Interobserver and Intraobserver Reproducibility with Volume Dynamic Contrast Enhanced Computed Tomography (DCE-CT) in Gastroesophageal Junction Cancer
Abstract
:1. Introduction
2. Experimental Section
2.1. Patients
2.2. Dynamic Contrast Enhanced CT Analysis
- 320-detector row CT scanner (Aquilion ONE, Toshiba Medical Systems, Ohtawara, Japan)
- z-axis coverage 12–16 cm
- 100 kV and 100 mA
- 0.5 s/rotation time and a fixed table position
- 19 consecutive scan volumes with variable start delay of 7.5 to 13.5 s determined by a test-bolus. Scan duration 55 to 60 s
- Iterative reconstruction (Adaptive Iterative Dose Reduction AIDR 3D (strong level) (Toshiba Medical Systems)
- Non-rigid 3D motion correction
- Image analysis on a stand-alone workstation (Vitrea 6.3, Vital Images, Toshiba Medical Systems, Minnetonka, MN, USA) for DCE-CT analysis
- Omnipaque 350; GE Healthcare, Milwaukee, WI, USA
- 30 to 40 mL, depending on bodyweight (<50 kg: 30 mL, 50–79 kg: 35 mL, >80 kg: 40 mL)
- Injection rate 5 to 8 mL/s (overall contrast injection time did not for any patient exceed 5 s)
- Saline flush of 30 mL
- Two hours fast prior to the examination
- 500 mL of water per oral prior to the examination
- 20 mg hyoscine butylbromide (Buscopan, Boehringer Ingelheim, Ingelheim, Germany) intravenously
- Shallow free breathing with an abdominal strap
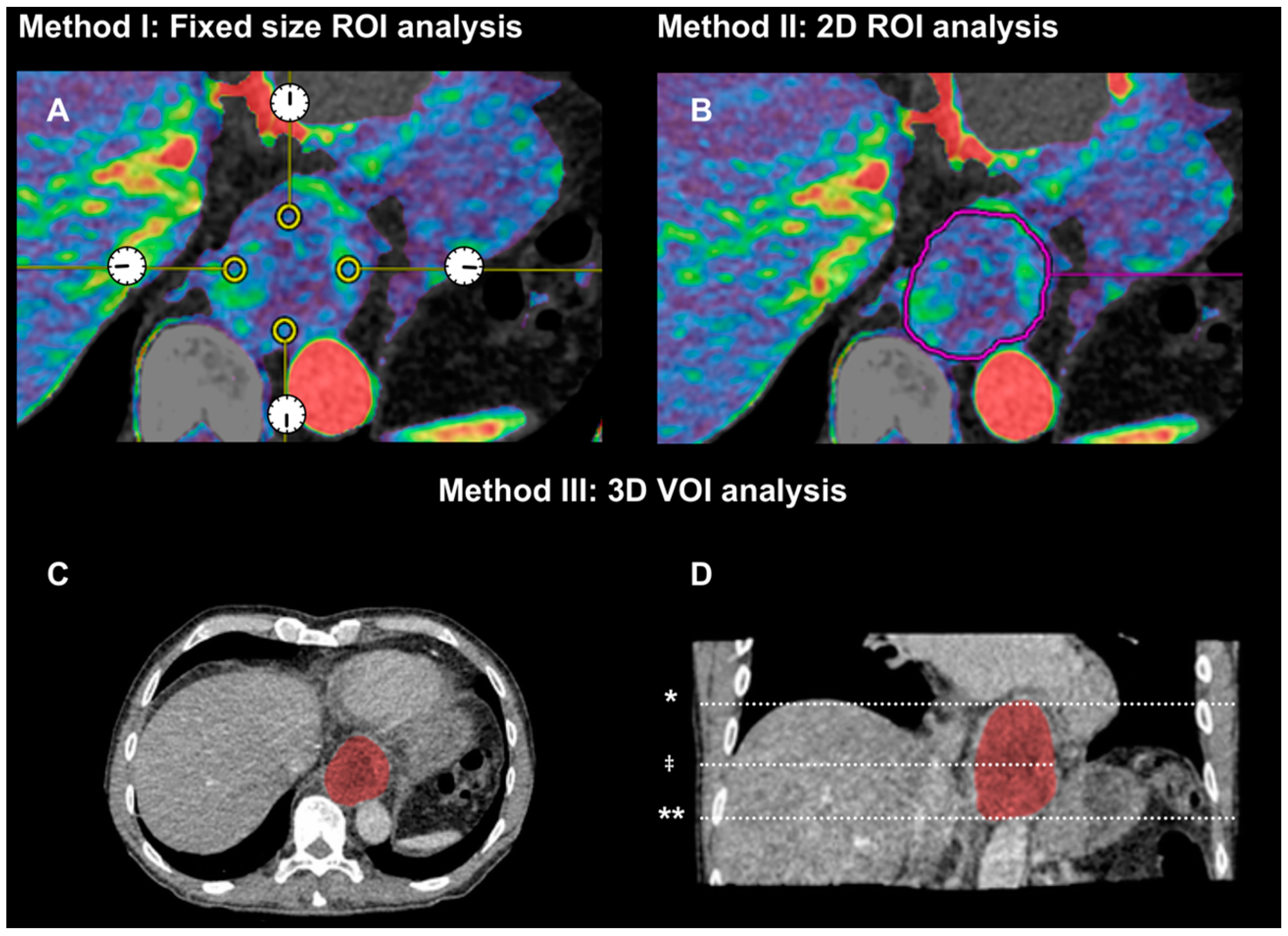
2.2.1. Method (I): Small Region of Interests of Fixed Size in Tumor Periphery (2D Fixed ROIs)
2.2.2. Method (II): 2D Region of Interest (2D-ROI) around Tumor Border at the Center Level of Tumor
2.2.3. Method (III): 3D Volume of Interest (3D-VOI) Encompassing Entire Tumor Volume
- Image number
- Average values for arterial flow (mL·min−1·100 g−1), blood volume (mL·100 g−1) and permeability (mL·min−1·100 g−1) from four ROIs at 12, 3, 6, and 9 o’clock
- Image number
- Tumor area (mm2)
- Average values for arterial flow, blood volume and permeability
- Start level (image number from top)
- End level (image number from top)
- Tumor length (mm)
- Tumor volume (mL)
- Average values for arterial flow, blood volume and permeability
2.3. Statistics
3. Results and Discussion
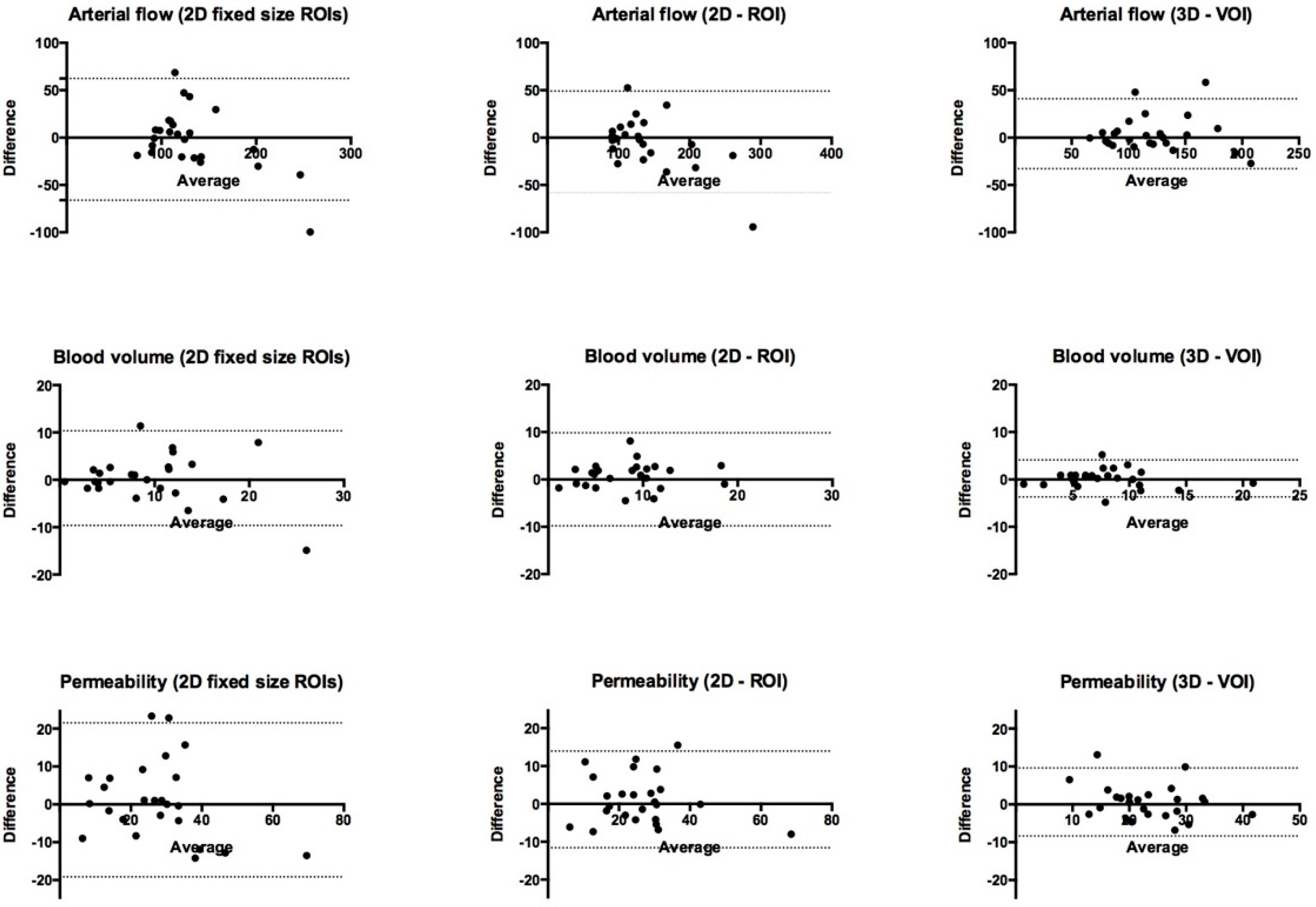
3.1. Bland–Altman Limits of Agreement and Intraclass Correlation Coefficient (ICC)
