Importance of Attenuation Correction (AC) for Small Animal PET Imaging
Abstract
:1. Introduction
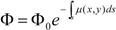
2. Material and Methods
2.1. Animal Preparations
| weight [g] | tumor [mL] | |
|---|---|---|
| mouse1 | 25.1 | 0.09 |
| mouse2 | 30.2 | 0.14 |
| mouse3 | 29.4 | 0.13 |
| mouse4 | 30.3 | 0.15 |
| mouse5 | 26.3 | 0.12 |
| mouse6 | 27.1 | 0.12 |
| mouse7 | 25.7 | 0.12 |
| mouse8 | 25.3 | 0.09 |
| mouse9 | 29.9 | 0.14 |
| mouse10 | 28.2 | 0.13 |
2.2. Imaging Experiments
2.3. PET Transmission (PET-Based) AC Method
2.4. CT Transmission (CT-Based) AC Method
2.5. Uniform Attenuation Map
2.6. Statistics
3. Results and Discussion
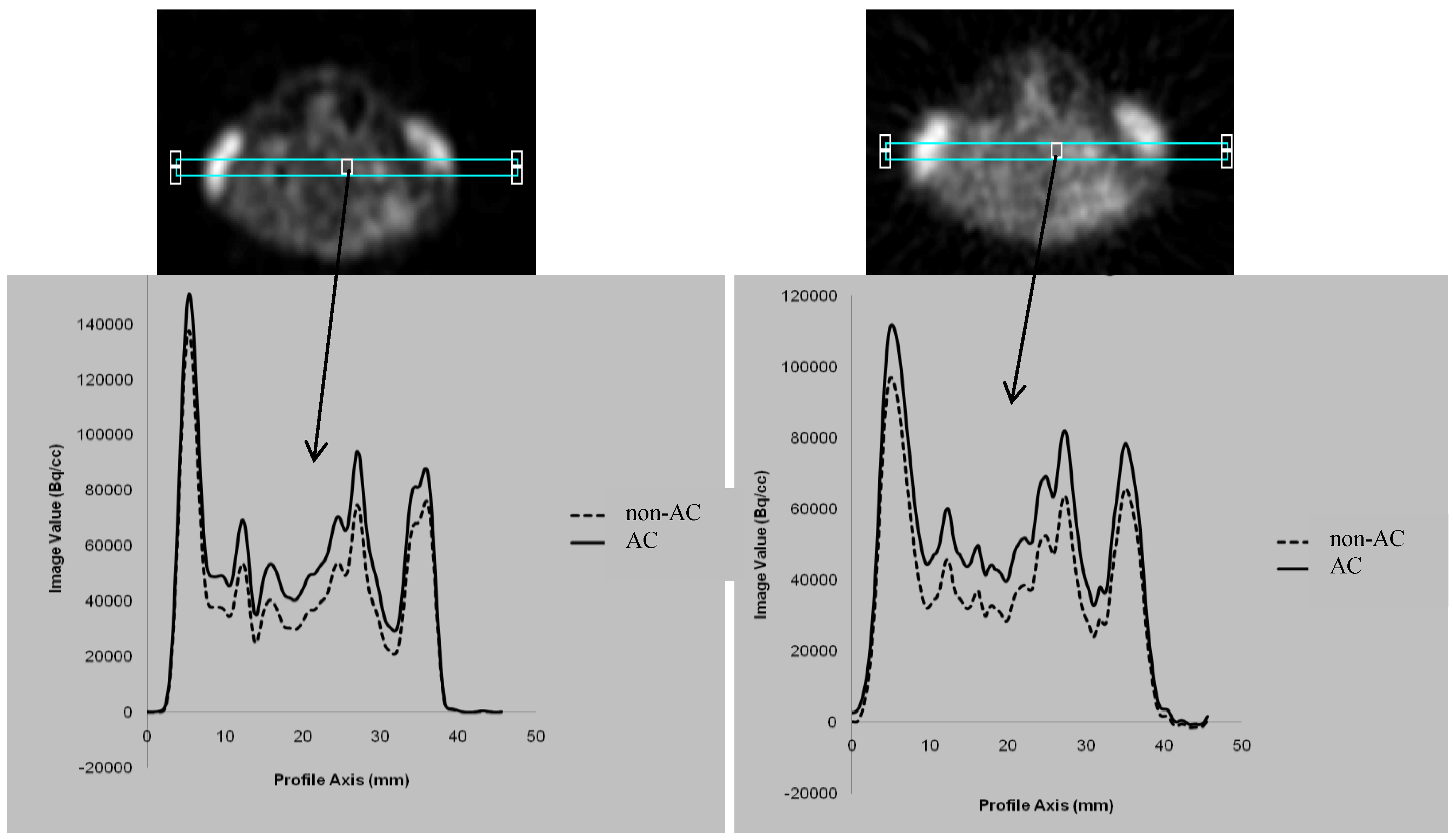
3.1. Comparison between the AC and non-AC PET Data
3.2. Attenuation Recovery versus the Choice of the Reconstruction Method

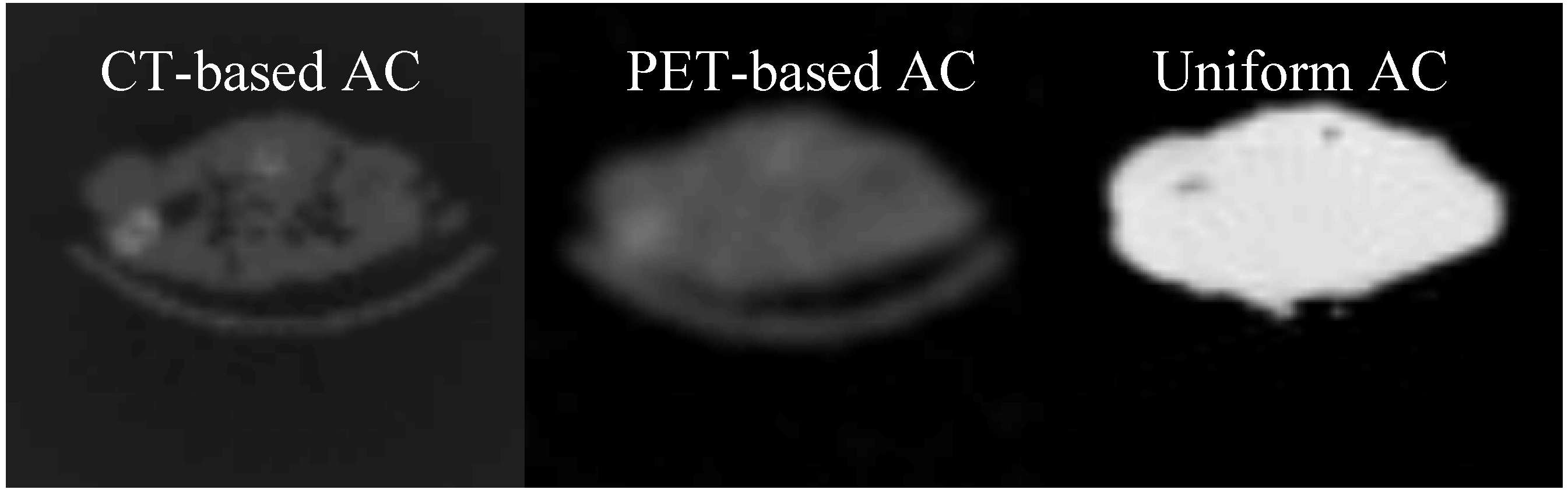
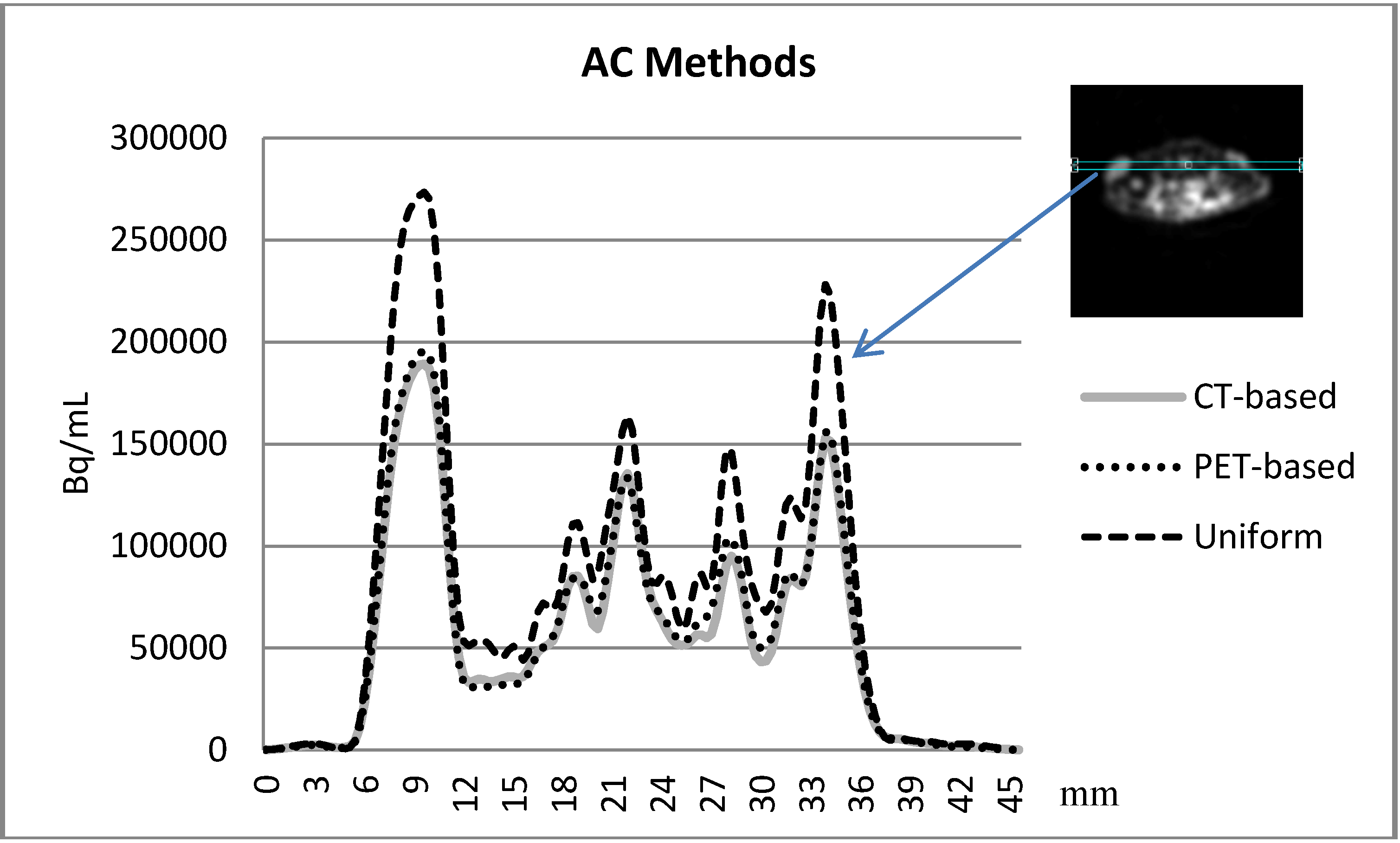
3.3. Comparison between the AC methods
3.4. The Attenuation Recovery of the Different Organs
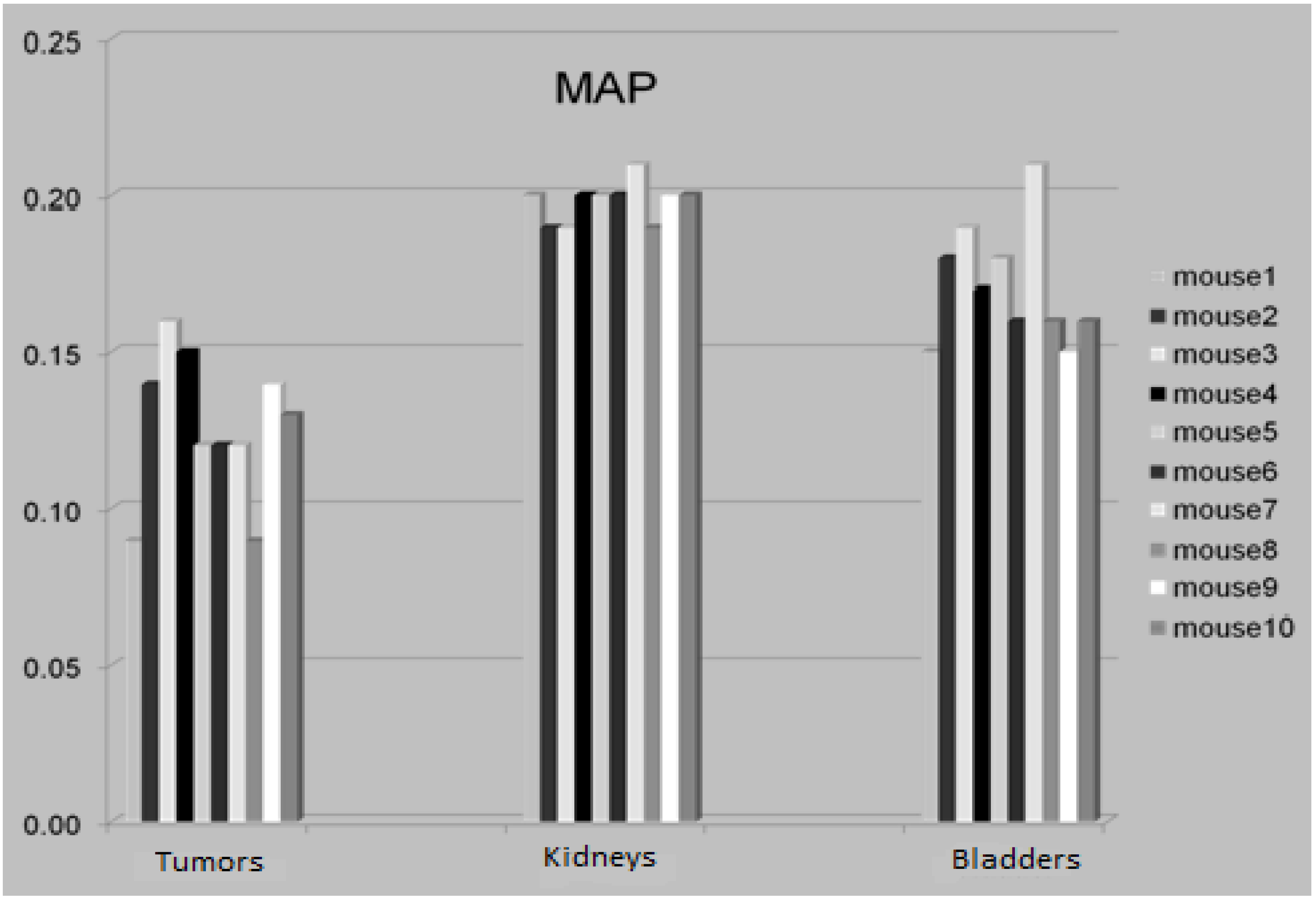
3.5. Discussion
4. Conclusions
Acknowledgments
References
