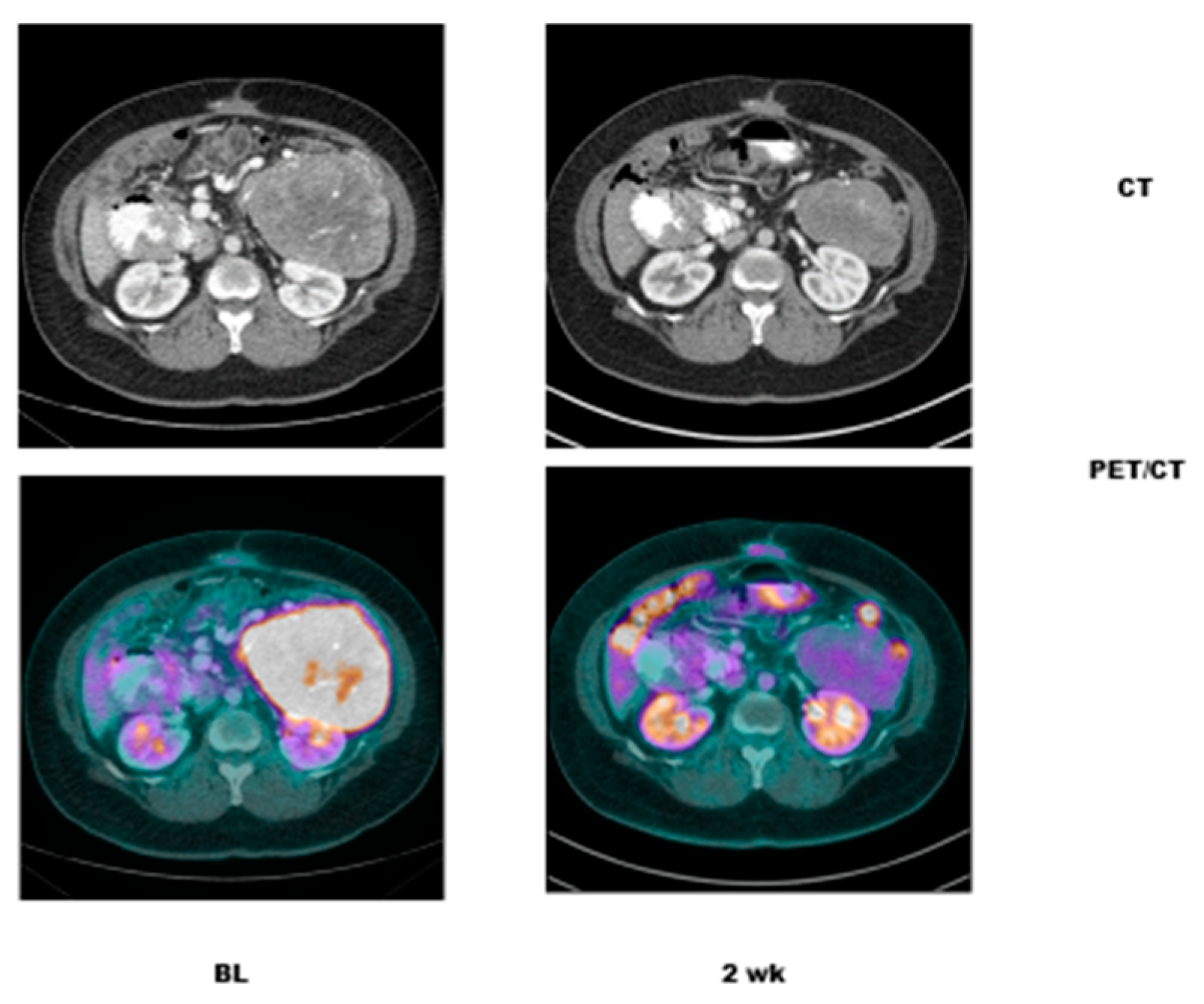
- Case # 2:
- This is an 82 year old female with gastro-intestinal stromal tumor (GIST). Baseline FDG/PET study was performed off tyrosine kinase inhibitor therapy and she was initiated on a therapy. Repeat study was performed two weeks after initiation of Gleevec® (Imatinib) a specific c-KIT inhibitor. Both anatomic and metabolic response was seen. Although there was still a residual anatomic abnormality, the tumor had complete metabolic response. This illustrates that the power of early functional imaging (Figure 3).