| CT Perfusion Parameter and Method | Bland–Altman 95% Limits of Agreement | Interobserver ICC |
|---|---|---|
| Arterial flow mL·min−1·100 g−1 | ||
| (I) 2D fixed ROIs | 128.4 (−66.1; 62.3) | 0.79 (0.57–0.90) |
| (II) 2D-ROI | 107.5 (−58.4; 49.1) | 0.88 (0.74–0.94) |
| (III) 3D-VOI | 73.8 (−32.8; 41.0) | 0.88 (0.75–0.95) |
| Blood volume mL·100 g−1 | ||
| (I) 2D fixed ROIs | 20.0 (−9.6; 10.4) | 0.70 (0.42–0.86) |
| (II) 2D-ROI | 19.6 (−9.8; 9.8) | 0.70 (0.42–0.86) |
| (III) 3D-VOI | 7.8 (−3.7; 4.1) | 0.89 (0.77–0.95) |
| Permeability (ktrans) mL·min−1·100 g−1 | ||
| (I) 2D fixed ROIs | 40.6 (−19.1; 21.5) | 0.76 (0.52–0.88) |
| (II) 2D-ROI | 25.6 (−11.6; 14.0) | 0.87 (0.73–0.94) |
| (III) 3D-VOI | 18.0 (−8.4; 9.6) | 0.91 (0.90–0.96) |
| CT Perfusion Parameter and Method | 95% Limits of Agreement | Intraobserver ICC |
|---|---|---|
| Arterial flow mL·min−1·100 g−1 | ||
| (I) 2D fixed ROIs | 176.1 (−92.5; 83.6) | 0.70 (0.42–0.85) |
| (II) 2D-ROI | 159.0 (−82.6; 76.4) | 0.72 (0.45–0.86) |
| (III) 3D-VOI | 76.6 (−37.7; 38.9) | 0.88 (0.75–0.95) |
| Blood volume mL·100 g−1 | ||
| (I) 2D fixed ROIs | 15.9 (−7.8; 8.1) | 0.77 (0.53–0.89) |
| (II) 2D-ROI | 12.3 (−5.8; 6.5) | 0.83 (0.65–0.92) |
| (III) 3D-VOI | 8.0 (−3.8; 4.2) | 0.89 (0.76–0.95) |
| Permeability (ktrans) mL·min−1·100 g−1 | ||
| (I) 2D fixed ROIs | 46.1 (−25.6; 20.5) | 0.76 (0.53–0.89) |
| (II) 2D-ROI | 43.6 (−24.0; 19.6) | 0.72 (0.46–0.87) |
| (III) 3D-VOI | 30.8 (−17.4; 13.4) | 0.80 (0.55–0.91) |
3.2. Tumor Definition and Delineation
3.3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nabavi, D.G.; Cenic, A.; Dool, J.; Smith, R.M.; Espinosa, F.; Craen, R.A.; Gelb, A.W.; Lee, T.Y. Quantitative assessment of cerebral hemodynamics using CT: Stability, accuracy, and precision studies in dogs. J. Comput. Assist. Tomogr. 1999, 23, 506–515. [Google Scholar] [CrossRef]
- Sahani, D.V.; Kalva, S.P.; Hamberg, L.M.; Hahn, P.F.; Willett, C.G.; Saini, S.; Mueller, P.R.; Lee, T.-Y. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: Initial observations. Radiology 2005, 234, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Curvo-Semedo, L.; Portilha, M.A.; Ruivo, C.; Borrego, M.; Leite, J.S.; Caseiro-Alves, F. Usefulness of perfusion CT to assess response to neoadjuvant combined chemoradiotherapy in patients with locally advanced rectal cancer. Acad. Radiol. 2012, 19, 203–213. [Google Scholar] [CrossRef]
