Manipulation of Pattern of Cell Differentiation in a hetR Mutant of Anabaena sp. PCC 7120 by Overexpressing hetZ Alone or with hetP
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Plasmid Construction and Conjugation
3. Results
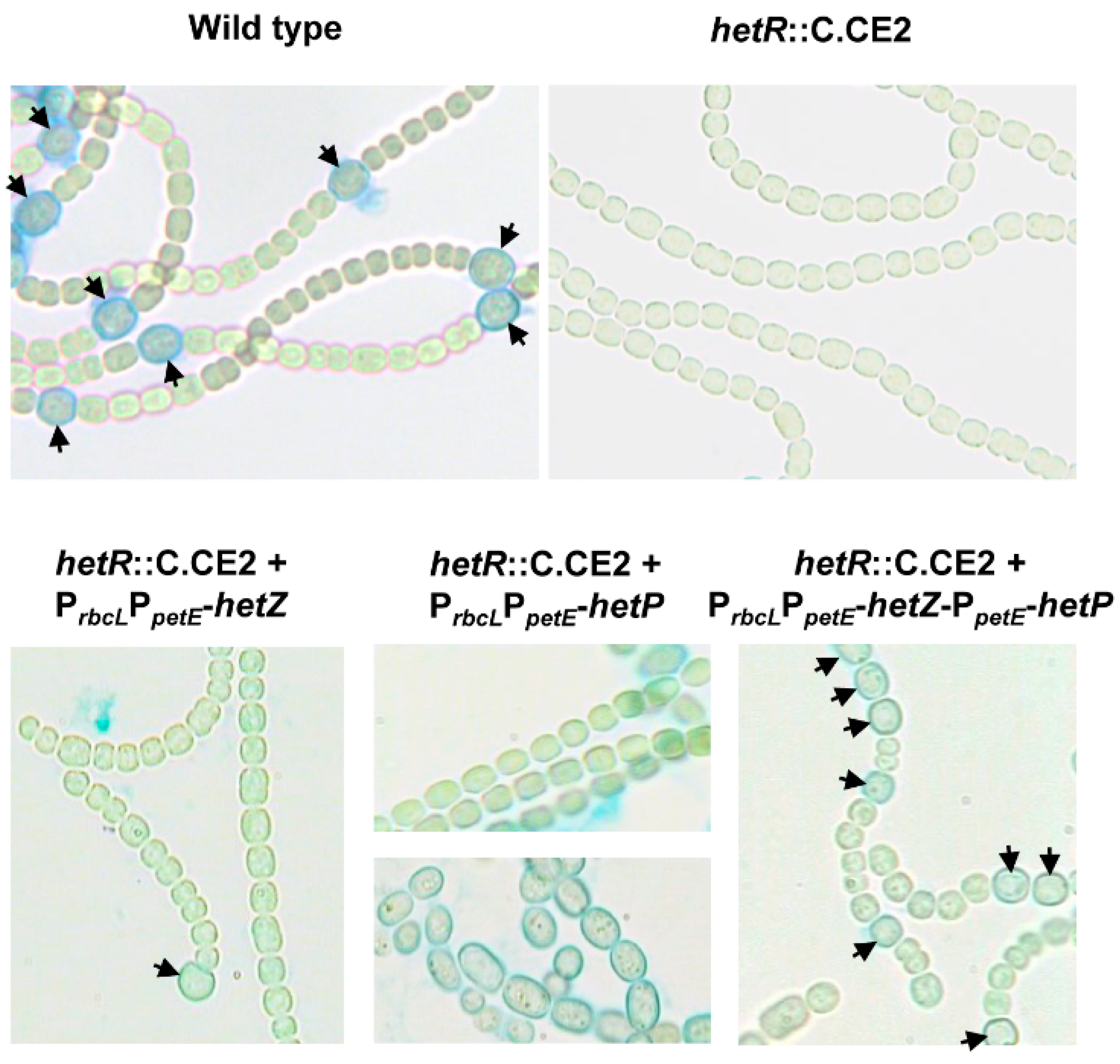
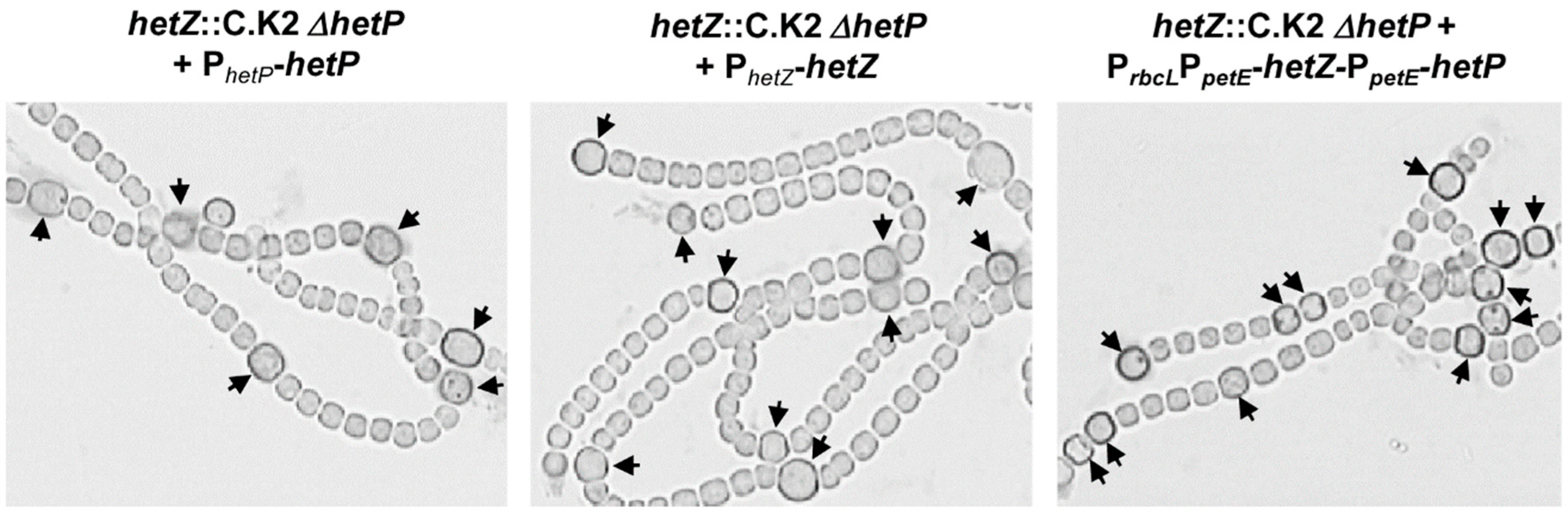
3.1. Formation of Mch in a hetR Mutant that Overexpresses hetZ and hetP
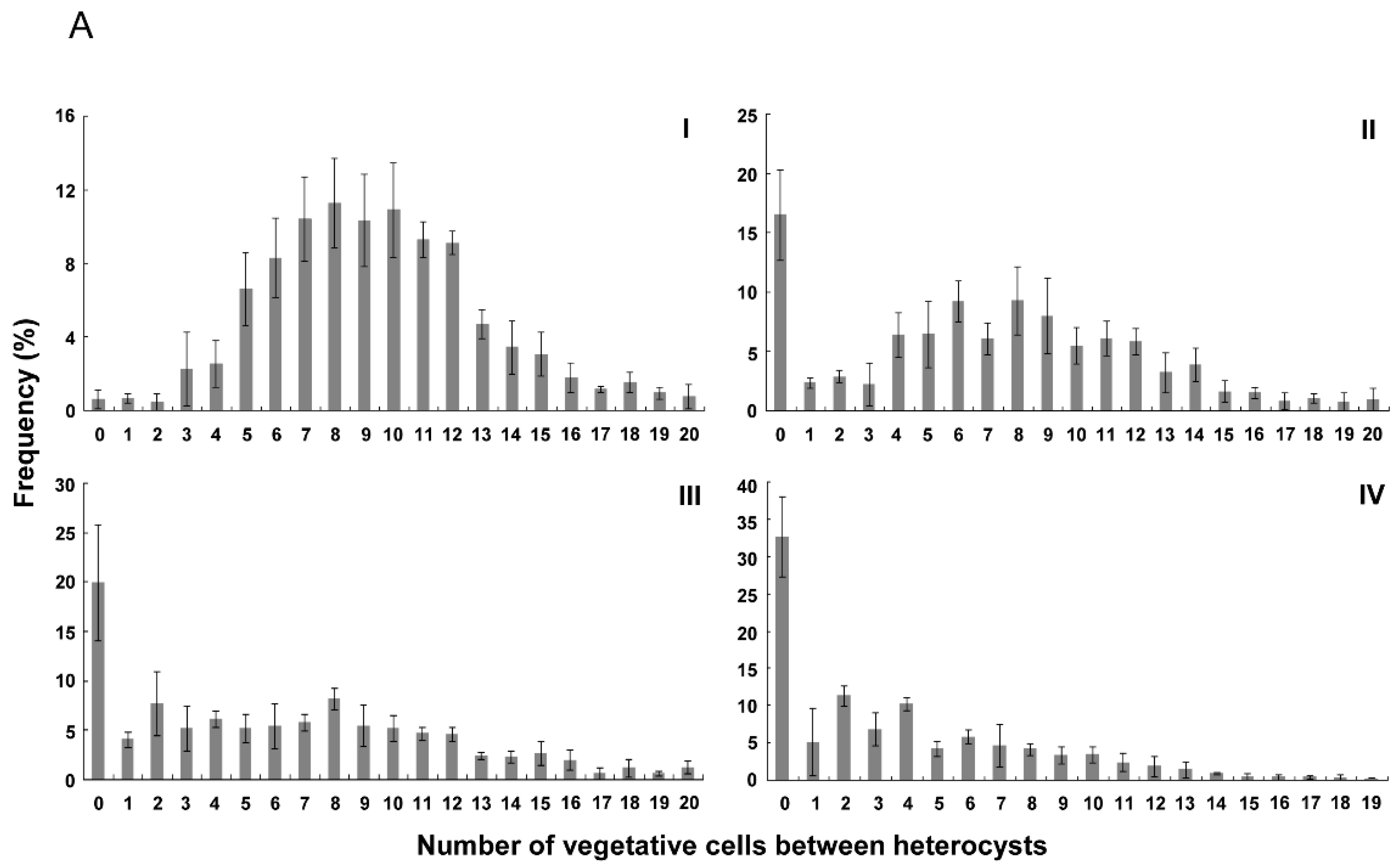
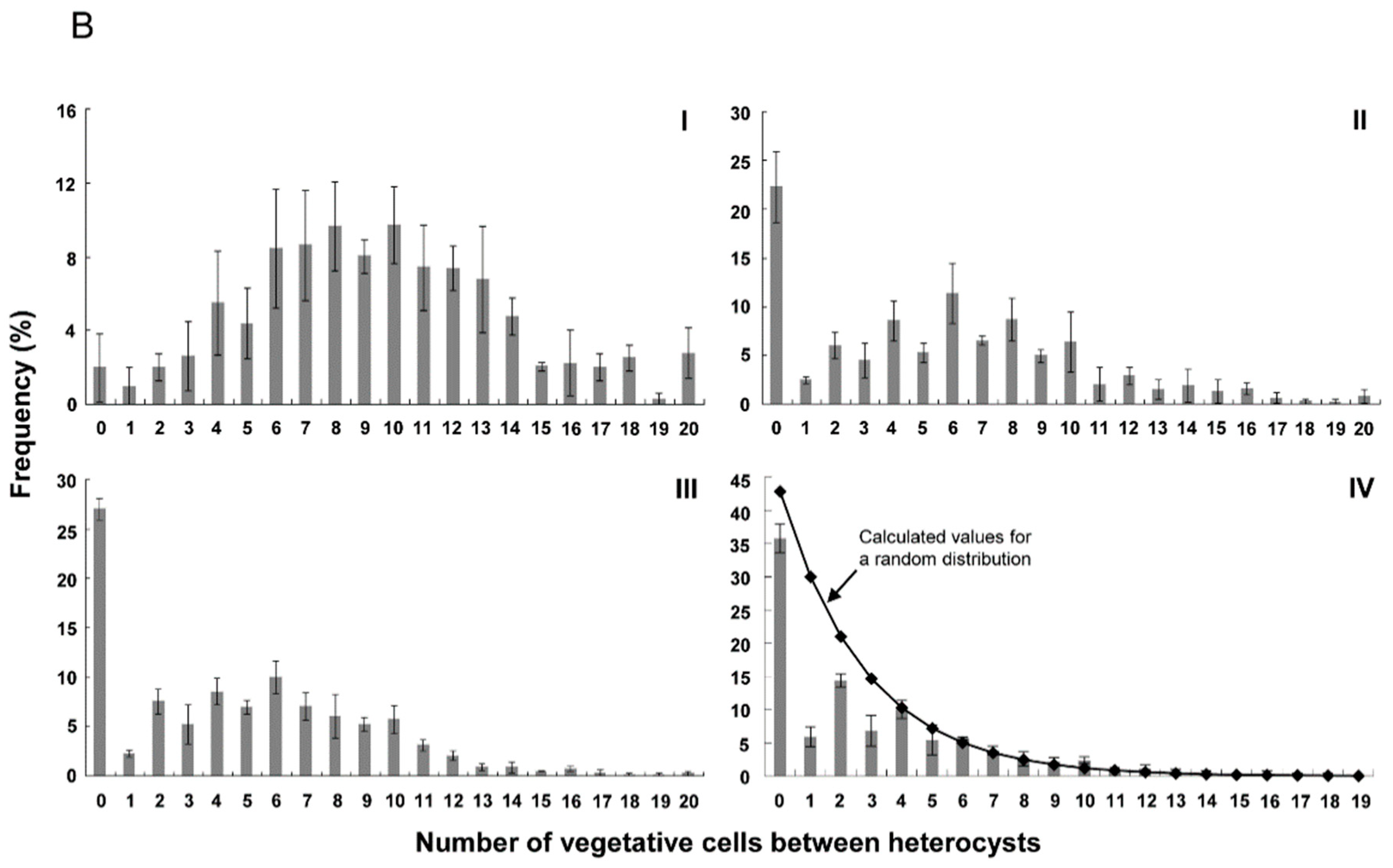
3.2. Distribution of Heterocysts along Filaments
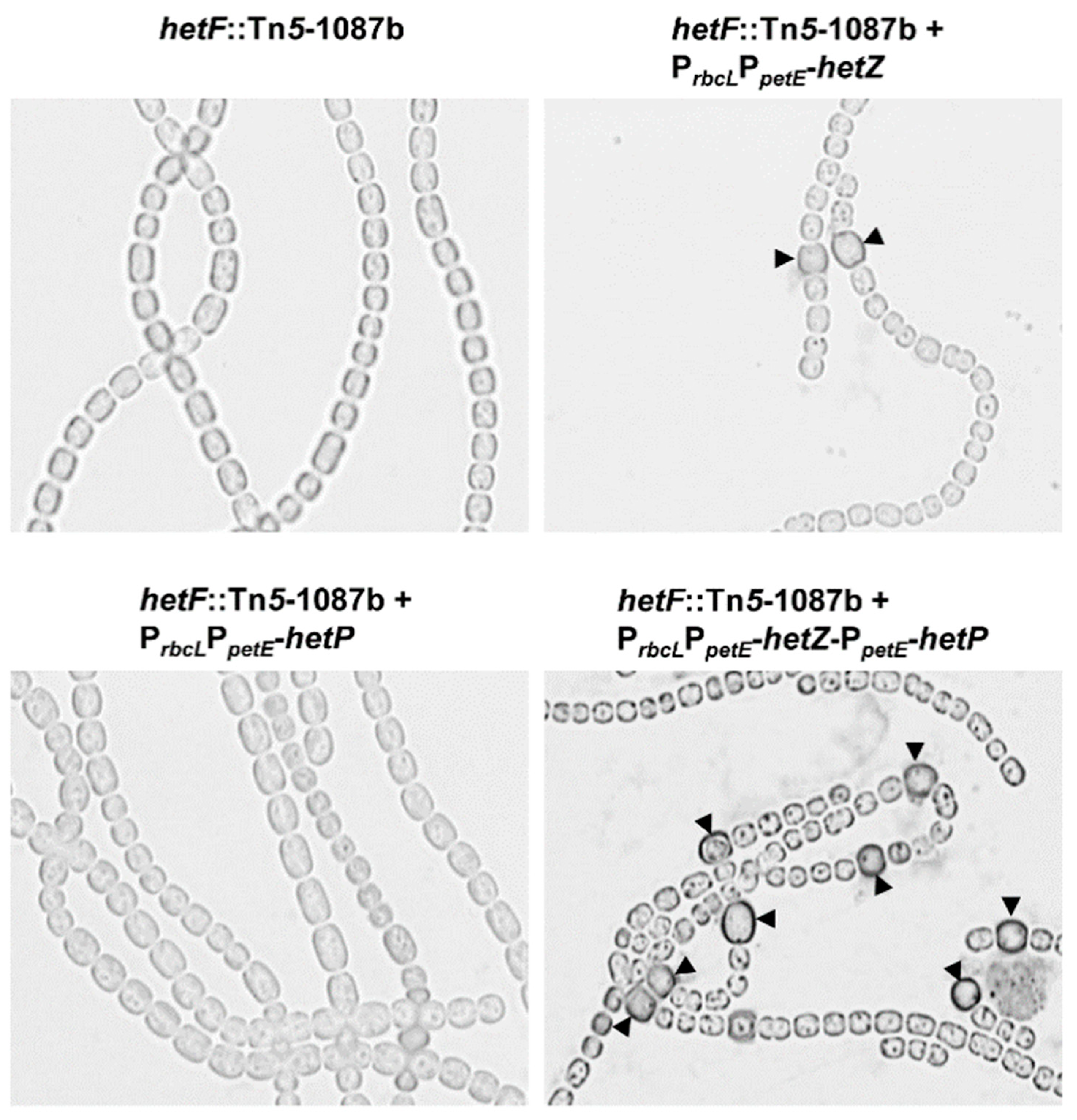
3.3. Heterocyst Formation in a hetF Background
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rasmussen, B.; Fletcher, I.R.; Brocks, J.J.; Kilburn, M.R. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 2008, 455, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W. The paleobiological record of photosynthesis. Photosynth. Res. 2011, 107, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Wolk, C.P.; Ernst, A.; Elhai, J. Heterocyst metabolism and development. In The Molecular Biology of Cyanobacteria; Bryant, D.A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 769–823. [Google Scholar]
- Castenholz, R.W. Oxygenic photosynthetic bacteria. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Boone, D.R., Castenholz, R.W., Eds.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 2001; pp. 474–487. [Google Scholar]
