Angiogenesis PET Tracer Uptake (68Ga-NODAGA-E[(cRGDyK)]2) in Induced Myocardial Infarction in Minipigs
Abstract
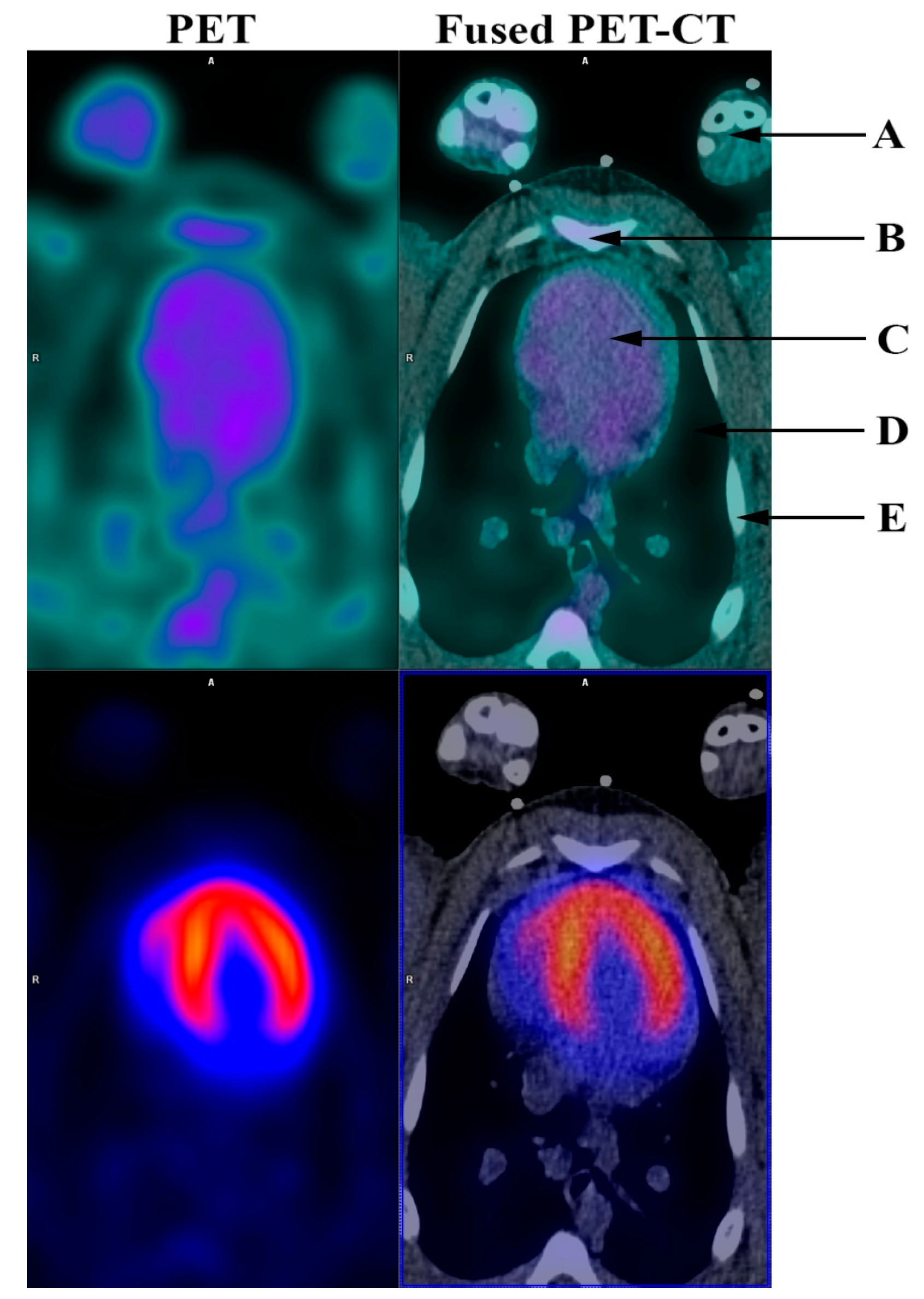
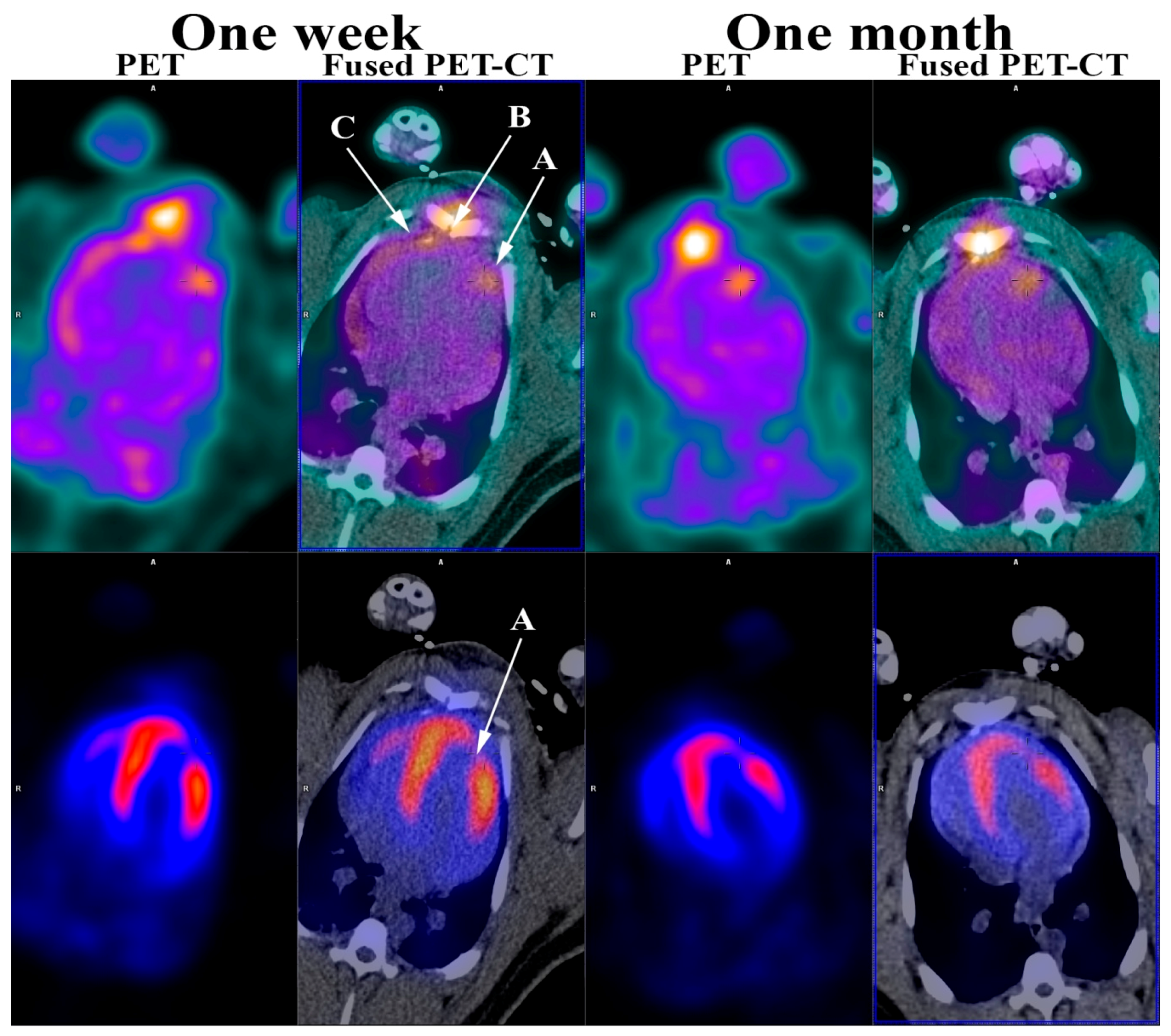
:
Acknowledgments
Conflicts of Interest
References
- Ja, K.M.M.; Miao, Q.; Zhen Tee, N.G.; Lim, S.Y.; Nandihalli, M.; Ramachandra, C.J.A.; Mehta, A.; Shim, W. iPSC-derived human cardiac progenitor cells improve ventricular remodelling via angiogenesis and interstitial networking of infarcted myocardium. J. Cell. Mol. Med. 2016, 20, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Niu, G.; Wu, H.; Chen, X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin alphavbeta3. Theranostics 2016, 6, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Dobrucki, L.W.; Sinusas, A.J. Imaging angiogenesis. Curr. Opin. Biotechnol. 2007, 18, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Ren, L.; Yin, X.; Guo, Z.; Li, Y.; He, T.; Tang, Y.; Long, T.; Liu, Y.; Liu, G.; et al. PET monitoring angiogenesis of infarcted myocardium after treatment with vascular endothelial growth factor and bone marrow mesenchymal stem cells. Amino Acids 2016, 48, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Kiugel, M.; Dijkgraaf, I.; Kyto, V.; Helin, S.; Liljenback, H.; Saanijoki, T.; Yim, C.; Oikonen, V.; Saukko, P.; Knuuti, J.; et al. Dimeric [68Ga]DOTA-RGD peptide targeting alphavbeta 3 integrin reveals extracellular matrix alterations after myocardial infarction. Mol. Imaging Biol. 2014, 16, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Eo, J.S.; Paeng, J.C.; Lee, S.; Lee, Y.S.; Jeong, J.M.; Kang, K.W.; Chung, J.K.; Lee, D.S. Angiogenesis imaging in myocardial infarction using 68Ga-NOTA-RGD PET: characterization and application to therapeutic efficacy monitoring in rats. Coron. Artery Dis. 2013, 24, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, I.; Notni, J.; Pohle, K.; Rudelius, M.; Farrell, E.; Nekolla, S.G.; Henriksen, G.; Neubauer, S.; Kessler, H.; Wester, H.-J.; et al. Comparison of cyclic RGD peptides for αvβ3 integrin detection in a rat model of myocardial infarction. EJNMMI Res. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, L.; Kusmic, C.; Panetta, D.; Arosio, D.; Petroni, D.; Matteucci, M.; Salvadori, P.A.; Casagrande, C.; L'Abbate, A.; Manzoni, L. MicroPET/CT imaging of αvβ3 integrin via a novel 68Ga-NOTA-RGD peptidomimetic conjugate in rat myocardial infarction. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Lang, L.; Guo, N.; Cao, F.; Quan, Q.; Hu, S.; Kiesewetter, D.O.; Niu, G.; Chen, X. PET imaging of angiogenesis after myocardial infarction/reperfusion using a one-step labeled integrin-targeted tracer 18F-AlF-NOTA-PRGD2. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Sherif, H.M.; Saraste, A.; Nekolla, S.G.; Weidl, E.; Reder, S.; Tapfer, A.; Rudelius, M.; Higuchi, T.; Botnar, R.M.; Wester, H.J.; et al. Molecular imaging of early αvβ3 integrin expression predicts long-term left-ventricle remodeling after myocardial infarction in rats. J. Nucl. Med. 2012, 53, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, I.; Saraste, A.; Weidl, E.; Poethko, T.; Weber, A.W.; Nekolla, S.G.; Leppänen, P.; Ylä-Herttuala, S.; Hölzlwimmer, G.; Walch, A.; et al. Evaluation of αvβ3 integrin-targeted positron emission tomography tracer 18F-galacto-RGD for imaging of vascular inflammation in atherosclerotic mice. Circ. Cardiovasc. Imaging 2009, 2, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Bengel, F.M.; Seidl, S.; Watzlowik, P.; Kessler, H.; Hegenloh, R.; Reder, S.; Nekolla, S.G.; Wester, H.J.; Schwaiger, M. Assessment of αvβ3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc. Res. 2008, 78, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Meoli, D.F.; Sadeghi, M.M.; Krassilnikova, S.; Bourke, B.N.; Giordano, F.J.; Dione, D.P.; Su, H.; Edwards, D.S.; Liu, S.; Harris, T.D.; et al. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J. Clin. Investig. 