- Case # 3:
- This is a 46 year old female with recurrent GIST. She had multiple prior therapies including imatinib, sunitinib, regorafenib, nilotinib, and pazopanib. After 2 weeks of new therapy with a novel TKI targeting c-KIT, she had a complete metabolic response, but only a partial anatomic response. This showcases early response can be seen as early as 2 weeks in GIST another sarcoma like Ewing’s sarcoma where in early responses as early as 9 days have been shown to predict survival [38] (Figure 4).
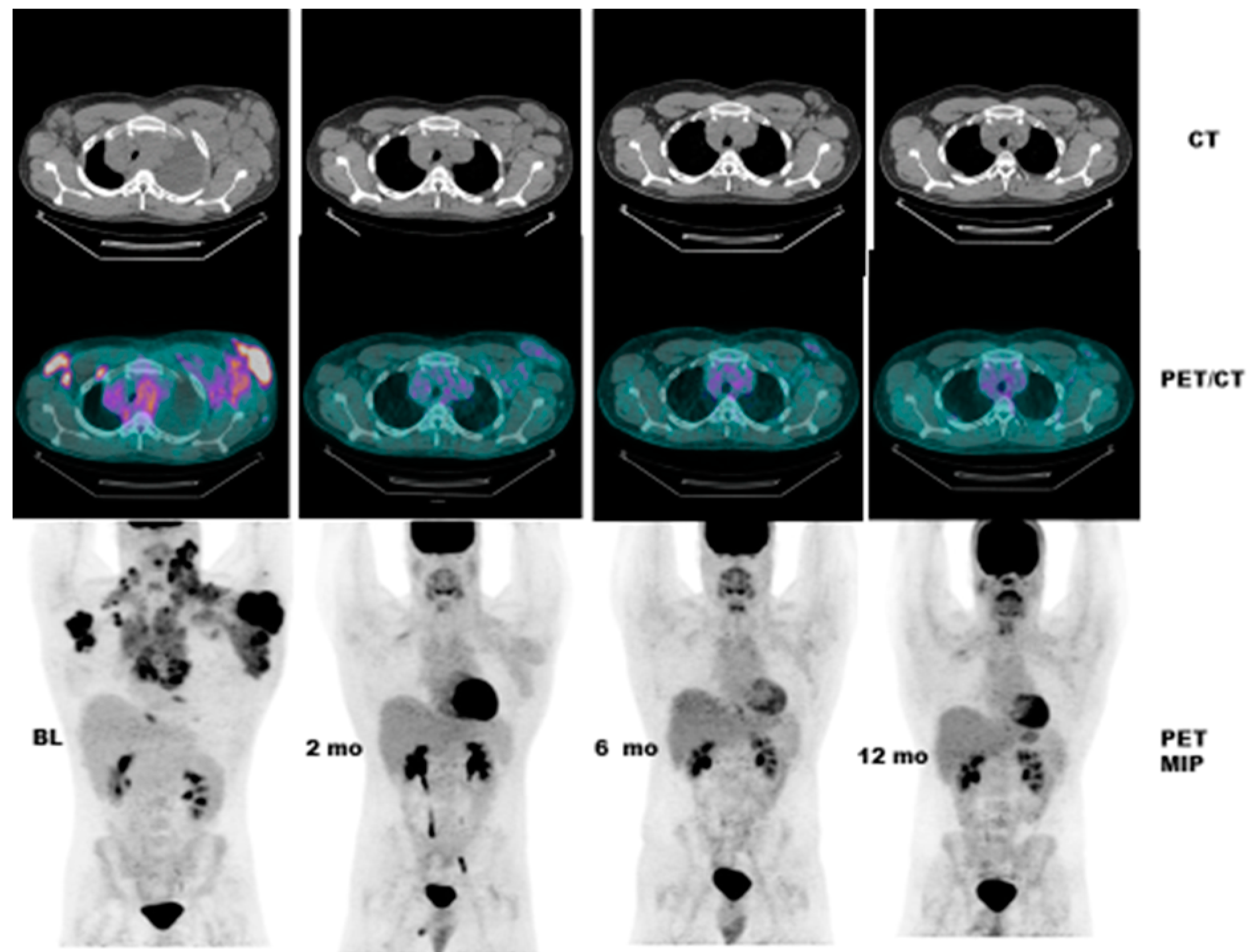
- Case # 4:
- This is a 22 y/o male with Hodgkin lymphoma (nodular sclerosis type). He had complete metabolic response after 2 cycles of chemotherapy (ABVD) but only partial anatomic response. He completed 6 cycles of therapy with complete metabolic response but residual anatomic abnormalities. He had consolidation radiation therapy, about 1 month after completing chemotherapy; about 2 months after completing radiation therapy, there was no change. It is not uncommon to have residual masses after therapy for lymphoma, especially Hodgkin lymphoma, that may complicate anatomic response assessment [35,40,41,42] (Figure 5).
8. Response Assessments in Radiology
Current State-of-the-Art
9. The Future of “OMICS” in Radiology—Radiomics
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment; World Health Organization: Geneva, Switzerland, 1979. [Google Scholar]
- Green, S.; Weiss, G.R. Southwest oncology group standard response criteria, endpoint definitions and toxicity criteria. Investig. New Drugs 1992, 10, 239–253. [Google Scholar] [CrossRef]
- Forner, A.; Ayuso, C.; Varela, M.; Rimola, J.; Hessheimer, A.J.; de Lope, C.R.; Reig, M.; Bianchi, L.; Llovet, J.M.; Bruix, J. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: Are response evaluation criteria in solid tumors reliable? Cancer 2009, 115, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Miller, A.B.; Hoogstraten, B.; Staquet, M.; Winkler, A. Reporting results of cancer treatment. Cancer 1981, 47, 207–214. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumors: Revised recist guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50, S122–S150. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbe, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef]
- Choi, H.; Charnsangavej, C.; Faria, S.C.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Benjamin, R.S. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J. Clin. Oncol. 2007, 25, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M.; Llovet, J.M.; Beaugrand, M.; Lencioni, R.; Burroughs, A.K.; Christensen, E.; Pagliaro, L.; Colombo, M.; Rodes, J.; et al. Clinical management of hepatocellular carcinoma. Conclusions of the barcelona-2000 easl conference. European association for the study of the liver. J. Hepatol. 2001, 35, 421–430. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Kubo, S.; Takayasu, K.; Sakamoto, M.; Tanaka, M.; Ikai, I.; Furuse, J.; Nakamura, K.; Makuuchi, M.; Liver Cancer Study Group of Japan. Response evaluation criteria in cancer of the liver (RECICL) proposed by the Liver Cancer Study Group of Japan (2009 revised version). Hepatol. Res. 2010, 40, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Han, K.H.; Kokudo, N.; Cheng, A.L.; Choi, B.I.; Furuse, J.; Izumi, N.; Park, J.W.; Poon, R.T.; Sakamoto, M. Liver cancer working group report. Jpn. J. Clin. Oncol. 