- Rowland, D.J.; Lewis, J.S.; Welch, M.J. Molecular imaging: The application of small animal positron emission tomography. J. Cell. Biochem. Suppl. 2002, 39, 110–115. [Google Scholar] [PubMed]
- Chow, P.L.; Rannou, F.R.; Chatziioannou, A.F. Attenuation correction for small animal PET tomographs. Phys. Med. Biol. 2005, 50, 1837–1850. [Google Scholar] [CrossRef]
- Zaidi, H.; Montandon, M.L.; Slosman, D.O. Attenuation compensation in cerebral 3D PET: Effect of the attenuation map on absolute and relative quantitation. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 52–63. [Google Scholar] [CrossRef]
- Burger, C.; Goerres, G.; Schoenes, S.; Buck, A.; Lonn, A.; von Schulthess, G. PET attenuation coefficients from CT images: Experimental evaluation of the transformation of CT into PET 511-keV attenuation coefficients. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 922–927. [Google Scholar] [CrossRef]
- Turkington, T.G. Attenuation correction in hybrid positron emission tomography. Semin. Nucl. Med. 2000, 30, 255–267. [Google Scholar] [CrossRef]
- Liang, H.; Yang, Y.; Yang, K.; Wu, Y.; Boone, J.M.; Cherry, S.R. A microPET/CT system for in vivo small animal imaging. Phys. Med. Biol. 2007, 52, 3881–3894. [Google Scholar] [CrossRef]