2004, 113, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Oxboel, J.; Brandt-Larsen, M.; Schjoeth-Eskesen, C.; Myschetzky, R.; El-Ali, H.H.; Madsen, J.; Kjaer, A. Comparison of two new angiogenesis PET tracers 68Ga-NODAGA-E[c(RGDyK)]2 and 64Cu-NODAGA-E[c(RGDyK)]2; in vivo imaging studies in human xenograft tumors. Nucl. Med. Biol. 2014, 41, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.; Follin, B.; Kastrup, J.; Christensen, T.E.; Hammelev, K.P.; Kjaer, A.; Hasbak, P. Myocardial perfusion of infarcted and normal myocardium in propofol-anesthetized minipigs using Rubidium PET. J. Nucl. Cardiol. 2016, 23, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Makowski, M.R.; Ebersberger, U.; Nekolla, S.; Schwaiger, M. In vivo molecular imaging of angiogenesis, targeting αvβ3 integrin expression, in a patient after acute myocardial infarction. Eur. Heart J. 2008, 29. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Sun, Y.; Zhu, Z.; Li, F. Is the change of integrin αvβ3 expression in the infarcted myocardium related to the clinical outcome? Clin. Nucl. Med. 2014, 39, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zeng, Y.; Zhu, Y.; Feng, F.; Xu, W.; Wu, C.; Xing, B.; Zhang, W.; Wu, P.; Cui, L.; et al. Application of 68Ga-PRGD2 PET/CT for αvβ3-integrin imaging of myocardial infarction and stroke. Theranostics 2014, 4, 778–786. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmussen, T.; Follin, B.; Kastrup, J.; Brandt-Larsen, M.; Madsen, J.; Emil Christensen, T.; Pharao Hammelev, K.; Hasbak, P.; Kjær, A. Angiogenesis PET Tracer Uptake (68Ga-NODAGA-E[(cRGDyK)]2) in Induced Myocardial Infarction in Minipigs. Diagnostics 2016, 6, 26. https://doi.org/10.3390/diagnostics6020026
Rasmussen T, Follin B, Kastrup J, Brandt-Larsen M, Madsen J, Emil Christensen T, Pharao Hammelev K, Hasbak P, Kjær A. Angiogenesis PET Tracer Uptake (68Ga-NODAGA-E[(cRGDyK)]2) in Induced Myocardial Infarction in Minipigs. Diagnostics. 2016; 6(2):26. https://doi.org/10.3390/diagnostics6020026
Chicago/Turabian StyleRasmussen, Thomas, Bjarke Follin, Jens Kastrup, Malene Brandt-Larsen, Jacob Madsen, Thomas Emil Christensen, Karsten Pharao Hammelev, Philip Hasbak, and Andreas Kjær. 2016. "Angiogenesis PET Tracer Uptake (68Ga-NODAGA-E[(cRGDyK)]2) in Induced Myocardial Infarction in Minipigs" Diagnostics 6, no. 2: 26. https://doi.org/10.3390/diagnostics6020026