- Tomitani, A.; Knoll, A.H.; Cavanaugh, C.M.; Ohno, T. The evolutionary diversification of cyanobacteria: Molecular-phylogenetic and paleontological perspectives. Proc. Natl. Acad. Sci. USA 2006, 103, 5442–5447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meinhardt, H.; Gierer, A. Pattern formation by local self-activation and lateral inhibition. Bioessays 1991, 22, 753–760. [Google Scholar] [CrossRef]
- Kondo, S.; Miura, T. Reaction-diffusion model as a framework for understanding biological pattern formation. Science 2006, 329, 1616–1620. [Google Scholar] [CrossRef] [PubMed]
- Buikema, W.J.; Haselkorn, R. Expression of the Anabaena hetR gene from a copper-regulated promoter leads to heterocyst differentiation under repressing conditions. Proc. Natl. Acad. Sci. USA 2001, 98, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Cai, Y.; Hou, S.; Xu, X. Identification of the HetR-recognition sequence upstream of hetZ in Anabaena sp. strain PCC 7120. J. Bacteriol. 2012, 194, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhou, F.; Peng, S.; Gao, H.; Xu, X. The HetR-binding site that activates expression of patA in vegetative cells is required for normal heterocyst patterning in Anabaena sp. PCC 7120. Sci. Bull. 2015, 60, 192–201. [Google Scholar] [CrossRef]
- Hu, H.X.; Jiang, Y.L.; Zhao, M.X.; Cai, K.; Liu, S.; Wen, B.; Lv, P.; Zhang, Y.; Peng, J.; Zhong, H.; et al. Structural insights 249 into HetR-PatS interaction involved in cyanobacterial pattern formation. Sci. Rep. 2015, 5, 16470. [Google Scholar] [CrossRef] [PubMed]
