Clinical Utility and Future Applications of PET/CT and PET/CMR in Cardiology
Abstract
:1. Introduction
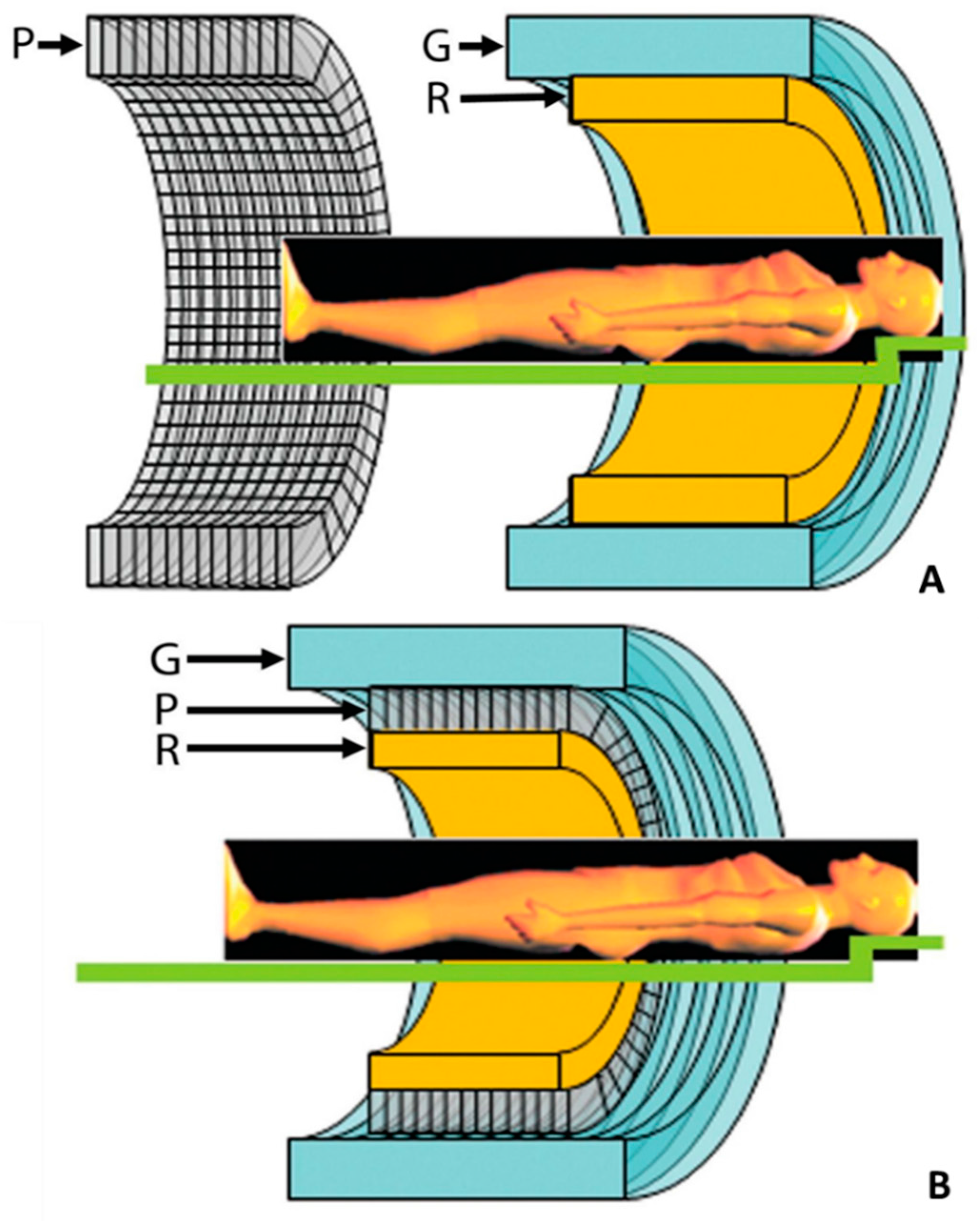
2. Technical Aspects of Hybrid Integration
2.1. PET/CT Technology
2.2. PET/CMR Technology
3. Radiotracers
3.1. PET Myocardial Perfusion Tracers
3.2. Myocardial Metabolism and Viability
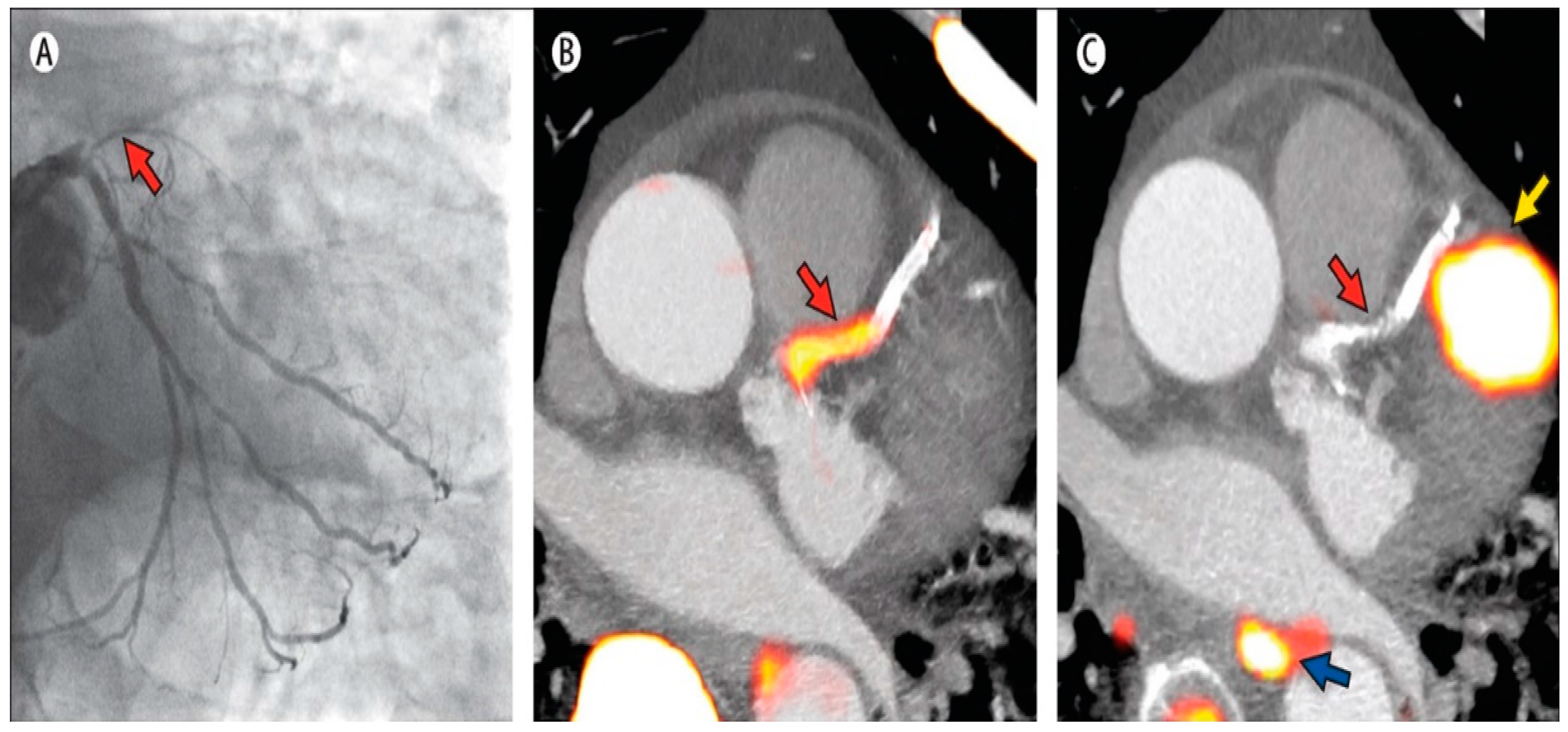
3.3. Inflammation and Plaque Characterization
3.4. New Tracer Options
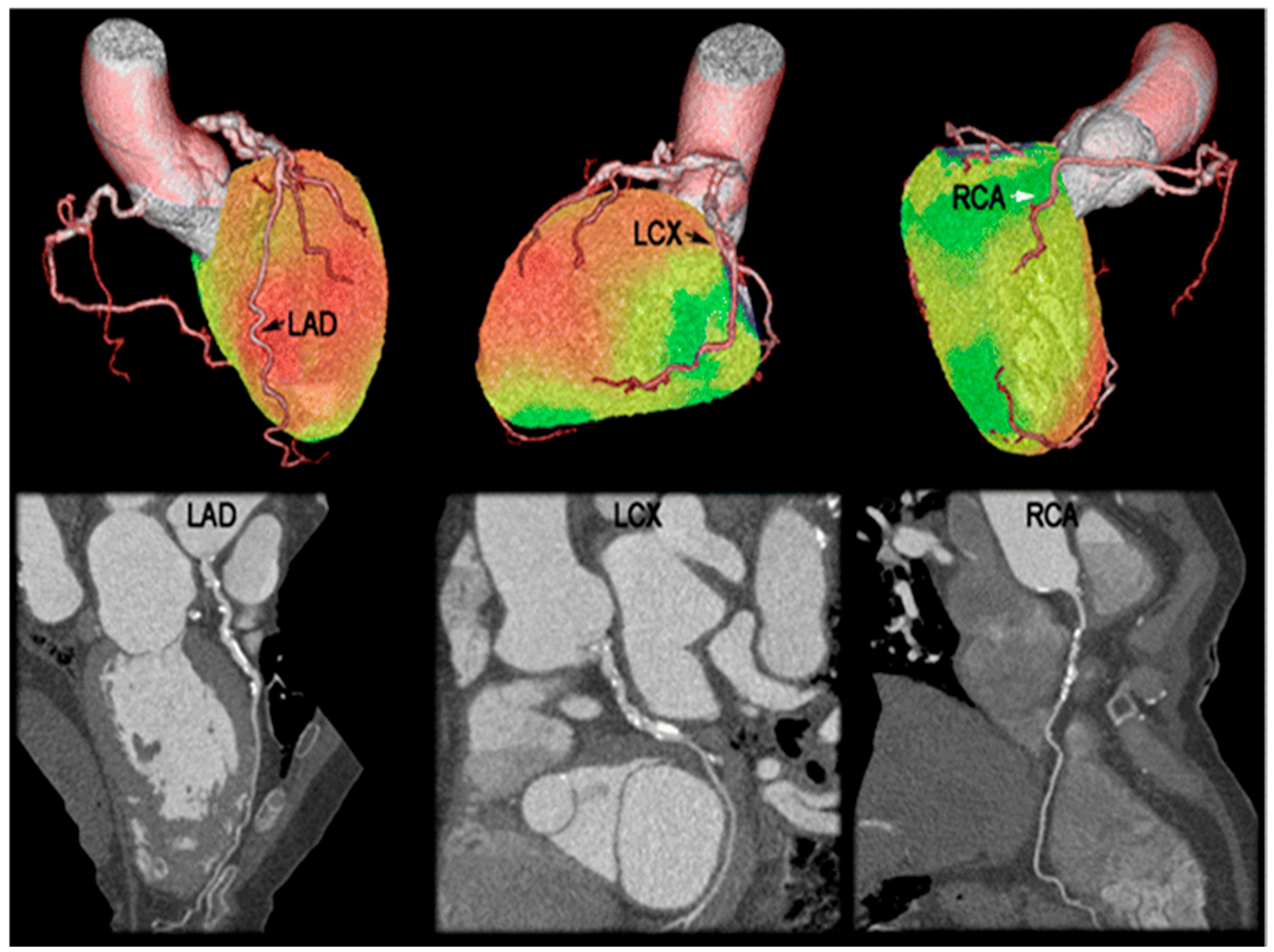
4. PET-CT Studies
4.1. MPI and Diagnostic Accuracy of CAD
4.2. Risk Stratification and Management
5. PET-CMR Studies
5.1. Stress Perfusion Imaging in CAD
5.2. Viability and Infarct Assessment
5.3. Heart Failure
5.4. Infiltrative and Inflammatory Processes
5.5. Molecular Imaging and Targeted Therapy
6. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| PET | Positron emission tomography |
| CT | Computed tomography |
| CMR | Cardiovascular magnetic resonance |
| MRI | Magnetic resonance imaging |
| CAD | Coronary artery disease |
| MPI | Myocardial perfusion imaging |
| CCTA | Coronary computed tomography angiography |
| CAC | Coronary artery calcium |
| μ-map | Attenuation coefficient map |
| 13NH3 | 13N-Ammonia |
| 82Rb | 82-Rubidium |
| FWHM | Full width half maximum |
| 82Sr | 82-Strontium |
| 18F | 18-Fluorine |
| FDG | Fluorodeoxyglucose |
| 11C-HED | 11C-Metahdyroxephedrine |
| 18F-galacto-RGD | 18F-galacto-cyclo(RGDfK) |
| MBF | Myocardial blood flow |
| MFR | Myocardial flow reserve |
| LVEF | Left ventricular ejection fraction |
| LGE | Late gadolinium enhancement |
| MVO | Microvascular obstruction |
| ECV | Extracellular volume |
| IE | Infective endocarditis |
| MMP | Matrix metalloproteinases |
References
- Adenaw, N.; Salerno, M. PET/MRI: Current state of the art and future potential for cardiovascular applications. J. Nucl. Cardiol. 2013, 20, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Danad, I.; Raijmakers, P.G.; Knaapen, P. Diagnosing coronary artery disease with hybrid PET/CT: It takes two to tango. J. Nucl. Cardiol. 2013, 20, 874–890. [Google Scholar] [CrossRef] [PubMed]
- Tarkin, J.M.; Joshi, F.R.; Rudd, J.H.F. PET imaging of inflammation in atherosclerosis. Nat. Rev. Cardiol. 2014, 11, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Judenhofer, M.S.; Cherry, S.R. Applications for preclinical PET/MRI. Semin. Nucl. Med. 2013, 43, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Nensa, F.; Beiderwellen, K.; Heusch, P.; Wetter, A. Clinical applications of PET/MRI: Current status and future perspectives. Diagn. Interv. Radiol. 2014, 20, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Pichler, B.J.; Kolb, A.; Nägele, T.; Schlemmer, H. PET/MRI: Paving the way for the next generation of clinical multimodality imaging applications. J. Nucl. Med. 2010, 51, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Pelizzari, C.A.; Chen, G.T.; Spelbring, D.R.; Weichselbaum, R.R.; Chen, C.T. Accurate three-dimensional registration of CT, PET, and/or MR images of the brain. J. Comput. Assist. Tomogr. 1989, 13, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Woods, R.P.; Mazziotta, J.C.; Cherry, S.R. MRI-PET registration with automated algorithm. J. Comput. Assist. Tomogr. 1993, 17, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, J.P. Cardiovascular magnetic resonance physics for clinicians: Part I. J. Cardiovasc. Magn. Reson. 