- Binderup, T.; H.El-Ali, H.; Ambrosini, V.; Skovgaard, D.; Munk Jensen, M.; Li, F.; Hesse, B.; Tranekjær Jørgensen, J.; Kjær, A. Molecular Imaging with Small Animal PET/CT. Curr. Med. Imaging Rev. 2011, 7, 234–247. [Google Scholar] [CrossRef]
- Daube-Witherspoon, M.E.; Muehllehner, G. Treatment of axial data in three-dimensional PET. J. Nucl. Med. 1987, 28, 1717–1724. [Google Scholar] [PubMed]
- Qi, J.; Leahy, R.M. Resolution and noise properties of MAP reconstruction for fully 3-D PET. IEEE Trans. Med. Imaging 2000, 19, 493–506. [Google Scholar] [CrossRef]
- Tohme, M.S.; Qi, J. Iterative image reconstruction for positron emission tomography based on a detector response function estimated from point source measurements. Phys. Med. Biol. 2009, 54, 3709–3725. [Google Scholar] [CrossRef]
- Cheng, J.C.; Rahmim, A.; Blinder, S.; Camborde, M.L.; Raywood, K.; Sossi, V. A scatter-corrected list-mode reconstruction and a practical scatter/random approximation technique for dynamic PET imaging. Phys. Med. Biol. 2007, 52, 2089–2106. [Google Scholar] [CrossRef]