- Risser, D.D.; Callahan, S.M. Genetic and cytological evidence that heterocyst patterning is regulated by inhibitor gradients that promote activator decay. Proc. Natl. Acad. Sci. USA 2009, 106, 19884–19888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Scappino, L.; Haselkorn, R. The patA gene product, which contains a region similar to CheY of Escherichia coli, controls heterocyst pattern formation in the cyanobacterium Anabaena 7120. Proc. Natl. Acad. Sci. USA 1992, 89, 5655–5659. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Koonin, E.V.; Haselkorn, R.; Galperin, M.Y. Cyanobacterial response regulator PatA contains a conserved N-terminal domain (PATAN) with an alpha-helical insertion. Bioinformatics 2006, 22, 1297–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.S.; Golden, J.W. Heterocyst pattern formation controlled by a diffusible peptide. Science 1998, 282, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Elhai, J.; Khudyakov, I. Ancient association of cyanobacterial multicellularity with the regulator HetR and an RGSGR pentapeptide-containing protein (PatX). Mol. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mitschke, J.; Vioque, A.; Haas, F.; Hess, W.R.; Muro-Pastor, A.M. Dynamics of transcriptional start site selection during nitrogen stress-induced cell differentiation in Anabaena sp. PCC7120. Proc. Natl. Acad. Sci. USA 2011, 108, 20130–20135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Cao, Z.; Zhao, J. Characterization of HetR protein turnover in Anabaena sp. PCC 7120. Arch. Microbiol. 1998, 169, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Black, T.A.; Cai, Y.; Wolk, C.P. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 1993, 9, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Higa, K.C.; Callahan, S.M. Ectopic expression of hetP can partially bypass the need for hetR in heterocyst differentiation by Anabaena sp. strain PCC 7120. Mol. Microbiol. 2010, 77, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, S.; Wang, Y.; Xu, X. Functional overlap of hetP and hetZ in regulation of heterocyst differentiation in Anabaena sp. strain PCC 7120. J. Bacteriol. 2018, 200, e00707-17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, Y.; Li, C.; Gao, H.; Zhang, C.-C.; Xu, X. Three substrains of the cyanobacterium Anabaena sp. PCC 7120 display divergence in genomic sequences and hetC function. J. Bacteriol. 2018, 200, e00076-18. [Google Scholar] [CrossRef] [PubMed]
- Videau, P.; Rivers, O.S.; Tom, S.K.; Oshiro, R.T.; Ushijima, B.; Swenson, V.A.; Philmus, B.; Gaylor, M.O.; Cozy, L.M. The hetZ gene indirectly regulates heterocyst development at the level of pattern formation in Anabaena sp. strain PCC 7120. Mol. Microbiol. 