Global Molecular Diversity of the Halotolerant Fungus Hortaea werneckii
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Growth at Different Salinities at 25 °C and 37 °C
2.3. DNA Extraction and Sequencing
2.4. Amplified Fragment Length Polymorphism Genotyping
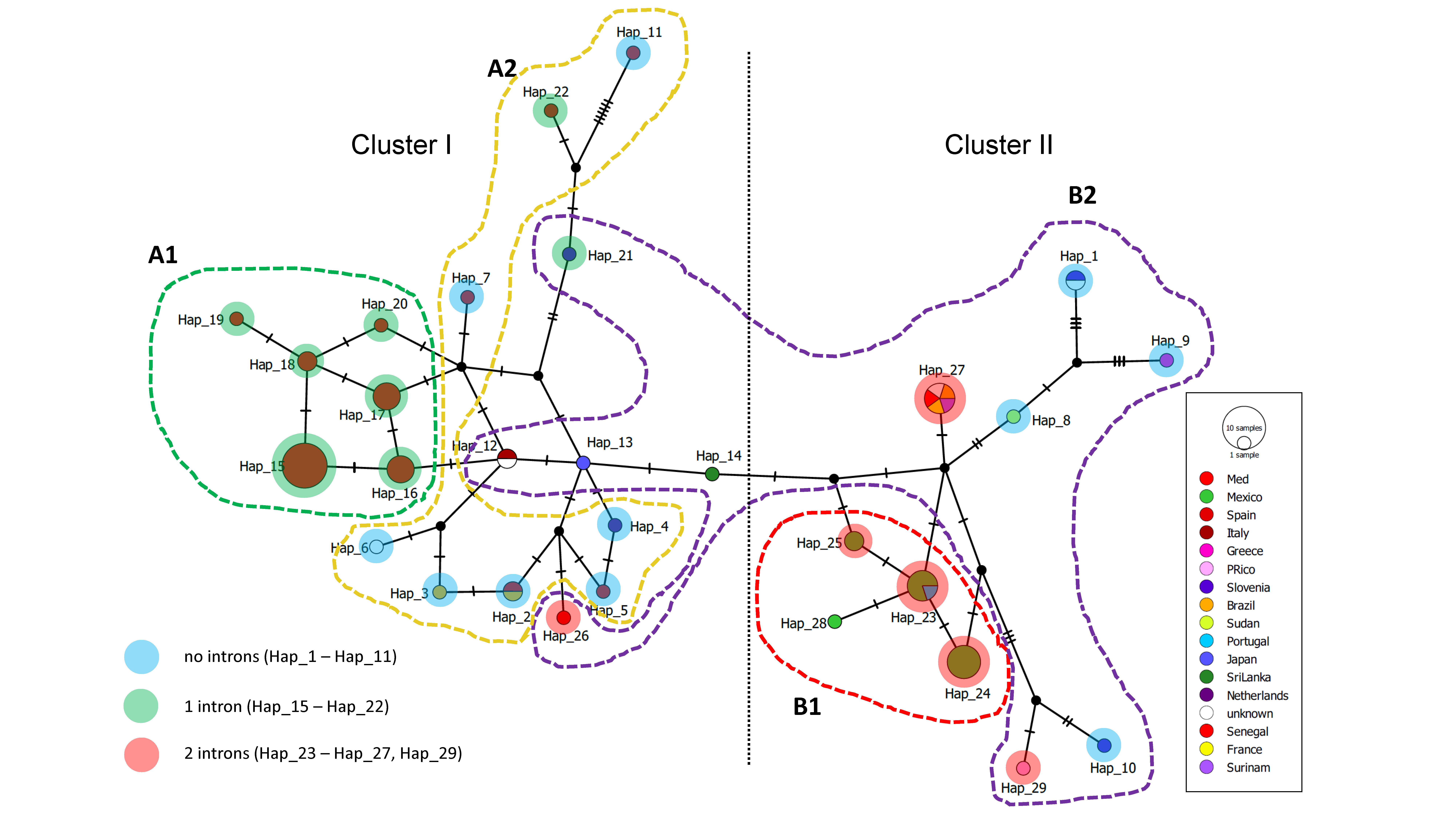
2.5. Haplotype Networks
3. Results
3.1. Sequencing
3.2. Physiology
3.3. Amplified Fragment Length Polymorphism Genotyping
3.4. Haplotype Network
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Selbmann, L.; de Hoog, G.S.; Mazzaglia, A.; Friedmann, E.I.; Onofri, S. Fungi at the edge of life: Cryptoendolithic black fungi from Antarctic desert. Stud. Mycol. 2005, 51, 1–32. [Google Scholar]
- Zalar, P.; Novak, M.; de Hoog, G.S.; Gunde-Cimerman, N. Dishwashers—A man-made ecological niche accommodating human opportunistic fungal pathogens. Fungal Biol. 2011, 115, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Parbery, D.G. Isolation of the kerosene fungus, Cladosporium resinae, from Australian soil. Trans. Br. Mycol. Soc. 1967, 50, 682–685. [Google Scholar] [CrossRef]
- Selbmann, L.; de Hoog, G.S.; Zucconi, L.; Isola, D.; Ruisi, S.; Gerrits van den Ende, A.H.G.; Ruibal, C.; De Leo, F.; Urzì, C.; Onofri, S. Drought meets acid: Three new genera in a dothidealean clade of extremotolerant fungi. Stud. Mycol. 2008, 61, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zajc, J.; Liu, Y.; Dai, W.; Yang, Z.; Hu, J.; Gostinčar, C.; Gunde-Cimerman, N. Genome and transcriptome sequencing of the halophilic fungus Wallemia ichthyophaga: Haloadaptations present and absent. BMC Genom. 2013, 14, 617. [Google Scholar] [CrossRef] [PubMed]
- Selbmann, L.; Zucconi, L.; Isola, D.; Onofri, S. Rock black fungi: Excellence in the extremes, from the Antarctic to space. Curr. Genet. 2015, 61, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Gostinčar, C.; Grube, M.; de Hoog, G.S.; Zalar, P.; Gunde-Cimerman, N. Extremotolerance in fungi: Evolution on the edge. FEMS Microbiol. Ecol. 2010, 71, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Gunde-Cimerman, N.; Plemenitaš, A. Ecology and molecular adaptations of the halophilic black yeast Hortaea werneckii. Rev. Environ. Sci. Biotechnol. 2006, 5, 323–331. [Google Scholar] [CrossRef]
- De Hoog, G.S.; McGinnis, M.R. Ascomycetous black yeasts. In The Expanding Realm of Yeast-Like Fungi; de Hoog, G.S., Smith, M.T., Weijman, A.C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 187–199. [Google Scholar]