- Trojanowska, A.; Grzycka-Kowalczyk, L.; Trojanowski, P.; Klatka, J.; Drop, A. Computed tomography perfusion examination is helpful in evaluating the extent of oropharyngeal and oral cavity cancer. Pol. J. Radiol. 2011, 76, 14–19. [Google Scholar] [PubMed]
- Betz, M.; Kopp, H.G.; Spira, D.; Claussen, C.D.; Horger, M. The benefit of using CT-perfusion imaging for reliable response monitoring in patients with gastrointestinal stromal tumor (GIST) undergoing treatment with novel targeted agents. Acta Radiol. 2013, 54, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Kambadakone, A.; Kulkarni, N.M.; Zhu, A.X.; Sahani, D.V. Monitoring response to antiangiogenic treatment and predicting outcomes in advanced hepatocellular carcinoma using image biomarkers, CT perfusion, tumor density, and tumor size (RECIST). Investig. Radiol. 2012, 47, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Norling, R.; Lauridsen, C.; Fallentin, E.; Bæksgaard, L.; Kofoed, K.; Svendsen, L.; Nielsen, M. Computed Tomography (CT) Perfusion in Abdominal Cancer: Technical Aspects. Diagnostics 2013, 3, 261–270. [Google Scholar] [CrossRef]
- Hsiao, E.M.; Rybicki, F.J.; Steigner, M. CT Coronary Angiography: 256-Slice and 320-Detector Row Scanners. Curr. Cardiol. Rep. 2010, 12, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Sahani, D.V.; Holalkere, N.-S.; Mueller, P.R.; Zhu, A.X. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue—Initial experience. Radiology 2007, 243, 736–743. [Google Scholar] [CrossRef]
- Goh, V.; Halligan, S.; Gartner, L.; Bassett, P.; Bartram, C.I. Quantitative colorectal cancer perfusion measurement by multidetector-row CT: Does greater tumor coverage improve measurement reproducibility? Br. J. Radiol. 2006, 79, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.-S.; Goh, V.; Klotz, E.; Fichte, H.; Saunders, M.I.; Hoskin, P.J.; Padhani, A.R. Quantitative assessment of lung cancer perfusion using MDCT: Does measurement reproducibility improve with greater tumor volume coverage? AJR 2006, 187, 1079–1084. [Google Scholar] [CrossRef]