2018, 109, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Risser, D.D.; Callahan, S.M. HetF and PatA control levels of HetR in Anabaena sp. strain PCC 7120. J. Bacteriol. 2008, 190, 7645–7654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, Y.; Khudyakov, I.; Fan, Q.; Gao, H.; Ning, D.; Wolk, C.P.; Xu, X. A gene cluster that regulates both heterocyst differentiation and pattern formation in Anabaena sp. strain PCC 7120. Mol. Microbiol. 2007, 66, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Hebbar, P.B.; Curtis, S.E. Characterization of devH, a gene encoding a putative DNA binding protein required for heterocyst function in Anabaena sp. strain PCC 7120. J. Bacteriol. 2000, 182, 3572–3581. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.; Hunt, H.D.; Ho, S.N.; Pullen, J.K.; Pease, L.R. Engineering hybrid genes without the use of restriction enzymes: Gene splicing by overlap extension. Gene 1989, 77, 61–68. [Google Scholar] [CrossRef]
- Wolk, C.P.; Cai, Y.; Cardemil, L.; Flores, E.; Hohn, B.; Murry, M.; Schmetterer, G.; Schrautemeier, B.; Wilson, R. Isolation and complementation of mutants of Anabaena sp. strain PCC 7120 unable to grow aerobically on dinitrogen. J. Bacteriol. 1988, 170, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Elhai, J.; Vepritskiy, A.; Muro-Pastor, A.M.; Flores, E.; Wolk, C.P. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J. Bacteriol. 1997, 179, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Elhai, J.; Wolk, C.P. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 1990, 9, 3379–3388. [Google Scholar] [CrossRef] [PubMed]
