Determination of Tear Lipid Film Thickness Based on a Reflected Placido Disk Tear Film Analyzer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Tests
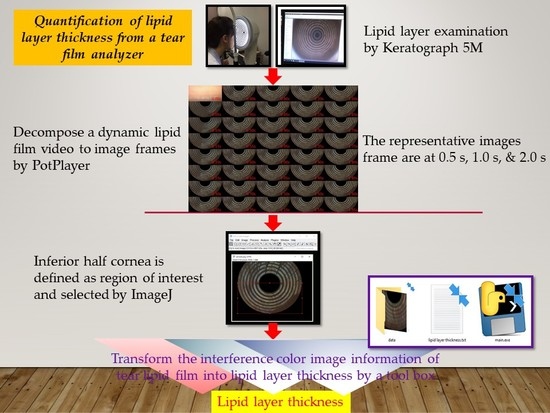
2.3. Examination of Dynamic Tear Lipid Layer
2.4. Selection of Tear Lipid Layer Image
2.5. Region of Interest and Ring Background Segmentation
2.6. Estimation of Lipid Layer Thickness
2.7. Statistical Analysis
3. Results
3.1. Subjects
3.2. Dynamic Lipid Layer Shown on the Placido Ring Tear Film Analyzer
3.3. Average Lipid Layer Thickness before and after Segmentation
3.4. Verification of LLT Detection on K5M Tear Film Analyzer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CGMH | Chang Gung Memorial Hospital |
| DED | dry eye disease |
| ICC | intraclass correlation coefficient |
| K5M | Keratograph® 5M |
| LL | lipid layer |
| LLT | lipid layer thickness |
| MGD | meibomian gland dysfunction |
| NIKBUT | non-invasive keratographic break-up time |
| OSDI | ocular surface disease index questionnaire |
| ROI | region of interest |
| RGB | red, green, and blue scales |
| TFOS DEWS | The Tear Film and Ocular Surface Society in the Dry Eye WorkShop |
| TMH | tear meniscus height |
References
- Tseng, S.C.; Tsubota, K. Important concepts for treating ocular surface and tear disorders. Am. J. Ophthalmol. 1997, 124, 825–835. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.D.; Shimazaki, J.; Benitez-del-Castillo, J.M.; Craig, J.P.; McCulley, J.P.; Den, S.; Foulks, G.N. The International Workshop on Meibomian Gland Dysfunction: Report of the Definition and Classification Subcommittee. Invest. Ophthalmol. Vis. Sci. 2011, 52, 1930–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, S.M.; Tong, L.; Duan, X.; Petznick, A.; Wenk, M.R.; Shui, G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J. Lipid Res. 2014, 55, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillon, J.P. Non-invasive Tearscope Plus routine for contact lens fitting. Cont. Lens Anterior Eye 1998, 21 (Suppl. 1), S31–S40. [Google Scholar] [CrossRef]
- Goto, E.; Tseng, S.C. Differentiation of lipid tear deficiency dry eye by kinetic analysis of tear interference images. Arch. Ophthalmol. 2003, 121, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.; Lee, J.S.; Kang, S.Y.; Kim, H.M.; Song, J.S. Correlation between quantitative measurements of tear film lipid layer thickness and meibomian gland loss in patients with obstructive meibomian gland dysfunction and normal controls. Am. J. Ophthalmol. 2013, 155, 1104–1110.e1102. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.T.; Fang, P.C.; Chao, T.L.; Chen, A.; Lai, Y.H.; Huang, Y.T.; Tseng, C.Y. Tear proteomics approach to monitoring Sjögren syndrome or dry eye disease. Int. J. Mol. Sci. 2019, 20, 1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, P.K.; Soltani, S.; Wong, A.K.C. A survey of thresholding techniques. Comput. Vis. Graph. Image Process. 1988, 41, 233–260. [Google Scholar] [CrossRef]