2010, 12, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Germano, G.; Berman, D.S.; Slomka, P. Technical aspects of cardiac PET imaging and recent advances. Cardiol. Clin. 2016, 34, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Di Carli, M.F.; Dorbala, S.; Meserve, J.; El Fakhri, G.; Sitek, A.; Moore, S.C. Clinical myocardial perfusion PET/CT. J. Nucl. Med. 2007, 48, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Becker, C.R.; Schoepf, U.J.; Ohnesorge, B.; Bruening, R.; Reiser, M.F. Coronary artery calcium: Absolute quantification in nonenhanced and contrast-enhanced multi-detector row CT studies. Radiology 2002, 223, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Dorbala, S.; Di Carli, M.F.; Delbeke, D.; Abbara, S.; DePuey, E.G.; Dilsizian, V.; Forrester, J.; Janowitz, W.; Kaufmann, P.A.; Mahmarian, J.; et al. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J. Nucl. Med. 2013, 54, 1485–1507. [Google Scholar] [CrossRef] [PubMed]
- Knaapen, P.; de Haan, S.; Hoekstra, O.S.; Halbmeijer, R.; Appelman, Y.E.; Groothuis, J.; Comans, E.F.; Meijerink, M.R.; Lammertsma, A.A.; Lubberink, M.; et al. Cardiac PET-CT: Advanced hybrid imaging for the detection of coronary artery disease. Neth. Heart J. 2010, 18, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Möller, A.; Souvatzoglou, M.; Navab, N.; Schwaiger, M.; Nekolla, S.G. Artifacts from misaligned CT in cardiac perfusion PET/CT Studies: Frequency, effects, and potential solutions. J. Nucl. Med. 2007, 48, 188–193. [Google Scholar] [PubMed]
- Gould, K.L.; Pan, T.; Loghin, C.; Johnson, N.P.; Guha, A.; Sdringola, S. Frequent diagnostic errors in cardiac PET/CT Due to misregistration of CT attenuation and emission PET images: A definitive analysis of causes, consequences, and corrections. J. Nucl. Med. 2007, 48, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Klingensmith, W.C.; Noonan, C.; Goldberg, J.H.; Buchwald, D.; Kimball, J.T.; Manson, S.M. Decreased perfusion in the lateral wall of the left ventricle in PET/CT studies with 13N-Ammonia: Evaluation in healthy adults. J. Nucl. Med. Technol. 2009, 37, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, S.; Marsden, P.K. PET-MRI: A review of challenges and solutions in the development of integrated multimodality imaging. Phys. Med. Biol. 2015, 60, R115–R154. [Google Scholar] [CrossRef] [PubMed]
- Delso, G.; Fürst, S.; Jakoby, B.; Ladebeck, R.; Ganter, C.; Nekolla, S.G.; Schwaiger, M.; Ziegler, S.I. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J. Nucl. Med. 2011, 52, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Delso, G.; Ziegler, S. PET/MRI system design. Eur. J. Nucl. Med. Mol. Imaging 2008, 36, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, H.; Ojha, N.; Morich, M.; Griesmer, J.; Hu, Z.; Maniawski, P.; Ratib, O.; Izquierdo-Garcia, D.; Fayad, Z.A.; Shao, L. Design and performance evaluation of a whole-body Ingenuity TF PET-MRI system. Phys. Med. Biol. 2011, 56, 3091–3106. [Google Scholar] [CrossRef] [PubMed]
- Griesmer, J.J.; Futey, J.; Ojha, N.; Morich, M. Whole-body PET-MR imaging system initial calibration results. In Proceedings of the IEEE Nuclear Science Symposuim & Medical Imaging Conference, Knoxville, TN, USA, 20 October–6 November 2010; pp. 2174–2176.
- Torigian, D.A.; Zaidi, H.; Kwee, T.C.; Saboury, B.; Udupa, J.K.; Cho, Z.; Alavi, A. PET/MR Imaging: Technical aspects and potential clinical applications. Radiology 2013, 267, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Veit-Haibach, P.; Kuhn, F.P.; Wiesinger, F.; Delso, G.; Schulthess, G. PET-MR imaging using a tri-modality PET/CT-MR system with a dedicated shuttle in clinical routine. Magn. Reson. Mater. Phys. Biol. Med. 2012, 26, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Savic, D.; Yang, J.; Shrestha, U.; Seo, Y. The effect of magnetic field on positron range and spatial resolution in an integrated whole-body time-of-flight PET/MRI system. In Proceedings of the 2014 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Seattle, WA, USA, 8–15 November 2014; pp. 1–4.