- Ay, M.R.; Zaidi, H. Assessment of errors caused by X-ray scatter and use of contrast medium when using CT-based attenuation correction in PET. Eur. J. Nucl. Med. Mol. Imaging. 2006, 33, 1301–1313. [Google Scholar] [CrossRef]
- Wegmann, K.; Brix, G. Evaluation of a scatter correction technique for single photon transmission measurements in PET by means of Monte Carlo simulations. Nuklearmedizin 2000, 39, 67–71. [Google Scholar] [PubMed]
- Zaidi, H. Scatter modelling and correction strategies in fully 3-D PET. Nucl. Med. Commun. 2001, 22, 1181–1184. [Google Scholar] [CrossRef]
- Karp, J.S.; Muehllehner, G.; Qu, H.; Yan, X.H. Singles transmission in volume-imaging PET with a 137Cs source. Phys. Med. Biol. 1995, 40, 929–944. [Google Scholar] [CrossRef]
- Chow, P.L.; Stout, D.B.; Komisopoulou, E.; Chatziioannou, A.F. A method of image registration for small animal, multi-modality imaging. Phys. Med. Biol. 2006, 51, 379–390. [Google Scholar] [CrossRef]
- Kinahan, P.E.; Townsend, D.W.; Beyer, T.; Sashin, D. Attenuation correction for a combined 3D PET/CT scanner. Med. Phys. 1998, 25, 2046–2053. [Google Scholar] [CrossRef]