- De Hoog, G.S.; Gerrits van den Ende, A.H.G. Nutritional pattern and ecophysiology of Hortaea werneckii, agent of human tinea nigra. Antonie Leeuwenhoek 1992, 62, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xing, X.K.; Zhang, L.C.; Xing, Y.M.; Guo, S.X. Identification of Hortaea werneckii isolated from mangrove plant Aegiceras comiculatum based on morphology and rDNA sequences. Mycopathologia 2012, 174, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Kogej, T.; Ramos, J.; Plemenitaš, A.; Gunde-Cimerman, N. The halophilic fungus Hortaea werneckii and the halotolerant fungus Aureobasidium pullulans maintain low intracellular cation concentrations in hypersaline environments. Appl. Environ. Microbiol. 2005, 71, 6600–6605. [Google Scholar] [CrossRef] [PubMed]
- Cabañes, F.J.; Bragulat, M.R.; Castellá, G. Hortaea werneckii isolated from silicone scuba diving equipment in Spain. Med. Mycol. 2012, 50, 852–857. [Google Scholar] [CrossRef] [PubMed]
- De Leo, F.; Lo Giudice, A.; Alaimo, C.; De Carlo, G.; Rappazzo, A.C.; Graziano, M.; De Domenico, E.; Urzì, C. A high abundance of the black yeast Hortaea werneckii occurs along the off-shore water column in the Mediterranean basin. Extrem. 2018. Submitted. [Google Scholar]
- Gunde-Cimerman, N.; Zalar, P.; de Hoog, G.S.; Plemenitaš, A. Hypersaline waters in salterns- Natural ecological niches for halophilic black yeasts. FEMS Microbiol. Ecol. 2000, 32, 235–240. [Google Scholar]
- Gunde-Cimerman, N.; Zalar, P. Extremely halotolerant and halophilic fungi inhabit brine in solar salterns around the globe. Food Technol. Biotechnol. 2014, 52, 170–179. [Google Scholar]
- Kogej, T.; Stein, M.; Volkmann, M.; Gorbushina, A.A.; Galinski, E.A.; Gunde-Cimerman, N. Osmotic adaptation of the halophilic fungus Hortaea werneckii: Role of osmolytes and melanization. Microbiology 2007, 153, 4261–4273. [Google Scholar] [CrossRef] [PubMed]
- Petrovič, U.; Gunde-Cimerman, N.; Plemenitaš, A. Cellular responses to environmental salinity in the halophilic black yeast Hortaea werneckii. Mol. Microbiol. 2002, 45, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Plemenitaš, A.; Vaupotič, T.; Lenassi, M.; Kogej, T.; Gunde-Cimerman, N. Adaptation of extremely halotolerant black yeast Hortaea werneckii to increased osmolarity: A molecular perspective at a glance. Stud. Mycol. 2008, 61, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Lenassi, M.; Zajc, J.; Gostinčar, C.; Gorjan, A.; Gunde-Cimerman, N.; Plemenitaš, A. Adaptation of the glycerol-3-phosphate dehydrogenase Gpd1 to high salinities in the extremely halotolerant Hortaea werneckii and halophilic Wallemia ichthyophaga. Fungal Biol. 2011, 115, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.; Colella, M.T.; Olaizola, C.; de Capriles, C.H.; Magaldi, S.; Mata-Essayag, S. Tinea nigra: Report of twelve cases in Venezuela. Mycopathologia 2005, 160, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Bonifaz, A.; Badali, H.; de Hoog, G.S.; Cruz, M.; Araiza, J.; Cruz, M.A.; Fierro, L.; Ponce, R.M. Tinea nigra by Hortaea werneckii, a report of 22 cases from Mexico. Stud. Mycol. 