2010, 40, i19–i27. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; Madewell, J.E.; Podoloff, D.A.; Hortobagyi, G.N.; Ueno, N.T. Bone imaging in metastatic breast cancer. J. Clin. Oncol. 2004, 22, 2942–2953. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.R.; Cascino, T.L.; Schold, S.C., Jr.; Cairncross, J.G. Response criteria for phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 1990, 8, 1277–1280. [Google Scholar] [PubMed]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.A.; Pruim, J.; Price, P.; European Organization for Research and Treatment of Cancer (EORTC) Pet Study Group. Measurement of clinical and subclinical tumor response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Costelloe, C.M.; Chuang, H.H.; Madewell, J.E.; Ueno, N.T. Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST. J. Cancer 2010, 1, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; Costelloe, C.M.; Madewell, J.E.; Liu, P.; Berry, D.A.; Islam, R.; Theriault, R.L.; Hortobagyi, G.N.; Ueno, N.T. Tumor response interpretation with new tumor response criteria vs the World Health Organisation criteria in patients with bone-only metastatic breast cancer. Br. J. Cancer 2010, 102, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Costelloe, C.M.; Hamaoka, T.; Wei, C.; Niikura, N.; Theriault, R.L.; Hortobagyi, G.N.; Madewell, J.E.; Ueno, N.T. A prospective study of bone tumor response assessment in metastatic breast cancer. Clin. Breast Cancer 2013, 13, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, V.; Andreopoulos, D. Assessment of therapeutic response in patients with metastatic skeletal disease: Suggested modifications for the MDA response classification criteria. Br. J. Cancer 2010, 103, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Van der Veldt, A.A.; Meijerink, M.R.; van den Eertwegh, A.J.; Haanen, J.B.; Boven, E. Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br. J. Cancer 2010, 102, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Negri, T.; Palassini, E.; Conca, E.; Gronchi, A.; Morosi, C.; Messina, A.; Pastorino, U.; Pierotti, M.A.; Casali, P.G.; et al. Sunitinib malate and figitumumab in solitary fibrous tumor: Patterns and molecular bases of tumor response. Mol. Cancer Ther. 2010, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Zappa, M.; Vilgrain, V.; Boucher, E.; Douillard, J.Y.; Lim, H.Y.; Kim, J.S.; Im, S.A.; Kang, Y.K.; Bouattour, M.; et al. Changes in tumor density in patients with advanced hepatocellular carcinoma treated with sunitinib. Clin. Cancer Res. 2011, 17, 4504–4512. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, H.J.; Spivey, J.R.; Hanish, S.I.; El-Rayes, B.F.; Kauh, J.S.; Chen, Z.; Kim, H.S. mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE). Ann. Oncol. 2013, 24, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Ziai, D.; Wagner, T.; El Badaoui, A.; Hitzel, A.; Woillard, J.B.; Melloni, B.; Monteil, J. Therapy response evaluation with FDG-PET/CT in small cell lung cancer: A prognostic and comparison study of the PERCIST and EORTC criteria. Cancer Imaging 2013, 13, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hoos, A.; Eggermont, A.M.; Janetzki, S.; Hodi, F.S.; Ibrahim, R.; Anderson, A.; Humphrey, R.; Blumenstein, B.; Old, L.; Wolchok, J. Improved endpoints for cancer immunotherapy trials. J. Natl. Cancer Inst. 2010, 102, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Hoos, A.; Wolchok, J.D.; Humphrey, R.W.; Hodi, F.S. CCR 20th anniversary commentary: Immune-related response criteria—Capturing clinical activity in immuno-oncology. Clin. Cancer Res. 2015, 21, 4989–4991. [Google Scholar] [CrossRef]