- Levin, C.S.; Zaidi, H. Current trends in preclinical PET system design. PET Clin. 2007, 2, 125–160. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
El Ali, H.H.; Bodholdt, R.P.; Jørgensen, J.T.; Myschetzky, R.; Kjaer, A. Importance of Attenuation Correction (AC) for Small Animal PET Imaging. Diagnostics 2012, 2, 42-51. https://doi.org/10.3390/diagnostics2040042
El Ali HH, Bodholdt RP, Jørgensen JT, Myschetzky R, Kjaer A. Importance of Attenuation Correction (AC) for Small Animal PET Imaging. Diagnostics. 2012; 2(4):42-51. https://doi.org/10.3390/diagnostics2040042
Chicago/Turabian StyleEl Ali, Henrik H., Rasmus Poul Bodholdt, Jesper Tranekjær Jørgensen, Rebecca Myschetzky, and Andreas Kjaer. 2012. "Importance of Attenuation Correction (AC) for Small Animal PET Imaging" Diagnostics 2, no. 4: 42-51. https://doi.org/10.3390/diagnostics2040042
APA StyleEl Ali, H. H., Bodholdt, R. P., Jørgensen, J. T., Myschetzky, R., & Kjaer, A. (2012). Importance of Attenuation Correction (AC) for Small Animal PET Imaging. Diagnostics, 2(4), 42-51. https://doi.org/10.3390/diagnostics2040042