2008, 61, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Kutty, S.N.; Philip, R. Marine yeasts—A review. Yeast 2008, 25, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.; Bonifaz, A.; de Hoog, G.S.; Vazquez-Maya, L.; Garcia-Carmona, K.; Meis, J.F.; van Diepeningen, A.D. Keratitis by Fusarium temperatum, a novel opportunist. BMC Infect. Dis. 2014, 14, 588. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny interfered from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.; Al-Hatmi, A.M.S.; Ilkit, M.; Gerrits van den Ende, A.H.G.; Hagen, F.; Meis, J.F.; de Hoog, G.S. Molecular Diagnostics of Arthroconidial Yeasts, Frequent Pulmonary Opportunists. J. Clin. Microbiol. 2017, 56, e01427-14. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Najafzadeh, M.J.; Dolatabadi, S.; Ran, Y.P.; Gerrits van den Ende, A.H.; Shen, Y.N.; Li, C.Y.; Xi, L.Y.; Hao, F.; Zhang, Q.Q.; et al. Taxonomy and epidemiology of Mucor irregularis, agent of chronic cutaneous mucormycosis. Persoonia 2013, 30, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSp v5: A software for comprehensive analysis of DNA polymorphism data. BioInformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Flibotte, S.; Neira, M.; Formby, S.; Plemenitaš, A.; Gunde Cimerman, N.; Lenassi, M.; Gostinčar, C.; Stajich, J.E.; Nislow, C. Insight into the recent genome duplication of the halophilic yeast Hortaea werneckii: Combining an improved genome with gene expression and chromatin structure. G3 Genes Genomes Genet. 2017, 7, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Schoch, C.L.; Hyde, K.D.; Wood, A.R.; Gueidan, C.; de Hoog, G.S.; Groenewald, J.Z. Phylogenetic lineages in the Capnodiales. Stud. Mycol. 2009, 64, 17–47. [Google Scholar] [CrossRef] [PubMed]
- Lenassi, M.; Gostinčar, C.; Jackman, S.; Turk, M.; Sadowski, I.; Nislow, C.; Jones, S.; Birol, I.; Gunde Cimerman, N.; Plemenitaš, A. Whole Genome Duplication and Enrichment of Metal Cation Transporters Revealed by De Novo Genome Sequencing of Extremely Halotolerant Black Yeast Hortaea werneckii. PLoS ONE 2013, 8, e71328. [Google Scholar] [CrossRef] [PubMed]
- Iwatsu, T.U.; Udagawa, S. Hortaea werneckii isolated from sea-water. Jpn. J. Med. Mycol. 1988, 29, 142–145. [Google Scholar] [CrossRef]
- Liu, D. Molecular Detection of Human Fungal Pathogens, 1st ed.; Taylor & Francis CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781439812402. [Google Scholar]
- Göttlich, E.; de Hoog, G.S.; Yoshida, S.; Takeo, K.; Nishimura, K.; Miyaji, M. Cell-surface hydrophobicity and lipolysis as essential factors in human tinea nigra. Mycoses 1995, 38, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Hagen, F.; Khayhan, K.; Theelen, B.; Kolecka, A.; Polacheck, I.; Sionov, E.; Falk, R.; Parnmen, S.; Lumbsch, H.T.; Boekhout, T. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 2015, 78, 16–48. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Dukik, K.; Li, D.; Sun, J.; Stielow, J.B.; Gerrits van den Ende, B.; Brankovics, B.; Menken, S.B.J.; Mei, H.; Bao, W.; et al. Phylogeny of dermatophytes with genomic character evaluation of clinically distinct Trichophyton rubrum and T. violaceum. Stud. Mycol. 2018, 89, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Warris, A.; Klaassen, C.H.W.; Meis, J.F.G.M.; de Ruiter, M.T.; de Valk, H.A.; Abrahamsen, T.G.; Gaustad, P.; Verweij, P.E. Molecular epidemiology of Aspergillus fumigatus isolates recovered from water, air, and patients shows two clusters of genetically distinct strains. J. Clin. Microbiol. 2003, 41, 4101–4106. [Google Scholar] [CrossRef] [PubMed]

| Collection Number | Country | Source | Gen Bank Accession Number | |
|---|---|---|---|---|
| ITS | TEF1 * | |||
| CBS 100455 | Slovenia | Seawater | AY128704 | MH259543 |
| CBS 100456 | Slovenia | Salt pan, saline water | MH028914 | MH259581 |
| CBS 100457 | Slovenia | Salt pan, saline water | MH028913 | MH259579 |
| CBS 100496 | Greece | Sea-sprayed marble | AY128703 | MH259542 |
| CBS 107.67 T | Portugal | Tinea nigra | AJ238468 | MH259537 |
| CBS 110352 | Sudan | Hollow tree | MH028917 | MH259577 |
| CBS 111.31 | Brazil | Tinea nigra | AJ238679 | MH259546 |
| CBS 115.90 | Brazil | Bufo granulosus kidney | AJ238470 | MH259548 |
| CBS 117.90 | Brazil | Osteoglossum bicirrhosum | AJ238472 | MH259526 |
| CBS 116.30 | Unknown | Tinea nigra | MH028923 | MH259521 |
| CBS 116.90 | Unknown | Chantarus chantarus eye infection | AJ238471 | MH259544 |
| CBS 120952 | Puerto Rico | Hypersaline water | MH028918 | MH259519 |
| CBS 122.32 | Unknown | Tinea nigra | AJ238473 | MH259574 |
| CBS 122340 | Mexico | Tinea nigra | MH028912 | MH249534 |
| CBS 122342 | Mexico | Tinea nigra | MH028899 | MH259529 |
| CBS 122344 | Mexico | Tinea nigra | MH028900 | MH259532 |
| CBS 122348 | Mexico | Tinea nigra | MH028911 | MH259528 |
| From CBS 123041 to CBS 123046 | Mexico | Tinea nigra | From MH028901 to MH028906 | MH259535, MH259540, MH259538, MH259533, MH259531, MH259536 |
| From CBS 126984 to CBS 126987 | Mexico | Tinea nigra | MH028907, MH028909, MH028910, MH028908 | n.d., MH259530, MH259539, MH259527 |
| CBS 123850 | Netherlands | Salt bath for salting cheeses | MH028916 | MH259550 |
| CBS 126.35 | Italy | Tinea nigra | MH028921 | MH259573 |
| CBS 132911 | Unknown | Atol | MH028924 | MH259547 |
| CBS 132930 | Spain | Silicone scuba diving mask | MH028925 | MH259578 |
| CBS 132931 | Spain | Silicone snorkel | MH028926 | MH259549 |
| CBS 132932 | Spain | Polyethylene plastic bag | MH028927 | MH259576 |
| CBS 255.96 | Spain | Casuarina equisetifolia | MH028928 | MH259541 |
| CBS 117931 | Spain | Limestone rock | MH028898 | MH259580 |
| CBS 373.92 | Spain | Beach soil | AJ238474 | MH259520 |
| CBS 359.66 | Suriname | Tinea nigra palmaris | AJ244249 | MH259524 |