- Patel, S.P.; Woodman, S.E. Profile of ipilimumab and its role in the treatment of metastatic melanoma. Drug Des. Dev. Ther. 2011, 5, 489–495. [Google Scholar]
- Hodi, F.S.; Hwu, W.J.; Kefford, R.; Weber, J.S.; Daud, A.; Hamid, O.; Patnaik, A.; Ribas, A.; Robert, C.; Gangadhar, T.C.; et al. Evaluation of immune-related response criteria and recist v1.1 in patients with advanced melanoma treated with pembrolizumab. J. Clin. Oncol. 2016, 34, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.K.; Coumou, A.W.; Kostkiewicz, M.; Kairemo, K. [18F]-2-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography imaging in oncology: Initial staging and evaluation of cancer therapy. Med. Princ. Pract. 2013, 22, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, R.F.; Dos Anjos, D.A.; Vieira, D.L.; Leite, A.F.; Figueiredo, P.T.; de Melo, N.S. Effectiveness of FDG-PET/CT for evaluating early response to induction chemotherapy in head and neck squamous cell carcinoma: A systematic review. Medicine 2016, 95, e4450. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Naing, A.; Brown, R.E.; Chen, H.; Doyle, L.; LoRusso, P.; Benjamin, R.; Anderson, P.; Kurzrock, R. Targeted morphoproteomic profiling of Ewing’s sarcoma treated with insulin-like growth factor 1 receptor (IGF1R) inhibitors: Response/resistance signatures. PLoS ONE 2011, 6, e18424. [Google Scholar] [CrossRef]
- Subbiah, V.; Murthy, R.; Anderson, P.M. [90Y]yttrium microspheres radioembolotherapy in desmoplastic small round cell tumor hepatic metastases. J. Clin. Oncol. 2011, 29, e292–e294. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Brown, R.E.; McGuire, M.F.; Buryanek, J.; Janku, F.; Younes, A.; Hong, D. A novel immunomodulatory molecularly targeted strategy for refractory hodgkin’s lymphoma. Oncotarget 2014, 5, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Schelhaas, S.; Held, A.; Wachsmuth, L.; Hermann, S.; Honess, D.J.; Heinzmann, K.; Smith, D.M.; Griffiths, J.R.; Faber, C.; Jacobs, A.H. Gemcitabine mechanism of action confounds early assessment of treatment response by 3’-deoxy-3’-[18F]fluorothymidine in preclinical models of lung cancer. Cancer Res. 2016, 76, 7096–7105. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Anderson, P.; Rohren, E. Alpha emitter radium 223 in high-risk osteosarcoma: First clinical evidence of response and blood-brain barrier penetration. JAMA Oncol. 2015, 1, 253–255. [Google Scholar] [CrossRef]
- Hyun, O.J.; Luber, B.S.; Leal, J.P.; Wang, H.; Bolejack, V.; Schuetze, S.M.; Schwartz, L.H.; Helman, L.J.; Reinke, D.; Baker, L.H.; et al. Response to early treatment evaluated with 18F-FDG PET and PERCIST 1.0 predicts survival in patients with ewing sarcoma family of tumors treated with a monoclonal antibody to the insulinlike growth factor 1 receptor. J. Nucl. Med. 2016, 57, 735–740. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Aboagye, E.O.; Adams, J.E.; Aerts, H.J.; Barrington, S.F.; Beer, A.J.; Boellaard, R.; Bohndiek, S.E.; Brady, M.; Brown, G.; et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Radford, J.A.; Cowan, R.A.; Flanagan, M.; Dunn, G.; Crowther, D.; Johnson, R.J.; Eddleston, B. The significance of residual mediastinal abnormality on the chest radiograph following treatment for hodgkin’s disease. J. Clin. Oncol. 1988, 6, 940–946. [Google Scholar] [PubMed]