- Videau, P.; Oshiro, R.T.; Cozy, L.M.; Callahan, S.M. Transcriptional dynamics of developmental genes assessed with an FMN-dependent fluorophore in mature heterocysts of Anabaena sp. strain PCC 7120. Microbiology 2014, 160, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Elhai, J. Strong and regulated promoters in the cyanobacterium Anabaena PCC 7120. FEMS Microbiol. Lett. 1993, 114, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Khudyakov, I.Y.; Golden, J.W. Different functions of HetR, a master regulator of heterocyst differentiation in Anabaena sp. PCC 7120, can be separated by mutation. Proc. Natl. Acad. Sci. USA 2004, 101, 16040–16045. [Google Scholar] [CrossRef] [PubMed]
| Strains | Derivation/Relevant Characteristics a | Reference or Source |
|---|---|---|
| Anabaena 7120 | Wild Type (WT) | FACHB b |
| hetF::Tn5-1087b | Emr, Tn5-1087b inserted within hetF 310 bp from its 3′ terminus | [9] |
| hetR::C.CE2 | CmrEmr, C.CE2 (a chloramphenicol-resistance and erythromycin-resistance cassette) inserted into the ClaI site of hetR | [9] |
| hetR::C.CE2 (pHB4382) | CmrEmrNmr, pHB4382 bearing PrbcLPpetE-hetZ introduced into the hetR::C.CE2 mutant | This study |
| hetR::C.CE2 (pHB4409) | CmrEmrNmrSmrSpr, pHB4409 bearing PrbcLPpetE-hetP introduced into the hetR::C.CE2 mutant | This study |