- Hofmann, M.; Steinke, F.; Scheel, V.; Charpiat, G.; Farquhar, J.; Aschoff, P.; Brady, M.; Schölkopf, B.; Pichler, B.J. MRI-based attenuation correction for PET/MRI: A novel approach combining pattern recognition and atlas registration. J. Nucl. Med. 2008, 49, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Bezrukov, I.; Mantlik, F.; Aschoff, P.; Steinke, F.; Beyer, T.; Pichler, B.J.; Schölkopf, B. MRI-Based Attenuation Correction for Whole-Body PET/MRI: Quantitative evaluation of segmentation- and atlas-based methods. J. Nucl. Med. 2011, 52, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Martinez-Möller, A.; Souvatzoglou, M.; Holzapfel, K.; Pickhard, A.; Löffelbein, D.; Santi, I.; Rummeny, E.J.; Ziegler, S.; Schwaiger, M. Value of a Dixon-based MR/PET attenuation correction sequence for the localization and evaluation of PET-positive lesions. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Möller, A.; Souvatzoglou, M.; Navab, N.; Schwaiger, M.; Nekolla, S. MR-based attenuation correction for whole-body MR/PET. J. Nucl. Med. Meet. Abstr. 2008, 49, 65P. [Google Scholar]
- Veltman, C.E.; de Roos, A.; Schuijf, J.D.; van der Wall, E.E. Myocardial perfusion imaging: The Role of SPECT, PET and CMR. In From Basic Cardiac Imaging to Image Fusion: Core Competencies versus Technological Progress; Marzullo, P., Mariani, G., Eds.; Springer Milan: Milano, Italy, 2013; pp. 29–49. [Google Scholar]
- Salerno, M.; Beller, G.A. Noninvasive assessment of myocardial perfusion. Circ. Cardiovasc. Imaging 2009, 2, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Schelbert, H.R.; Phelps, M.E.; Huang, S.C.; MacDonald, N.S.; Hansen, H.; Selin, C.; Kuhl, D.E. N-13 ammonia as an indicator of myocardial blood flow. Circulation 1981, 63, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Mullani, N.; Gould, K.L. Coronary flow and flow reserve by PET simplified for clinical applications using rubidium-82 or nitrogen-13-ammonia. J. Nucl. Med. 1996, 37, 1701–1712. [Google Scholar] [PubMed]
- Machac, J. Radiopharmaceuticals for clinical cardiac PET Imaging. In Cardiac PET and PET/CT Imaging; Carli, M.F., Lipton, M.J., Eds.; Springer New York: New York, NY, USA, 2007; pp. 73–82. [Google Scholar]
- Lecomte, R. Technology challenges in small animal PET imaging. In Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, In Proceedings of the 2nd International Conference on Imaging Technologies in Biomedical Sciences, Athens, Greece, 26–30 May 2003; pp. 157–165.
- Yoshinaga, K.; Klein, R.; Tamaki, N. Generator-produced rubidium-82 positron emission tomography myocardial perfusion imaging—From basic aspects to clinical applications. J. Cardiol. 2010, 55, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Guaraldi, M.T.; Mistry, M.; Kagan, M.; McDonald, J.L.; Drew, K.; Radeke, H.; Azure, M.; Purohit, A.; Casebier, D.S.; et al. BMS-747158-02: A novel PET myocardial perfusion imaging agent. J. Nucl. Cardiol. 2007, 14, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Sherif, H.M.; Saraste, A.; Weidl, E.; Weber, A.W.; Higuchi, T.; Reder, S.; Poethko, T.; Henriksen, G.; Casebier, D.; Robinson, S.; et al. Evaluation of a novel 18F-labeled positron-emission tomography perfusion tracer for the assessment of myocardial infarct size in rats. Circ. Cardiovasc. Imaging 2009, 2, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Nekolla, S.G.; Reder, S.; Saraste, A.; Higuchi, T.; Dzewas, G.; Preissel, A.; Huisman, M.; Poethko, T.; Schuster, T.; Yu, M.; et al. Evaluation of the novel myocardial perfusion positron-emission tomography tracer 18F-BMS-747158-02: Comparison to 13N-Ammonia and validation with microspheres in a pig model. Circulation 2009, 119, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Yalamanchili, P.; Wexler, E.; Hayes, M.; Yu, M.; Bozek, J.; Kagan, M.; Radeke, H.S.; Azure, M.; Purohit, A.; Casebier, D.S.; et al. Mechanism of uptake and retention of F-18 BMS-747158–02 in cardiomyocytes: A novel PET myocardial imaging agent. J. Nucl. Cardiol. 2007, 14, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Huisman, M.C.; Higuchi, T.; Reder, S.; Nekolla, S.G.; Poethko, T.; Wester, H.; Ziegler, S.I.; Casebier, D.S.; Robinson, S.P.; Schwaiger, M. Initial characterization of an 18F-labeled myocardial perfusion tracer. J. Nucl. Med. 2008, 49, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.S.; Maddahi, J.; Tamarappoo, B.K.; Czernin, J.; Taillefer, R.; Udelson, J.E.; Gibson, C.M.; Devine, M.; Lazewatsky, J.; Bhat, G.; et al. Phase II safety and clinical comparison with single-photon emission computed tomography myocardial perfusion imaging for detection of coronary artery disease: Flurpiridaz F 18 positron emission tomography. J. Am. Coll. Cardiol. 2013, 61, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Krivokapich, J.; Huang, S.C.; Selin, C.E.; Phelps, M.E. Fluorodeoxyglucose rate constants, lumped constant, and glucose metabolic rate in rabbit heart. Am. J. Phys. Heart Circ. Phys. 1987, 252, H777–H787. [Google Scholar]
- Kouijzer, I.J.E.; Vos, F.J.; Janssen, M.J.R.; Dijk, A.P.J.; Oyen, W.J.G.; Bleeker-Rovers, C. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.M.; Williams, K.A. “Mass-ive” infarction: Case report and review of myocardial metastatic malignancies. J. Nucl. Cardiol. 2008, 15, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Skali, H.; Schulman, A.R.; Dorbala, S. 18F-FDG PET/CT for the assessment of myocardial sarcoidosis. Curr. Cardiol. Rep. 2013, 15, 352. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Seifarth, H.; Schäfers, M.; Stegger, L.; Hoffmeier, A.; Spieker, T.; Tiemann, K.; Maintz, D.; Scheld, H.H.; Schober, O.; et al. Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT. J. Nucl. Med. 2012, 53, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Yen, R.; Chen, Y.; Wu, Y.; Pan, M.; Chang, S. Using 18-fluoro-2-deoxyglucose positron emission tomography in detecting infectious endocarditis/endoarteritis: A preliminary report. Acad. Radiol. 2004, 11, 316–321. [Google Scholar] [CrossRef]
- Beanlands, R.S.; Ruddy, T.D.; deKemp, R.A.; Iwanochko, R.M.; Coates, G.; Freeman, M.; Nahmias, C.; Hendry, P.; Burns, R.J.; Lamy, A.; et al. PARR Investigators Positron emission tomography and recovery following revascularization (PARR-1): The importance of scar and the development of a prediction rule for the degree of recovery of left ventricular function. J. Am. Coll. Cardiol. 2002, 40, 1735–1743. [Google Scholar] [CrossRef]