- Goh, V.; Halligan, S.; Hugill, J.-A.; Bartram, C.I. Quantitative assessment of tissue perfusion using MDCT: Comparison of colorectal cancer and skeletal muscle measurement reproducibility. AJR 2006, 187, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.L.; Fallentin, E.E.; Lauridsen, C.; Law, I.; Svendsen, L.B.; Bæksgaard, L.L.; Federspiel, B.B.; Nielsen, M.B. Computed Tomography (CT) Perfusion as an early prognostic marker for treatment response to neoadjuvant chemotherapy in gastroesophageal junction cancer and gastric cancer—A prospective study. PLoS ONE 2014, e97605. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Chalian, H.; Tochetto, S.M.; Töre, H.G.; Rezai, P.; Yaghmai, V. Hepatic tumors: Region-of-interest versus volumetric analysis for quantification of attenuation at CT. Radiology 2012, 262, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.P.; Dankbaar, J.W.; Bredno, J.; Cheng, S.; Bhogal, S.; Dillon, W.P.; Wintermark, M. Automated versus manual post-processing of perfusion-CT data in patients with acute cerebral ischemia: Influence on interobserver variability. Neuroradiology 2009, 51, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Petralia, G.; Summers, P.; Viotti, S.; Montefrancesco, R.; Raimondi, S.; Bellomi, M. Quantification of variability in breath-hold perfusion CT of hepatocellular carcinoma: A step toward clinical use. Radiology 2012, 265, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Harders, S.W.; Madsen, H.H.; Nellemann, H.M.; Rasmussen, T.R.; Thygesen, J.; Hager, H.; Andersen, N.T.; Rasmussen, F. Dynamic contrast-enhanced CT in suspected lung cancer: Quantitative results. Br. J. Radiol. 2013, 86. [Google Scholar] [CrossRef]
- Miles, K.A.; Lee, T.Y.; Goh, V.; Klotz, E.; Cuenod, C.; Bisdas, S.; Groves, A.M.; Hayball, M.P.; Alonzi, R.; Brunner, T. Current status and guidelines for the assessment of tumor vascular support with dynamic contrast-enhanced computed tomography. Eur. Radiol. 2012, 22, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.; Meyer, H.; Hein, P.; Lembcke, A.; Rueckert, J.-C.; Rogalla, P. Comparison of free breathing versus breath-hold in perfusion imaging using dynamic volume CT. Insights Imag. 2012, 3, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Petralia, G.; Bonello, L.; Viotti, S.; Preda, L.; d’Andrea, G.; Bellomi, M. CT perfusion in oncology: How to do it. Cancer Imag. 2010, 10, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Goh, V.; Halligan, S.; Hugill, J.