- Subbiah, V. Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N. Engl. J. Med. 2008, 358, 743. [Google Scholar] [PubMed]
- Subbiah, V.; Ly, U.K.; Khiyami, A.; O’Brien, T. Tissue is the issue-sarcoidosis following ABVD chemotherapy for Hodgkin’s lymphoma: A case report. J. Med. Case Rep. 2007, 1, 148. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]

| Number | Criteria | Development Rational | Brief Description | Reference |
|---|---|---|---|---|
| 1 | WHO Criteria | To develop a common language to describe cancer treatment and to agree on internationally acceptable general principles for evaluating data. | Recommendations have been developed for standardized approaches to the recording and reporting of cancer treatments. Objective definitions of response using reduction tumor volume were published using bidimensional approach. | WHO Handbook (1979), Miller, A.B. et al. (1981) [5] |
| 2 | RECIST | To review the response definitions in use and to create a revision of the WHO criteria that, far as possible, addressed areas of conflict and inconsistency. | Response rates were derived from unidimensional measurements of tumor lesions and sum of diameters. | Therasse, P. et al. (2000) [4] |
| 3 | RECIST 1.1 | To address questions raised after extensive use of RECIST 1.0 and to newer developments in imaging technologies and targeted therapies. | This revision of the RECIST guidelines includes updates that touch on all points like fewer than 10 lesions can be assessed, how to apply RECIST in randomized phase III trials where progression, not response, is the primary endpoint, how to utilize newer imaging technologies such as FDG-PET and MRI; how to handle assessment of lymph nodes; whether response confirmation is truly needed; and, not least, the applicability of RECIST in trials of targeted non-cytotoxic drugs. | Eisenhauer, E.A. et al. (2009) [6] |
| 4 | PERCIST | To Propose quantitative PET response assessment guideline. | Qualitative and quantitative approaches to metabolic tumor response assessment with 18F-FDG PET and a draft framework for PET Response Criteria in Solid Tumors. | Wahl, R.L. et al. (2009) [7] |
| 5 | irRC | Novel criteria for the evaluation of antitumor responses with immunotherapeutic agents. | The core novelty of the irRC is the incorporation of measurable new lesions into “total tumor burden” and comparison of this variable to baseline measurements. | Wolchok, J.D. et al. (2009) [8] |
| 6 | CHOI Criteria | To determine if CT criteria could be used in quantitative response evaluation in GIST. | A combination of the values of tumor size and tumor density on CT (a 10% decrease in tumor size or a more than 15% decrease in tumor density at 2 months of treatment) were used. | Choi, H. et al. [9] |
| 7 | EASL | Recommendations for response evaluation in HCC by European Association for the Study of the Liver while using WHO criteria. | Measurement of tumor load by simple bi-dimensional determinations of diameter is not accurate enough, since tumor necrosis due to treatment is not taken into account. To address this concern method of estimation of the reduction in viable tumor volume was suggested. | Bruix, J. et al. (2001) [10] |