- Schinkel, A.F.L.; Poldermans, D.; Elhendy, A.; Bax, J.J. Assessment of myocardial viability in patients with heart failure. J. Nucl. Med. 2007, 48, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Rogers, I.S.; Nasir, K.; Figueroa, A.L.; Cury, R.C.; Hoffmann, U.; Vermylen, D.A.; Brady, T.J.; Tawakol, A. Feasibility of FDG imaging of the coronary arteries comparison between acute coronary syndrome and stable angina. JACC Cardiovasc. Imaging 2010, 3, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K.; Falk, E.; Badimon, J.J.; Fernandez-Ortiz, A.; Mailhac, A.; Villareal-Levy, G.; Fallon, J.T.; Regnstrom, J.; Fuster, V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation 1995, 92, 1565–1569. [Google Scholar] [PubMed]
- Demeure, F.; Hanin, F.; Bol, A.; Vincent, M.; Pouleur, A.; Gerber, B.; Pasquet, A.; Jamar, F.; Vanoverschelde, J.J.; Vancraeynest, D. A Randomized trial on the optimization of 18F-FDG myocardial uptake suppression: Implications for vulnerable coronary plaque imaging. J. Nucl. Med. 2014, 55, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Kolodny, G.M. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. Am. J. Roentgenol. 2008, 190, W151–W156. [Google Scholar] [CrossRef] [PubMed]
- Oshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.; Calvert, P.A.; Craighead, F.H.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014, 383, 705–713. [Google Scholar]
- New, S.E.P.; Goettsch, C.; Aikawa, M.; Marchini, J.F.; Shibasaki, M.; Yabusaki, K.; Libby, P.; Shanahan, C.M.; Croce, K.; Aikawa, E. Macrophage-derived matrix vesicles: An alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ. Res. 2013, 113, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, E.; Nahrendorf, M.; Figueiredo, J.; Swirski, F.K.; Shtatland, T.; Kohler, R.H.; Jaffer, F.A.; Aikawa, M.; Weissleder, R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 2007, 116, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Rosenspire, K.C.; Haka, M.S.; Van Dort, M.; Jewett, D.M.; Gildersleeve, D.L.; Schwaiger, M.; Wieland, D.M. Synthesis and preliminary evaluation of carbon-11-meta-hydroxyephedrine: A false transmitter agent for heart neuronal imaging. J. Nucl. Med. 1990, 31, 1328–1334. [Google Scholar] [PubMed]
- Schwaiger, M.; Kalff, V.; Rosenspire, K.; Haka, M.S.; Molina, E.; Hutchins, G.D.; Deeb, M.; Wolfe, E., Jr.; Wieland, D.M. Noninvasive evaluation of sympathetic nervous system in human heart by positron emission tomography. Circulation 1990, 82, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Allman, K.C.; Wieland, D.M.; Muzik, O.; Degrado, T.R.; Wolfe, E.R., Jr.; Schwaiger, M. Carbon-11 hydroxyephedrine with positron emission tomography for serial assessment of cardiac adrenergic neuronal function after acute myocardial infarction in humans. J. Am. Coll. Cardiol. 1993, 22, 368–375. [Google Scholar] [CrossRef]
- Backs, J.; Haunstetter, A.; Gerber, S.H.; Metz, J.; Borst, M.M.; Strasser, R.H.; Kübler, W.; Haass, M. The neuronal norepinephrine transporter in experimental heart failure: Evidence for a posttranscriptional downregulation. J. Mol. Cell. Cardiol. 2001, 33, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, S.; Nanni, C.; Marini, C.; Bonfiglioli, R.; Martignani, C.; Dib, B.; Fuccio, C.; Boriani, G.; Picori, L.; Boschi, S.; et al. Heterogeneous response of cardiac sympathetic function to cardiac resynchronization therapy in heart failure documented by 11[C]-hydroxy-ephedrine and PET/CT. Nucl. Med. Biol. 2015, 42, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Opavsky, M.A.; Stewart, D.J.; Rabinovitch, M.; Dawood, F.; Wen, W.H.; Liu, P.P. Temporal response and localization of integrins β1 and β3 in the heart after myocardial infarction: Regulation by cytokines. Circulation 2003, 107, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Sherif, H.M.; Saraste, A.; Nekolla, S.G.; Weidl, E.; Reder, S.; Tapfer, A.; Rudelius, M.; Higuchi, T.; Botnar, R.M.; Wester, H.J.; et al. Molecular imaging of early αvβ3 integrin expression predicts long-term left-ventricle remodeling after myocardial infarction in rats. J. Nucl. Med. 2012, 53, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Bengel, F.M.; Seidl, S.; Watzlowik, P.; Kessler, H.; Hegenloh, R.; Reder, S.; Nekolla, S.G.; Wester, H.J.; Schwaiger, M. Assessment of αvβ3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc. Res. 2008, 78, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Lang, L.; Guo, N.; Cao, F.; Quan, Q.; Hu, S.; Kiesewetter, D.O.; Niu, G.; Chen, X. PET imaging of angiogenesis after myocardial infarction/reperfusion using a one-step labeled integrin-targeted tracer 18F-AlF-NOTA-PRGD2. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Nandalur, K.R.; Dwamena, B.A.; Choudhri, A.F.; Nandalur, S.R.; Reddy, P.; Carlos, R.C. Diagnostic performance of positron emission tomography in the detection of coronary artery disease: A Meta-analysis. Acad. Radiol. 2008, 15, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma, C.; Leiner, T.; Bekkers, S.C.; Crijns, H.J.; Wildberger, J.E.; Nagel, E.; Nelemans, P.J.; Schalla, S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery diseasea meta-analysis. J. Am. Coll. Cardiol. 2012, 59, 1719–1728. [Google Scholar] [PubMed]
- Gonzalez, J.A.; Lipinski, M.J.; Flors, L.; Shaw, P.W.; Kramer, C.M.; Salerno, M. Meta-analysis of diagnostic performance of coronary computed tomography angiography, computed tomography perfusion, and computed tomography-fractional flow reserve in functional myocardial ischemia assessment versus invasive fractional flow reserve. Am. J. Cardiol. 2015, 116, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Danad, I.; Raijmakers, P.G.; Appelman, Y.E.; Harms, H.J.; de Haan, S.; van den Oever, M.L.; Heymans, M.W.; Tulevski, I.I.; van Kuijk, C.; Hoekstra, O.S.; et al. Hybrid imaging using quantitative H215O PET and CT-based coronary angiography for the detection of coronary artery disease. J. Nucl. Med. 2013, 54, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kajander, S.; Joutsiniemi, E.; Saraste, M.; Pietila, M.; Ukkonen, H.; Saraste, A.; Sipila, H.T.; Teras, M.; Maki, M.; Airaksinen, J.; et al. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation 2010, 122, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.M.; Speechly-Dick, M.E.; Kayani, I.; Pugliese, F.; Endozo, R.; McEwan, J.; Menezes, L.J.; Habib, S.B.; Prvulovich, E.; Ell, P.J. First experience of combined cardiac PET/64-detector CT angiography with invasive angiographic validation. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Kajander, S.A.; Joutsiniemi, E.; Saraste, M.; Pietila, M.; Ukkonen, H.; Saraste, A.; Sipila, H.T.; Teras, M.; Maki, M.; Airaksinen, J.; et al. Clinical value of absolute quantification of myocardial perfusion with (15)O-water in coronary artery disease. Circ. Cardiovasc. Imaging 2011, 4, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Fiechter, M.; Ghadri, J.R.; Gebhard, C.; Fuchs, T.A.; Pazhenkottil, A.P.; Nkoulou, R.N.; Herzog, B.A.; Wyss, C.A.; Gaemperli, O.; Kaufmann, P.A. Diagnostic value of 13N-ammonia myocardial perfusion PET: Added value of myocardial flow reserve. J. Nucl. Med. 2012, 53, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Parkash, R.; deKemp, R.A.; Ruddy, T.D.; Kitsikis, A.; Hart, R.; Beauchesne, L.; Williams, K.; Davies, R.A.; Labinaz, M.; Beanlands, R.S. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J. Nucl. Cardiol. 2004, 11, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Danad, I.; Raijmakers, P.G.; Appelman, Y.E.; Harms, H.J.; Haan, S.; Oever, M.L.P.; Kuijk, C.; Allaart, C.P.; Hoekstra, O.S.; Lammertsma, A.A.; et al. Coronary risk factors and myocardial blood flow in patients evaluated for coronary artery disease: A quantitative [15O]H2O PET/CT study. Eur. J. Nucl. Med. Mol. Imaging 2011, 39, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Valenta, I.; Quercioli, A.; Vincenti, G.; Nkoulou, R.; Dewarrat, S.; Rager, O.; Zaidi, H.; Seimbille, Y.; Mach, F.; Ratib, O.; et al. Structural epicardial disease and microvascular function are determinants of an abnormal longitudinal myocardial blood flow difference in cardiovascular risk individuals as determined with PET/CT. J. Nucl. Cardiol. 2010, 17, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Chow, B.J.; Williams, K.; Chen, L.; deKemp, R.A.; Garrard, L.; Lok-Tin Szeto, A.; Aung, M.; Davies, R.A.; Ruddy, T.D.; et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J. Am. Coll. Cardiol. 2006, 48, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Herzog, B.A.; Husmann, L.; Valenta, I.; Gaemperli, O.; Siegrist, P.T.; Tay, F.M.; Burkhard, N.; Wyss, C.A.; Kaufmann, P.A. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J. Am. Coll. Cardiol. 2009, 54, 150–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lertsburapa, K.; Ahlberg, A.W.; Bateman, T.M.; Katten, D.; Volker, L.; Cullom, S.J.; Heller, G.V. Independent and incremental prognostic value of left ventricular ejection fraction determined by stress gated rubidium 82 PET imaging in patients with known or suspected coronary artery disease. J. Nucl. Cardiol. 2008, 15, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Dorbala, S.; Hachamovitch, R.; Curillova, Z.; Thomas, D.; Vangala, D.; Kwong, R.Y.; di Carli, M.F. Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. JACC Cardiovasc. Imaging 2009, 2, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Dorbala, S.; di Carli, M.F.; Beanlands, R.S.; Merhige, M.E.; Williams, B.A.; Veledar, E.; Chow, B.J.W.; Min, J.K.; Pencina, M.J.; Berman, D.S.; et al. Prognostic value of stress myocardial perfusion positron emission tomography results from a multicenter observational registry. J. Am. Coll. Cardiol. 2013, 61, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.H.; Nitzsche, E.U.; Schelbert, H.R.; Olschewski, M.; Sayre, J.; Mix, M.; Brink, I.; Zhang, X.L.; Kreissl, M.; Magosaki, N.; et al. Positron emission tomography-measured abnormal responses of myocardial blood flow to sympathetic stimulation are associated with the risk of developing cardiovascular events. J. Am. Coll. Cardiol. 2005, 45, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Javadi, M.S.; Higuchi, T.; Lautamaki, R.; Merrill, J.; Nekolla, S.G.; Bengel, F.M. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J. Nucl. Med. 2011, 52, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Ziadi, M.C.; Dekemp, R.A.; Williams, K.A.; Guo, A.; Chow, B.J.; Renaud, J.M.; Ruddy, T.D.; Sarveswaran, N.; Tee, R.E.; Beanlands, R.S. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J. Am. Coll. Cardiol. 2011, 58, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Ziadi, M.C.; Dekemp, R.A.; Williams, K.; Guo, A.; Renaud, J.M.; Chow, B.J.; Klein, R.; Ruddy, T.D.; Aung, M.; Garrard, L.; et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J. Nucl. Cardiol. 2012, 19, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Graf, S.; Khorsand, A.; Gwechenberger, M.; Novotny, C.; Kletter, K.; Sochor, H.; Pirich, C.; Maurer, G.; Porenta, G.; Zehetgruber, M. Typical chest pain and normal coronary angiogram: Cardiac risk factor analysis versus PET for detection of microvascular disease. J. Nucl. Med. 2007, 48, 175–181. [Google Scholar] [PubMed]
- Sdringola, S.; Loghin, C.; Boccalandro, F.; Gould, K.L. Mechanisms of progression and regression of coronary artery disease by PET related to treatment intensity and clinical events at long-term follow-up. J. Nucl. Med. 2006, 47, 59–67. [Google Scholar] [PubMed]
- Dorbala, S.; Vangala, D.; Sampson, U.; Limaye, A.; Kwong, R.; di Carli, M.F. Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: A 82Rb PET/CT study. J. Nucl. Med. 2007, 48, 349–358. [Google Scholar] [PubMed]
- Bittencourt, M.S.; Hulten, E.; Ghoshhajra, B.; O’Leary, D.; Christman, M.P.; Montana, P.; Truong, Q.A.; Steigner, M.; Murthy, V.L.; Rybicki, F.J.; et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ. Cardiovasc. Imaging 2014, 7, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.M.; Dowe, D.A.; Brink, J.A. Traditional clinical risk assessment tools do not accurately predict coronary atherosclerotic plaque burden: A CT angiography study. AJR Am. J. Roentgenol. 2009, 192, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Rumberger, J.A.; Simons, D.B.; Fitzpatrick, L.A.; Sheedy, P.F.; Schwartz, R.S. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation 1995, 92, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- Brodov, Y.; Gransar, H.; Dey, D.; Shalev, A.; Germano, G.; Friedman, J.D.; Hayes, S.W.; Thomson, L.E.J.; Rogatko, A.; Berman, D.S.; et al. Combined quantitative assessment of myocardial perfusion and coronary artery calcium score by hybrid 82Rb PET/CT improves detection of coronary artery disease. J. Nucl. Med. 2015, 56, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Schenker, M.P.; Dorbala, S.; Hong, E.C.; Rybicki, F.J.; Hachamovitch, R.; Kwong, R.Y.; di Carli, M.F. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: A combined positron emission tomography/computed tomography study. Circulation 2008, 117, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, G.; Shin, V.; Punjani, S.; Ritter, N.; Sharma, S.C.; Wu, W.C. The role of calcium score and CT angiography in the medical management of patients with normal myocardial perfusion imaging. J. Nucl. Cardiol. 