- Hwang, H.; Jeon, H.J.; Yow, K.C.; Hwang, H.S.; Chung, E. Image-based quantitative analysis of tear film lipid layer thickness for meibomian gland evaluation. Biomed. Eng. Online 2017, 16, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.Y.; Ho, R.W.; Fang, P.C.; Yu, H.J.; Chien, C.C.; Hsiao, C.C.; Kuo, M.T. The function and morphology of meibomian glands in patients with thyroid eye disease: A preliminary study. BMC Ophthalmol. 2018, 18, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, R.W.; Fang, P.C.; Chao, T.L.; Chien, C.C.; Kuo, M.T. Increase lipid tear thickness after botulinum neurotoxin A injection in patients with blepharospasm and hemifacial spasm. Sci. Rep. 2018, 8, 8367. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.H.; Lai, I.C.; Fang, P.C.; Chien, C.C.; Tseng, S.L.; Lai, Y.H.; Huang, Y.T.; Kuo, M.T. Meibomian gland performance in glaucomatous patients with long-term instillation of IOP-lowering medications. J. Glaucoma 2018, 27, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Takehisa, Y.; Kinoshita, S. Correlation of tear lipid layer interference patterns with the diagnosis and severity of dry eye. Am. J. Ophthalmol. 1996, 122, 818–824. [Google Scholar] [CrossRef]
- Korb, D.R.; Greiner, J.V. Increase in tear film lipid layer thickness following treatment of meibomian gland dysfunction. Adv. Exp. Med. Biol. 1994, 350, 293–298. [Google Scholar] [PubMed]
- Napoli, P.E.; Nioi, M.; Mangoni, L.; Gentile, P.; Braghiroli, M.; d’Aloja, E.; Fossarello, M. Fourier-domain OCT imaging of the ocular surface and tear film dynamics: A review of the state of the art and an integrative model of the tear behavior during the inter-blink period and visual fixation. J. Clin. Med. 2020, 9, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cwiklik, L. Tear film lipid layer: A molecular level view. Biochim. Biophys. Acta. 2016, 1858, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Goto, E.; Tseng, S.C. Kinetic analysis of tear interference images in aqueous tear deficiency dry eye before and after punctal occlusion. Invest. Ophthalmol. Vis. Sci. 2003, 44, 1897–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuoka, S.; Arita, R. Increase in tear film lipid layer thickness after instillation of 3% diquafosol ophthalmic solution in healthy human eyes. Ocul. Surf. 2017, 15, 730–735. [Google Scholar] [CrossRef] [PubMed]









| Baseline Characteristics | Data |
|---|---|
| No. of subjects, N | 28 |
| Sex, F:M | 20:8 |
| Eye, OD:OS | 20:8 |
| Age, median (range), years | 41 (21–68) |
| OSDI a, median (range), scores | 26.1 (6.3–95.8) |
| NIKBUT b, median (range), s | 6.9 (1.0–24.0) |
| TMH c, median (range), mm | 0.24 (0.9–0.35) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, P.-I.; Fang, P.-C.; Ho, R.-W.; Chao, T.-L.; Cho, W.-H.; Lai, H.-Y.; Hsiao, Y.-T.; Kuo, M.-T. Determination of Tear Lipid Film Thickness Based on a Reflected Placido Disk Tear Film Analyzer. Diagnostics 2020, 10, 353. https://doi.org/10.3390/diagnostics10060353
Fu P-I, Fang P-C, Ho R-W, Chao T-L, Cho W-H, Lai H-Y, Hsiao Y-T, Kuo M-T. Determination of Tear Lipid Film Thickness Based on a Reflected Placido Disk Tear Film Analyzer. Diagnostics. 2020; 10(6):353. https://doi.org/10.3390/diagnostics10060353
Chicago/Turabian StyleFu, Pin-I, Po-Chiung Fang, Ren-Wen Ho, Tsai-Ling Chao, Wan-Hua Cho, Hung-Yin Lai, Yu-Ting Hsiao, and Ming-Tse Kuo. 2020. "Determination of Tear Lipid Film Thickness Based on a Reflected Placido Disk Tear Film Analyzer" Diagnostics 10, no. 6: 353. https://doi.org/10.3390/diagnostics10060353