| 8 | mRECIST | Recommendations for response evaluation in HCC while using RECIST criteria. | To address limitations of anatomic tumor response metrics when applied to molecular-targeted therapies or locoregional therapies in HCC. | Lencioni, R. et al. (2010) [11] |
| 9 | RECICL | Response evaluation criteria solely devoted for HCC. | HCC specific criteria to address the direct effects of treatment on the hepatocellular carcinoma (HCC) by locoregional therapies such as radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE) and molecular targeted therapies, which cause necrosis of the tumor in the clinical practice as well as in the clinical trials. | Kudo, M. et al. (2010) [12,13] |
| 10 | MDA Criteria | To develop a practical approach for diagnosis and assessment of bone metastasis. | The MDA criteria divide response into 4 standard categories (CR, PR, PD, and SD) and include quantitative and qualitative assessments of the behavior of bone metastases. | Hamaoka, T. et al. (2004) [14] |
| 11 | The Macdonald Criteria | New criteria based on modern scanning and a fuller appreciation of the influence of steroids on neurologic findings and brain tumor images. | These criteria provided an objective radiologic assessment of tumor response and were based primarily on contrast-enhanced computed tomography (CT) and the two-dimensional WHO oncology response criteria using enhancing tumor area (the product of the maximal cross-sectional enhancing diameters) as the primary tumor measure. These criteria also considered the use of corticosteroids and changes in the neurologic status of the patient. | Macdonald, D.R. et al. (1990) [15] |
| 12 | RANO criteria | To address significant limitations of McDonalds criteria, which only address the contrast-enhancing component of the tumor. | The criteria included new information provided by MRI like T1, T2 images, Standardization of imaging definitions and measurement rules. | Wen, P.Y. et al. (2010) [16] |
| 13 | EORTC PET response criteria | To summarize the status of the technique and recommendations on the measurement of [18F]-FDG uptake for tumor response. | The EORTC PET study group has proposed a common method of assessing tumor [18F]-FDG uptake and reporting of response data. | Young, H. et al. (1999) [17] |
| Parameter | RECIST 1.0 | RECIST 1.1 |
|---|---|---|
| Minimum size measurable lesions | CT: 10 mm spiral, 20 mm non-spiral Clinical: 20 mm Lymph node: not mentioned | CT: 10 mm Clinical: 10 mm (must be measurable with calipers) Lymph node by CT *: ≥$15 mm short axis for target ≥10$–<15 mm for non-target <10 mm is non-pathological |
| Overall tumor burden | Up to 10 target lesions, maximum 5 per organ | Up to 5 target lesions, maximum 2 per organ |
| Response criteria Lymph node | Not defined | For CR lymph nodes must be <10 mm short axis |
| Progressive disease | 20% increase over smallest sum on study or new lesions | 20% increase over smallest sum on study and at least 5 mm increase or new lesions |
| Response criteria non-target disease | “unequivocal progression” considered as PD | More detailed description of “unequivocal progression” it must be representative of overall disease status change, not a single lesion increase |