2010, 17, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, A.; Petersen, H.; Diederichsen, A.C.P.; Mickley, H.; Jensen, L.O.; Johansen, A.; Gerke, O.; Braad, P.; Thayssen, P.; Hoilund-Carlsen, M.M.; et al. Hybrid CT angiography and quantitative 15O-water PET for assessment of coronary artery disease: Comparison with quantitative coronary angiography. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, A.; Servadei, F.; Zoccai, G.B.; Giacobbi, E.; Anemona, L.; Bonanno, E.; Casella, S. Coronary calcification identifies the vulnerable patient rather than the vulnerable Plaque. Atherosclerosis 2013, 229, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Finn, A.V.; Virmani, R. Do vulnerable and ruptured plaques hide in heavily calcified arteries? Atherosclerosis 2013, 229, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Wykrzykowska, J.; Lehman, S.; Williams, G.; Parker, J.A.; Palmer, M.R.; Varkey, S.; Kolodny, G.; Laham, R. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J. Nucl. Med. 2009, 50, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.Y.; Slomka, P.J.; le Meunier, L.; Tamarappoo, B.K.; Nakazato, R.; Dey, D.; Berman, D.S. Coronary arterial 18F-FDG Uptake by fusion of PET and coronary ct angiography at sites of percutaneous stenting for acute myocardial infarction and stable coronary artery disease. J. Nucl. Med. 2012, 53, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Gholami, S.; Salavati, A.; Houshmand, S.; Werner, T.J.; Alavi, A. Assessment of atherosclerosis in large vessel walls: A comprehensive review of FDG-PET/CT image acquisition protocols and methods for uptake quantification. J. Nucl. Cardiol. 2015, 22, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.; Kidambi, A.; Sourbron, S.; Fairbairn, T.A.; Uddin, A.; Kozerke, S.; Greenwood, J.P.; Plein, S. Quantitative three-dimensional cardiovascular magnetic resonance myocardial perfusion imaging in systole and diastole. J. Cardiovasc. Magn. Reson. 2014, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fair, M.J.; Gatehouse, P.D.; DiBella, E.V.R.; Firmin, D.N. A review of 3D first-pass, whole-heart, myocardial perfusion cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2015, 17, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.R.; Jha, S. Diagnostic performance of cardiac stress perfusion MRI in the detection of coronary artery disease using fractional flow reserve as the reference standard: A meta-analysis. Am. J. Roentgenol. 2013, 201, W245–W252. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.; Chiribiri, A.; Ishida, M.; Hussain, S.T.; Schuster, A.; Indermuehle, A.; Perera, D.; Knuuti, J.; Baker, S.; Hedström, E.; et al. Quantification of absolute myocardial perfusion in patients with coronary artery diseasecomparison between cardiovascular magnetic resonance and positron emission tomography. J. Am. Coll. Cardiol. 2012, 60, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.A.; Hasbak, P.; Larsson, H.B.W.; Christensen, T.E.; Ghotbi, A.A.; Mathiasen, A.B.; Vejlstrup, N.G.; Kjaer, A.; Kastrup, J. Quantification of myocardial perfusion using cardiac magnetic resonance imaging correlates significantly to rubidium-82 positron emission tomography in patients with severe coronary artery disease: A preliminary study. Eur. J. Radiol. 2014, 83, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, G.; Betocchi, S.; Pace, L.; Losi, M.A.; Perrone-Filardi, P.; Soricelli, A.; Piscione, F.; Taube, J.; Squame, F.; Salvatore, M.; et al. Prolonged impairment of regional contractile function after resolution of exercise-induced angina: Evidence of myocardial stunning in patients with coronary artery disease. Circulation 1996, 94, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.; Hall, R.J.C.; Dutka, D.P.; Camici, P.G. Absolute blood flow and oxygenconsumption in stunned myocardiumin patients with coronary artery disease. J. Am. Coll. Cardiol. 2002, 39, 420–427. [Google Scholar] [CrossRef]
- Diamond, G.A.; Forrester, J.S.; deLuz, P.L.; Wyatt, H.L.; Swan, H.J. Post-extrasystolic potentiation of ischemic myocardium by atrial stimulation. Am. Heart J. 1978, 95, 204–209. [Google Scholar] [CrossRef]
- Rahimtoola, S.H. A perspective on the three large multicenter randomized clinical trials of coronary bypass surgery for chronic stable angina. Circulation 1985, 72, V123–V135. [Google Scholar] [PubMed]
- Schinkel, A.F.L.; Bax, J.J.; Poldermans, D.; Elhendy, A.; Ferrari, R.; Rahimtoola, S.H. Hibernating Myocardium: Diagnosis and Patient Outcomes. Curr. Probl. Cardiol. 2007, 32, 375–410. [Google Scholar] [CrossRef] [PubMed]
- Di Carli, M.F.; Asgarzadie, F.; Schelbert, H.R.; Brunken, R.C.; Laks, H.; Phelps, M.E.; Maddahi, J. Quantitative relation between myocardial viability and improvement in heart failure symptoms after revascularization in patients with ischemic cardiomyopathy. Circulation 1995, 92, 3436–3444. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.; Wu, E.; Rafael, A.; Chen, E.L.; Parker, M.A.; Simonetti, O.; Klocke, F.J.; Bonow, R.O.; Judd, R.M. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N. Engl. J. Med. 2000, 343, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Hunold, P.; Brandt-Mainz, K.; Freudenberg, L.; Vogt, F.M.; Neumann, T.; Knipp, S.; Barkhausen, J. Evaluation of myocardial viability with contrast-enhanced magnetic resonance imaging—Comparison of the late enhancement technique with positronemission tomography. RoFo 2002, 174, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, H.P.; Beek, A.M.; van der Weerdt, A.P.; Hofman, M.B.; Visser, C.A.; Lammertsma, A.A.; Heussen, N.; Visser, F.C.; van Rossum, A.C. Myocardial viability in chronic ischemic heart disease: Comparison of contrast-enhanced magnetic resonance imaging with 18F-fluorodeoxyglucose positron emission tomography. J. Am. Coll. Cardiol. 2003, 41, 1341–1348. [Google Scholar] [CrossRef]