-A.; Bassett, P.; Bartram, C.I. Quantitative assessment of colorectal cancer perfusion using MDCT: Inter- and intraobserver agreement. AJR 2005, 185, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Schornack, P.A.; Secomb, T.W.; Raghunand, N. Causes and effects of heterogeneous perfusion in tumors. Neoplasia 1999, 1, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Sheiman, R.G.; Sitek, A. CT perfusion imaging: Know its assumptions and limitations. Radiology 2008, 246, 649–650. [Google Scholar] [CrossRef]
- Sun, C.; Liu, C.; Wang, X.-M.; Chen, J.; Wang, D.; Merges, R. Functional CT in a rabbit model: Evaluation of the perfusion characteristics before and after Ar-He cryoablation therapy. J. Med. Imag. Radiat. Oncol. 2008, 52, 351–357. [Google Scholar] [CrossRef]
- Ganeshan, B.; Miles, K.A. Quantifying tumor heterogeneity with CT. Cancer Imag. 2013, 13, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Goh, V.; Sanghera, B.; Wellsted, D.M.; Sundin, J.; Halligan, S. Assessment of the spatial pattern of colorectal tumor perfusion estimated at perfusion CT using two-dimensional fractal analysis. Eur. Radiol. 2009, 19, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundsgaard Hansen, M.; Fallentin, E.; Axelsen, T.; Lauridsen, C.; Norling, R.; Svendsen, L.B.; Nielsen, M.B. Interobserver and Intraobserver Reproducibility with Volume Dynamic Contrast Enhanced Computed Tomography (DCE-CT) in Gastroesophageal Junction Cancer. Diagnostics 2016, 6, 8. https://doi.org/10.3390/diagnostics6010008
Lundsgaard Hansen M, Fallentin E, Axelsen T, Lauridsen C, Norling R, Svendsen LB, Nielsen MB. Interobserver and Intraobserver Reproducibility with Volume Dynamic Contrast Enhanced Computed Tomography (DCE-CT) in Gastroesophageal Junction Cancer. Diagnostics. 2016; 6(1):8. https://doi.org/10.3390/diagnostics6010008
Chicago/Turabian StyleLundsgaard Hansen, Martin, Eva Fallentin, Thomas Axelsen, Carsten Lauridsen, Rikke Norling, Lars Bo Svendsen, and Michael Bachmann Nielsen. 2016. "Interobserver and Intraobserver Reproducibility with Volume Dynamic Contrast Enhanced Computed Tomography (DCE-CT) in Gastroesophageal Junction Cancer" Diagnostics 6, no. 1: 8. https://doi.org/10.3390/diagnostics6010008