| hetR::C.CE2 (pHB4551) | CmrEmrNmrSmrSpr, pHB4551 bearing PrbcLPpetE-hetZ-PpetE-hetP introduced into the hetR::C.CE2 mutant | This study |
| hetZ::C.K2 ΔhetP | CmrEmrNmr, hetZ hetP double mutant | [21] |
| hetZ::C.K2 ΔhetP (pHB1462) | CmrEmrNmrSmrSpr, pHB1462 bearing PhetZ-hetZ [25] introduced into the hetZ hetP double mutant | This study |
| hetZ::C.K2 ΔhetP (pHB4550) | CmrEmrNmrSmrSpr, pHB4550 bearing PhetP-hetP introduced into the hetZ hetP double mutant | This study |
| hetZ::C.K2 ΔhetP (pHB4551) | CmrEmrNmrSmrSpr, pHB4551 bearing PrbcLPpetE-hetZ-PpetE-hetP introduced into the hetZ hetP double mutant | This study |
| WT (pHB4551) | NmrSmrSpr, pHB4551 bearing PrbcLPpetE-hetZ-PpetE-hetP introduced into Anabaena 7120 | This study |
| Strains | Hours | Nitrogenase Activity (μmol C2H4 mg Chla−1 h−1) a | Heterocyst Frequency (%) b | Diazotrophic Growth | ||
|---|---|---|---|---|---|---|
| Anoxic | Aerobic | −RGSGR | +RGSGR c | |||
| WT d | 24 | 3.65 ± 0.62 | 2.83 ± 0.11 | 9.4 ± 0.4 | 0 | Good |
| 48 | 6.74 ± 0.63 | 3.50 ± 0.57 | 10.6 ± 0.7 | Not tested | ||
| WT + PrbcLPpetE-hetZ-PpetE-hetP e1 | 24 | Not measured | Not measured | 11.9 ± 1.2 | 11.4 ± 1.3 | Moderate |
| 48 | Not measured | Not measured | 15.8 ± 2.8 | Not tested | ||
| hetR::C.CE2 | 24 | 0 | 0 | 0 | Not tested | No |
| 48 | 0 | 0 | 0 | Not tested | ||