- Klein, C.; Nekolla, S.G.; Bengel, F.M.; Momose, M.; Sammer, A.; Haas, F.; Schnackenburg, B.; Delius, W.; Mudra, H.; Wolfram, D.; et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: Comparison with positron emission tomography. Circulation 2002, 105, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Nensa, F.; Poeppel, T.D.; Beiderwellen, K.; Schelhorn, J.; Mahabadi, A.A.; Erbel, R.; Heusch, P.; Nassenstein, K.; Bockisch, A.; Forsting, M.; et al. Hybrid PET/MR Imaging of the heart: Feasibility and initial results. Radiology 2013, 268, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Rischpler, C.; Langwieser, N.; Souvatzoglou, M.; Batrice, A.; van Marwick, S.; Snajberk, J.; Ibrahim, T.; Laugwitz, K.; Nekolla, S.G.; Schwaiger, M. PET/MRI early after myocardial infarction: Evaluation of viability with late gadolinium enhancement transmurality vs. 18F-FDG uptake. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Hadamitzky, M.; Langhans, B.; Hausleiter, J.; Sonne, C.; Byrne, R.A.; Mehilli, J.; Kastrati, A.; Schömig, A.; Martinoff, S.; Ibrahim, T. Prognostic value of late gadolinium enhancement in cardiovascular magnetic resonance imaging after acute ST-elevation myocardial infarction in comparison with single-photon emission tomography using Tc99m-Sestamibi. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; de Waha, S.; Wöhrle, J.; Fuernau, G.; Lurz, P.; Pauschinger, M.; Desch, S.; Schuler, G.; Thiele, H. Comprehensive prognosis assessment by CMR Imaging after ST-Segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2014, 64, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.E. Magnetic resonance imaging for area at risk, myocardial infarction, and myocardial salvage. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Friedrich, M.G. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J. Cardiovasc. Magn. Reson. 2011, 13. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; White, S.K.; Fröhlich, G.M.; Casson, S.G.; O’Meara, C.; Newton, A.; Nicholas, J.; Weale, P.; Wan, S.M.Y.; Sirker, A.; et al. Quantifying the area at risk in reperfused ST-Segment–Elevation myocardial infarction patients using hybrid cardiac positron emission tomography–magnetic resonance imaging. Circ. Cardiovasc. Imaging 2016, 9, e003900. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Kramer, C.M. Advances in parametric mapping with CMR imaging. JACC Cardiovasc. Imaging 2013, 6, 806–822. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; Rogers, W.J.; Theobald, T.M.; Power, T.P.; Petruolo, S.; Reichek, N. Remote noninfarcted region dysfunction soon after first anterior myocardial infarction: A magnetic resonance tagging study. Circulation 1996, 94, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Rosen, B.D.; Fernandes, V.R.S.; Yan, R.T.; Yoneyama, K.; Donekal, S.; Opdahl, A.; Almeida, A.L.C.; Wu, C.O.; Gomes, A.S.; et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: The Multi-Ethnic Study of Atherosclerosis. Eur. Heart J. 2013, 34, 2354–2361. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 Mapping: Basic techniques and clinical applications. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.C.; Piehler, K.; Meier, C.G.; Testa, S.M.; Klock, A.M.; Aneizi, A.A.; Shakesprere, J.; Kellman, P.; Shroff, S.G.; Schwartzman, D.S.; et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation 2012, 126, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Mascherbauer, J.; Marzluf, B.A.; Tufaro, C.; Pfaffenberger, S.; Grad, A.; Schreiber, C.; Karakus, G.; Hülsman, M.; Pacher, R.; Lang, I.M.; et al. Extent of diffuse myocardial fibrosis determines outcome in patients with heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 2013, 61. [Google Scholar] [CrossRef]
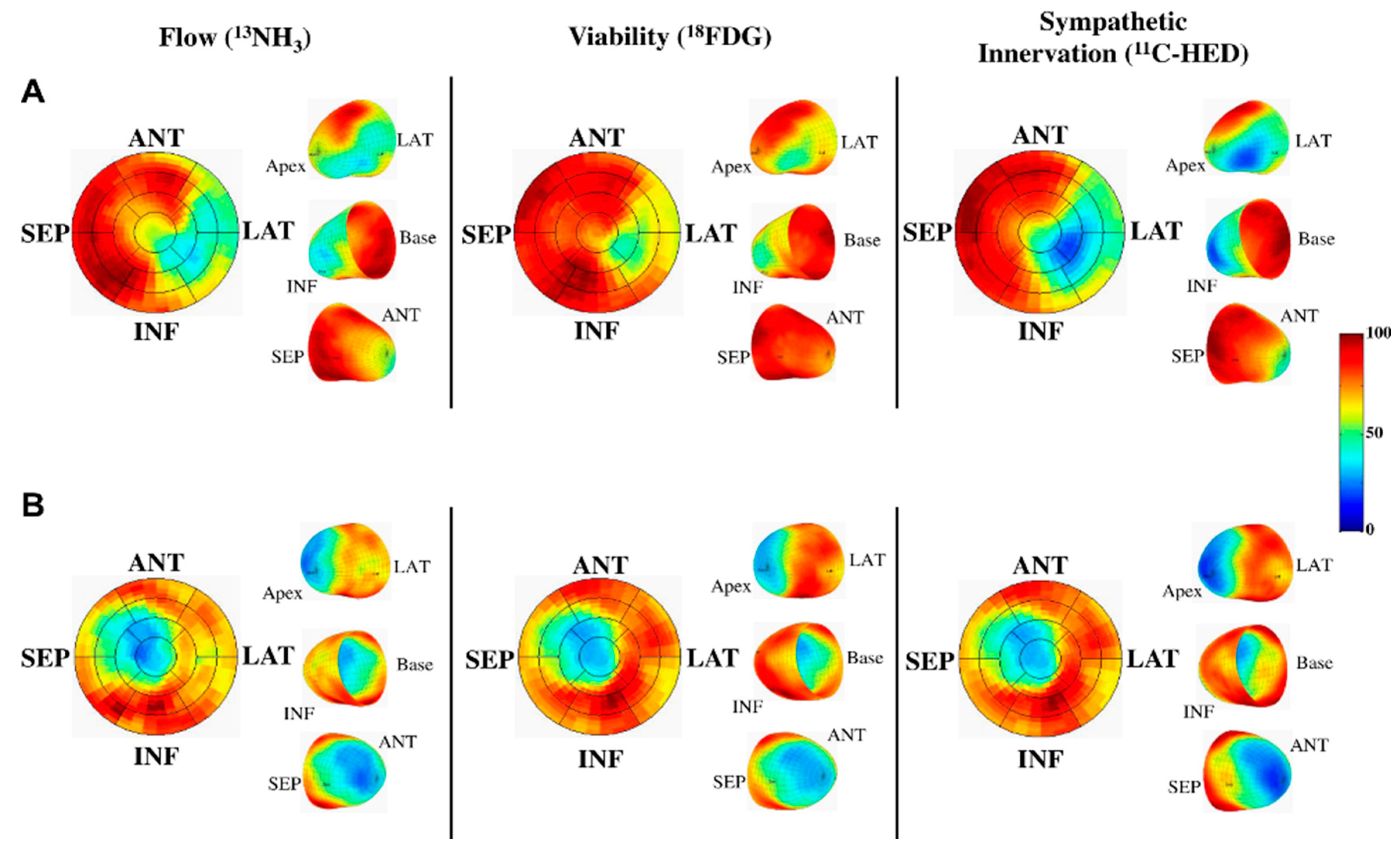
- Thackeray, J.T.; Bengel, F.M. Assessment of cardiac autonomic neuronal function using PET imaging. J. Nucl. Cardiol. 2012, 20, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, F.; Ziegler, S.; Nekolla, S.; Hadamitzky, M.; Seyfarth, M.; Richardt, G.; Schwaiger, M. Regional patterns of myocardial sympathetic denervation in dilated cardiomyopathy: An analysis using carbon-11 hydroxyephedrine and positron emission tomography. Heart 1999, 81, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Pietila, M.; Malminiemi, K.; Ukkonen, H.; Saraste, M.; Nagren, K.; Lehikoinen, P.; Voipio-Pulkki, L.M. Reduced myocardial carbon-11 hydroxyephedrine retention is associated with poor prognosis in chronic heart failure. Eur. J. Nucl. Med. 2001, 28, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Fallavollita, J.A.; Heavey, B.M.; Luisi, A.J., Jr.; Michalek, S.M.; Baldwa, S.; Mashtare, T.L., Jr.; Hutson, A.D.; Dekemp, R.A.; Haka, M.S.; Sajjad, M.; et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 141–149. [Google Scholar] [CrossRef] [PubMed]
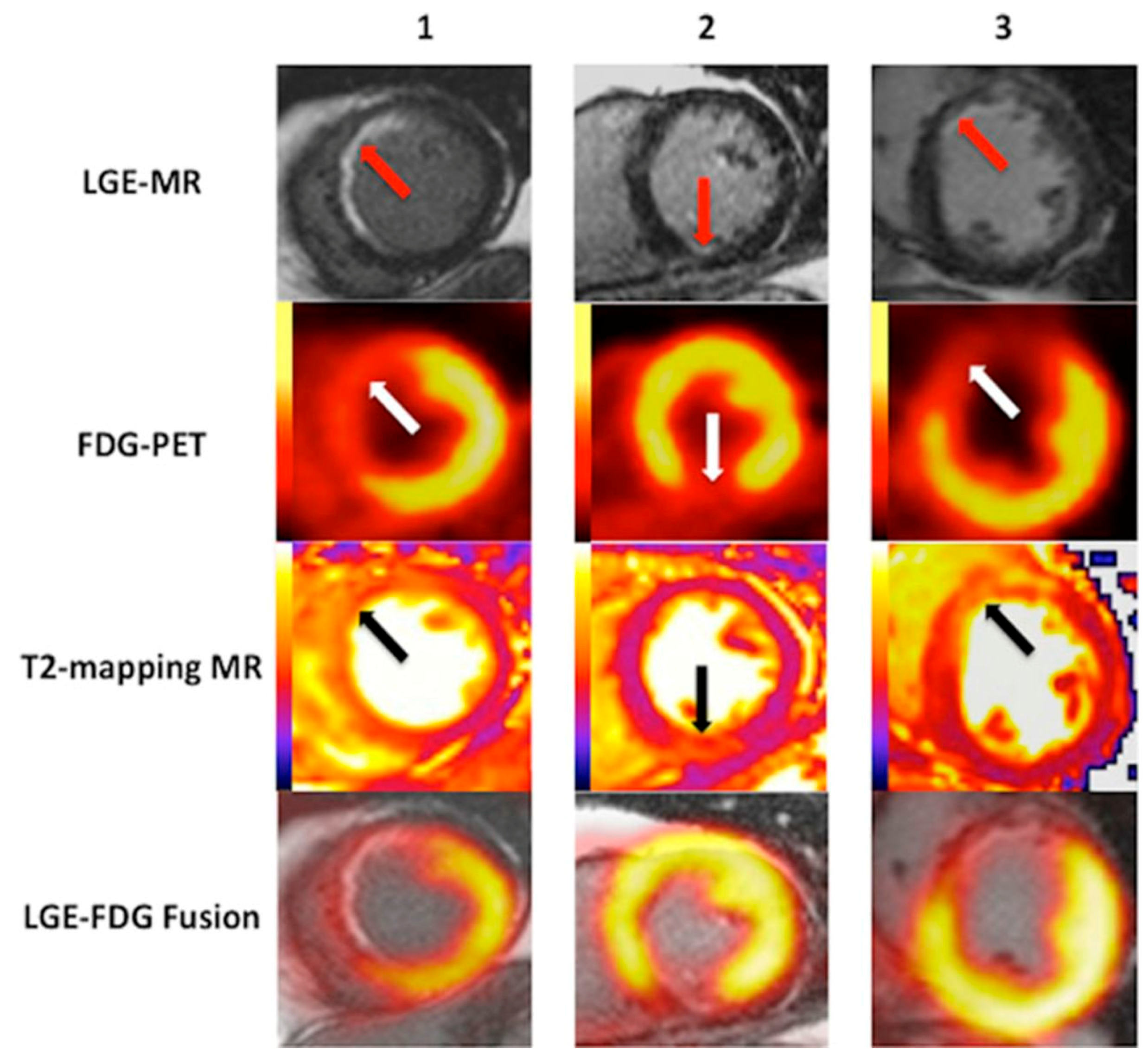
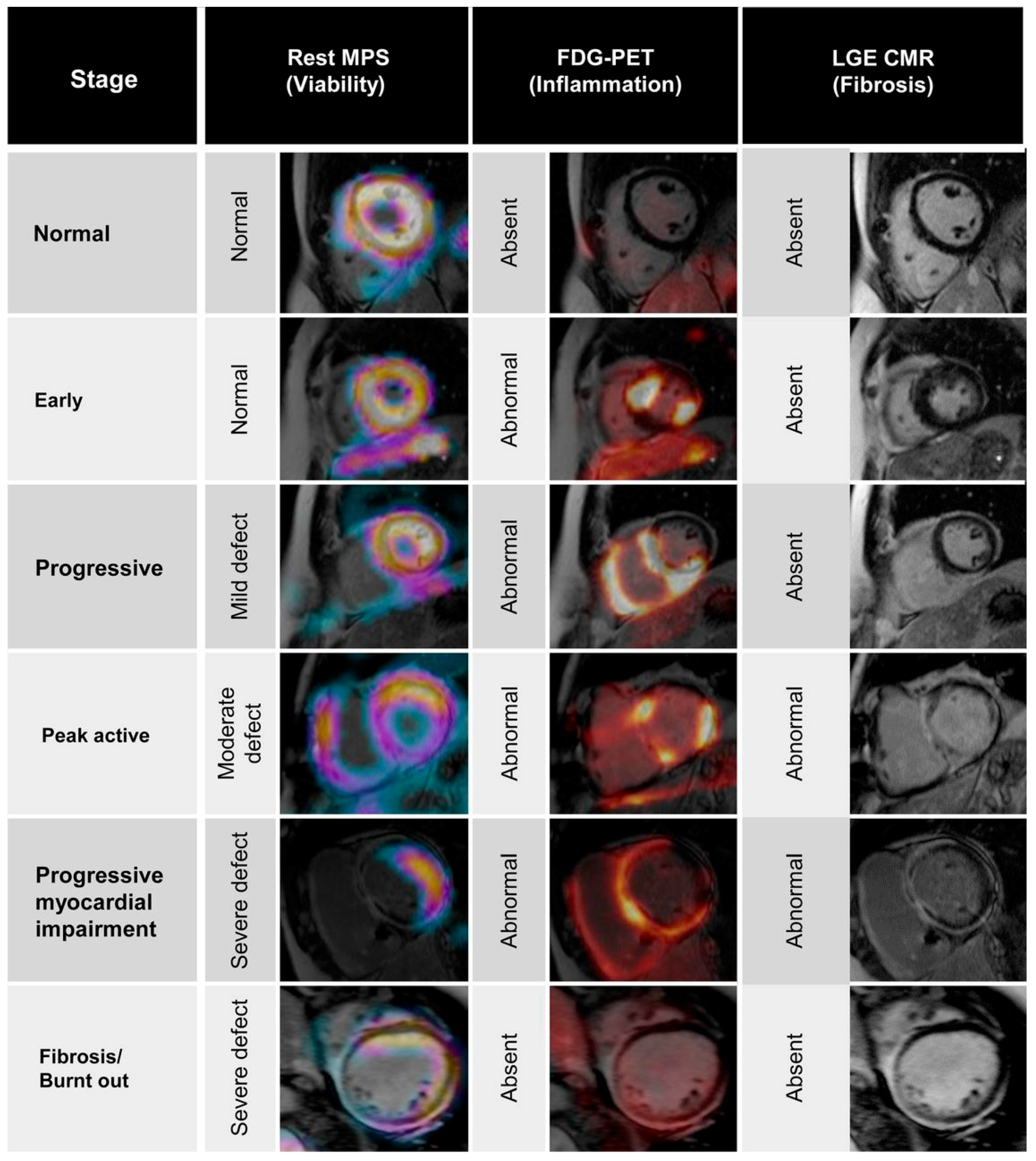
- Schatka, I.; Bengel, F.M. Advanced imaging of cardiac sarcoidosis. J. Nucl. Med. 2014, 55, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Cawley, P.J.; Heitner, J.F.; Klem, I.; Parker, M.A.; Jaroudi, W.A.; Meine, T.J.; White, J.B.; Elliott, M.D.; Kim, H.W.; et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009, 120, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, G.; Laffin, L.J.; Beshai, J.F.; Maffessanti, F.; Bonham, C.A.; Patel, A.V.; Yu, Z.; Addetia, K.; Mor-Avi, V.; Moss, J.D.; et al. Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: Risk stratification using cardiovascular magnetic resonance. Circ. Cardiovasc. Imaging 2016, 9, e003738. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Ono, C.; Tran, T.; He, X.; Raman, S.V. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am. J. Respir. Crit. Care Med. 2014, 189, 109–112. [Google Scholar] [PubMed]
- Okumura, W.; Iwasaki, T.; Toyama, T.; Iso, T.; Arai, M.; Oriuchi, N.; Endo, K.; Yokoyama, T.; Suzuki, T.; Kurabayashi, M. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J. Nucl. Med. 2004, 45, 1989–1998. [Google Scholar] [PubMed]
- Kouranos, V.; Wells, A.U.; Sharma, R.; Underwood, S.R.; Wechalekar, K. Advances in radionuclide imaging of cardiac sarcoidosis. Br. Med. Bull. 2015, 115, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Syed, I.S.; Glockner, J.F.; Feng, D.; Araoz, P.A.; Martinez, M.W.; Edwards, W.D.; Gertz, M.A.; Dispenzieri, A.; Oh, J.K.; Bellavia, D.; et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc. Imaging 2010, 3, 155–164. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Kim, H.W.; Shah, D.; Fine, N.; Kim, K.; Wendell, D.C.; Al-Jaroudi, W.; Parker, M.; Patel, M.; Gwadry-Sridhar, F.; et al. CMR imaging with rapid visual T1 assessment predicts mortality in patients suspected of cardiac amyloidosis. JACC Cardiovasc. Imaging 2014, 7, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Banypersad, S.M.; Treibel, T.A.; Maestrini, V.; Sado, D.M.; White, S.K.; Pica, S.; Castelletti, S.; Piechnik, S.K.; Robson, M.D.; et al. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc. Imaging 2014, 7, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D.; Piechnik, S.K.; Banypersad, S.M.; Fontana, M.; Ntusi, N.B.; Ferreira, V.M.; Whelan, C.J.; Myerson, S.G.; Robson, M.D.; Hawkins, P.N.; et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc. Imaging 2013, 6, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Merlini, G.; Quarta, C.C.; Riva, L.; Longhi, S.; Leone, O.; Salvi, F.; Ciliberti, P.; Pastorelli, F.; Biagini, E.; et al. Systemic cardiac amyloidoses: Disease profiles and clinical courses of the 3 main types. Circulation 2009, 120, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Banypersad, S.M.; Treibel, T.A.; Abdel-Gadir, A.; Maestrini, V.; Lane, T.; Gilbertson, J.A.; Hutt, D.F.; Lachmann, H.J.; Whelan, C.J.; et al. Differential myocyte responses in patients with cardiac transthyretin amyloidosis and light-chain amyloidosis: A cardiac MR imaging study. Radiology 2015, 277, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Chung, R.; Hawkins, P.N.; Moon, J.C. Cardiovascular magnetic resonance for amyloidosis. Heart Fail. Rev. 2015, 20, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dilsizian, V. Molecular imaging of amyloidosis: Will the heart be the next target after the brain? Curr. Cardiol. Rep. 2011, 14, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Dorbala, S.; Vangala, D.; Semer, J.; Strader, C.; Bruyere, J.R.; Carli, M.F.; Moore, S.C.; Falk, R.H. Imaging cardiac amyloidosis: A pilot study using 18F-florbetapir positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Dursun, M.; Yılmaz, S.; Yılmaz, E.; Yılmaz, R.; Onur, Ä.; Oflaz, H.; Dindar, A. The utility of cardiac MRI in diagnosis of infective endocarditis: Preliminary results. Diagn. Interv. Radiol. 2014, 21, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Graziosi, M.; Nanni, C.; Lorenzini, M.; Diemberger, I.; Bonfiglioli, R.; Pasquale, F.; Ziacchi, M.; Biffi, M.; Martignani, C.; Bartoletti, M.; et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: A prospective study. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Saby, L.; Laas, O.; Habib, G.; Cammilleri, S.; Mancini, J.; Tessonnier, L.; Casalta, J.; Gouriet, F.; Riberi, A.; Avierinos, J.; et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: Increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J. Am. Coll. Cardiol. 2013, 61, 2374–2382. [Google Scholar] [CrossRef] [PubMed]
- Bruun, N.E.; Habib, G.; Thuny, F.; Sogaard, P. Cardiac imaging in infectious endocarditis. Eur. Heart J. 2014, 35, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mitterhauser, M.; Loewe, C.; Heber, D.; Hacker, M. Quantitative assessment of FDG PET/MRI in atherosclerotic plaque: Comparison with a PET/CT hybrid system. J. Nucl. Med. 2015, 56, 187. [Google Scholar]
- Petibon, Y.; El Fakhri, G.; Nezafat, R.; Johnson, N.; Brady, T.; Ouyang, J. Towards coronary plaque imaging using simultaneous PET-MR: A simulation study. Phys. Med. Biol. 2014, 59, 1203. [Google Scholar] [CrossRef] [PubMed]
- Millon, A.; Dickson, S.D.; Klink, A.; Izquierdo-Garcia, D.; Bini, J.; Lancelot, E.; Ballet, S.; Robert, P.; Mateo de Castro, J.; Corot, C.; et al. Monitoring plaque inflammation in atherosclerotic rabbits with an iron oxide (P904) and 18F-FDG using a combined PET/MR scanner. Atherosclerosis 2013, 228, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, I.; Saura, M.; Castejón, B.; Martin, A.M.; Herruzo, I.; Balatsos, N.; Zamorano, J.L.; Zaragoza, C. Preclinical models of atherosclerosis. The future of Hybrid PET/MR technology for the early detection of vulnerable plaque. Expert Rev. Mol. Med. 2016, 18, e6. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Wu, H.; Liu, G. Advanced tracers in PET imaging of cardiovascular disease. BioMed Res. Int. 2014, 2014, 504532. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Sun, M.; Sader, S. Matrix metalloproteinases in cardiovascular disease. Can. J. Cardiol. 2006, 22, 25B–30B. [Google Scholar] [CrossRef]
- Beutel, B.; Daniliuc, C.G.; Riemann, B.; Schäfers, M.; Haufe, G. Fluorinated matrix metalloproteinases inhibitors—Phosphonate based potential probes for positron emission tomography. Bioorg. Med. Chem. 2016, 24, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Hartung, D.; Schäfers, M.; Fujimoto, S.; Levkau, B.; Narula, N.; Kopka, K.; Virmani, R.; Reutelingsperger, C.; Hofstra, L.; Kolodgie, F.D.; et al. Targeting of matrix metalloproteinase activation for noninvasive detection of vulnerable atherosclerotic lesions. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Breyholz, H.; Höltke, C.; Faust, A.; Schober, O.; Schäfers, M.; Kopka, K. A new 18F-labelled derivative of the MMP inhibitor CGS 27023A for PET: Radiosynthesis and initial small-animal PET studies. Appl. Radia. Isot. 2009, 67, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Faust, A.; Breyholz, H.; Schober, O.; Schäfers, M.; Kopka, K. The MMP inhibitor (R)-2-(N-benzyl-4-(2-18Ffluoroethoxy)phenylsulphonamido)-N-hydroxy-3-methylbutanamide: Improved precursor synthesis and fully automated radiosynthesis. Appl. Radiat. Isot. 2011, 69, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Woo, S.; Lee, K.C.; An, G.I.; Pandya, D.; Park, N.W.; Nahm, S.; Eom, K.D.; Kim, K.I.; Lee, T.S.; et al. Longitudinal monitoring adipose-derived stem cell survival by PET imaging hexadecyl-4–124I-iodobenzoate in rat myocardial infarction model. Biochem. Biophys. Res. Commun. 2015, 456, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Narsinh, K.H.; Lan, F.; Wang, L.; Nguyen, P.K.; Hu, S.; Lee, A.; Han, L.; Gong, Y.; Huang, M.; et al. Early stem cell engraftment predicts late cardiac functional recovery: Preclinical insights from molecular imaging. Circ. Cardiovasc. Imaging 2012, 5, 481–490. [Google Scholar] [CrossRef] [PubMed]
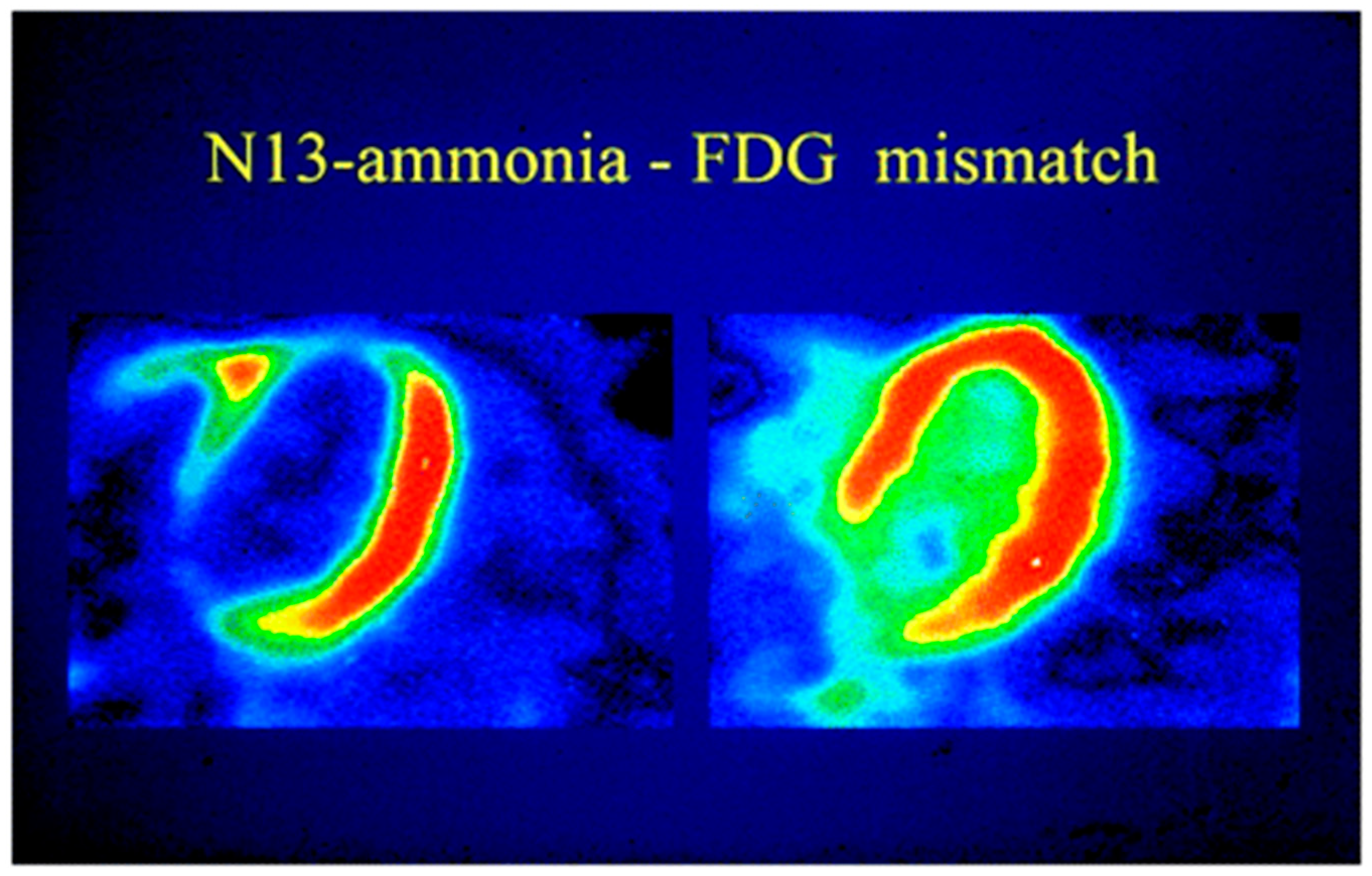
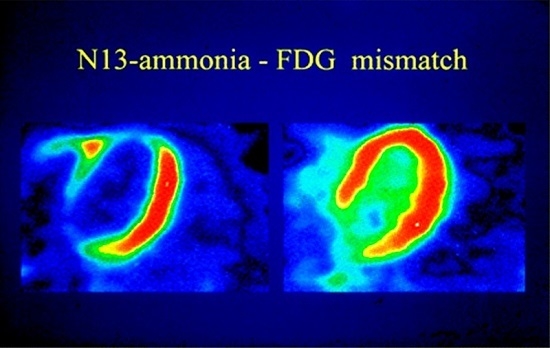
| Radiopharmaceuticals | Half-Life (min) | Mean Positron Range (mm) | First Pass Extraction | Availability | Comments |
|---|---|---|---|---|---|
| 15O-Water | 2.07 | 0.9 | 95% | Cyclotron | Not FDA approved, linear uptake, freely diffusible |
| 13N-Ammonia (13NH3) | 9.96 | 0.7 | 80% | Cyclotron | High myocardial retention due to intracellular trapping |
| Rubidium-82 (82Rb) | 1.25 | 2.6 | 60% | 82Sr/82Rb Generator | Short half-life for sequential imaging, not generated from cyclotron |
| 18F-Flurpiridaz | 110 | 0.2 | 94% | Cyclotron | Long half-light, high extraction, no onsite cyclotron required |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, J.A.; Salerno, M. Clinical Utility and Future Applications of PET/CT and PET/CMR in Cardiology. Diagnostics 2016, 6, 32. https://doi.org/10.3390/diagnostics6030032
Pan JA, Salerno M. Clinical Utility and Future Applications of PET/CT and PET/CMR in Cardiology. Diagnostics. 2016; 6(3):32. https://doi.org/10.3390/diagnostics6030032
Chicago/Turabian StylePan, Jonathan A., and Michael Salerno. 2016. "Clinical Utility and Future Applications of PET/CT and PET/CMR in Cardiology" Diagnostics 6, no. 3: 32. https://doi.org/10.3390/diagnostics6030032