| Overall response | Table integrated target and non-target lesions | Additional table with non-target lesion only. Guidance on CR in face of residual tissue |
| Confirmation of response | For CR and PR criteria must be met again 4 weeks after initial documentation | Required only for non-randomized trials with primary endpoint of response |
| Reporting of response results | 9 categories suggested for reporting phase II results | Divided into phase II and phase III. 9 categories collapsed into 5 |
| Guidance for imaging | Limited | updated with detailed guidance on use of MRI, PET/CT |
| Response Category | Criteria |
|---|---|
| Complete response | Disappearance of all target lesions |
| Reduction in short axis of target lymph nodes to <10 mm | |
| Partial response | Decrease in target lesion diameter sum > 30% † |
| Progressive disease | Increase in target lesion diameter sum > 20% ‡ |
| >5 mm increase in target lesion diameter sum | |
| New, malignant FDG uptake in the absence of other indications of progressive disease or an anatomically stable lesion, and confirmed on contemporaneous or follow-up CT | |
| Unequivocal progression of nontarget lesions | |
| Stable disease | Does not meet other criteria ‡ |
| Response Category | Criteria |
|---|---|
| Complete metabolic response | Normalization of all lesions (target and nontarget) to SUL less than mean liver SUL and equal to normal surrounding tissue SUL |
| Verification with follow-up study in 1 month if anatomic criteria indicate disease progression | |
| Partial metabolic response | >30% decrease in SUL peak; minimum 0.8 unit decrease * |
| Verification with follow-up study if anatomic criteria indicate disease progression | |
| Progressive metabolic disease | >30% increase in SUL peak; minimum 0.8 unit increase in SUL peak * |
| >75% increase in TLG of the 5 most active lesions | |
| Visible increase in extent of FDG uptake | |
| New lesions | |
| Verification with follow-up study if anatomic criteria indicate complete or partial response | |
| Stable metabolic disease | Does not meet other criteria |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subbiah, V.; Chuang, H.H.; Gambhire, D.; Kairemo, K. Defining Clinical Response Criteria and Early Response Criteria for Precision Oncology: Current State-of-the-Art and Future Perspectives. Diagnostics 2017, 7, 10. https://doi.org/10.3390/diagnostics7010010
Subbiah V, Chuang HH, Gambhire D, Kairemo K. Defining Clinical Response Criteria and Early Response Criteria for Precision Oncology: Current State-of-the-Art and Future Perspectives. Diagnostics. 2017; 7(1):10. https://doi.org/10.3390/diagnostics7010010
Chicago/Turabian StyleSubbiah, Vivek, Hubert H. Chuang, Dhiraj Gambhire, and Kalevi Kairemo. 2017. "Defining Clinical Response Criteria and Early Response Criteria for Precision Oncology: Current State-of-the-Art and Future Perspectives" Diagnostics 7, no. 1: 10. https://doi.org/10.3390/diagnostics7010010
APA StyleSubbiah, V., Chuang, H. H., Gambhire, D., & Kairemo, K. (2017). Defining Clinical Response Criteria and Early Response Criteria for Precision Oncology: Current State-of-the-Art and Future Perspectives. Diagnostics, 7(1), 10. https://doi.org/10.3390/diagnostics7010010