| hetR::C.CE2 + PrbcLPpetE-hetZ | 24 | 1.04 ± 0.14 | 0 | 2.9 ± 0.9 | 3.6 ± 0.4 | No |
| 48 | 1.20 ± 0.46 | 0.81 ± 0.13 | 3.5 ± 0.9 | Not tested | ||
| hetR::C.CE2 + PrbcLPpetE-hetP | 24 | 0.17 ± 0.10 | 0 | Not counted f | Not tested | No |
| 48 | 1.57 ± 0.26 | 0 | Not counted | Not tested | ||
| hetR::C.CE2 + PrbcLPpetE-hetZ-PpetE-hetP e2 | 24 | 4.00 ± 1.86 | 0.90 ± 0.07 | 11.5 ± 2.0 | 12.9 ± 0.9 | No |
| 48 | 4.12 ± 0.27 | 1.52 ± 0.78 | 17.5 ± 2.0 | Not tested | ||
| hetZ::C.K2 ΔhetP | 24 | 0 | 0 | 0 | Not tested | No |
| 48 | 0 | 0 | 0 | Not tested | ||
| hetZ::C.K2 ΔhetP + PrbcLPpetE-hetZ-PpetE-hetP e3 | 24 | Not measured | Not measured | 14.3 ± 1.2 | 15.7 ± 0.7 | Moderate |
| 48 | Not measured | Not measured | 17.6 ± 0.4 | Not tested | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xu, X. Manipulation of Pattern of Cell Differentiation in a hetR Mutant of Anabaena sp. PCC 7120 by Overexpressing hetZ Alone or with hetP. Life 2018, 8, 60. https://doi.org/10.3390/life8040060
Zhang H, Xu X. Manipulation of Pattern of Cell Differentiation in a hetR Mutant of Anabaena sp. PCC 7120 by Overexpressing hetZ Alone or with hetP. Life. 2018; 8(4):60. https://doi.org/10.3390/life8040060
Chicago/Turabian StyleZhang, He, and Xudong Xu. 2018. "Manipulation of Pattern of Cell Differentiation in a hetR Mutant of Anabaena sp. PCC 7120 by Overexpressing hetZ Alone or with hetP" Life 8, no. 4: 60. https://doi.org/10.3390/life8040060