| CBS 410.51 | Japan | Air | MH028919 | MH259571 |
| CBS 705.76 | France | Tinea nigra | MH028920 | MH259522 |
| CBS 706.76 | Senegal | Rhizophora mangle leaf | MH028955 | MH259523 |
| CBS 707.76 | Sri Lanka | Sooty mould | MH028915 | MH259572 |
| CBS 708.76 | Unknown | Tinea nigra | MH028922 | MH259525 |
| MC 846 and MC 847 | Italy | Seawater (Mediterranean Sea, depth 25 m, “Vector” station) | KX427192 KX427193 | MH259569 MH259545 |
| MC 848 | Italy | Seawater (Mediterranean Sea, depth 2500 m, “Vector” station) | KX427194 | n.d. |
| MC 849 | Italy | Seawater (Mediterranean Sea, depth 200 m, “KM3” station) | KX427195 | MH259551 |
| MC 850 | Italy | Seawater (Mediterranean Sea, depth 94 m, “Medee” station) | KX427196 | MH259582 |
| From MC 854 to MC 859 | Italy | Seawater (Mediterranean Sea, depth 0 m, “Sn2” station) | From MH028934 to MH028939 | MH259558, MH259557, MH259555, MH259567, MH259554, MH259556 |
| From MC 860 to MC 862 | Italy | Seawater (Mediterranean Sea, depth 100-250 m, “Sn2” station) | From MH028940 to MH028942 | MH259559, MH259570, MH259566 |
| MC 863 | Italy | Seawater (Mediterranean Sea, depth 2218 m, “Sn2” station) | MH028943 | MH259561 |
| From MC 865 to MC 874 | Italy | Seawater (Mediterranean Sea, depth 3402 m, “Geostar” station) | From MH028944 to MH028953 | MH259575, MH259565, MH259552, MH259560, MH259553, MH259564, MH259568, MH259563, n.d., MH259562 |
| 25 °C | 37 °C | |||||||
|---|---|---|---|---|---|---|---|---|
| 0% NaCl | 15% NaCl | 20% NaCl | 25% NaCl | 0% NaCl | 15% NaCl | 20% NaCl | 25% NaCl | |
| Strains from the Mediterranean Sea | ||||||||
| MC 846 | ++++ | +++ | + | w | ++ | +++ | + | - |
| MC 848 | ++++ | ++ | + | w | ++ | ++ | + | - |
| MC 849 | ++++ | ++ | + | w | ++ | ++ | + | - |
| MC 850 | ++++ | ++ | + | w | ++ | ++ | + | - |
| MC 854 | ++++ | ++ | + | w | ++ | ++ | + | - |
| MC 858 | ++++ | ++ | + | w | ++ | ++ | + | - |
| MC 859 | ++++ | ++ | + | w | ++ | ++ | + | - |
| MC 860 | ++++ | ++ | + | w | ++ | ++ | + | - |
| MC 861 | ++++ | ++ | + | w | ++ | ++ | + | - |
| MC 862 | ++++ | ++ | + | w | ++ | ++ | + | - |
| MC 863 | ++++ | ++ | + | w | ++ | ++ | + | - |
| MC 865 | ++++ | ++ | + | w | ++ | ++ | + | - |
| MC 873 | ++++ | ++ | + | w | ++ | ++ | + | - |
| MC874 | ++++ | ++ | + | w | ++ | ++ | + | - |
| Clinical strains | ||||||||
| CBS 107.67 | ++ | +++ | + | - | - | ++++ | + | - |
| CBS 111.31 | ++++ | ++++ | ++ | w | + | ++++ | ++ | - |
| CBS 116.30 | ++++ | + | + | - | - | + | - | - |
| CBS 122.32 | +++ | +++ | ++ | w | + | +++ | ++ | - |
| CBS 126.35 | ++++ | ++ | + | - | - | + | - | - |
| CBS 359.66 | ++ | ++ | + | w | - | ++ | + | - |
| CBS 705.76 | +++ | ++++ | +++ | w | + | ++++ | ++ | - |
| CBS 708.76 | ++++ | +++ | ++ | w | - | + | - | - |
| CBS 122348 | ++++ | +++ | + | w | ++ | ++++ | ++ | - |
| CBS 123041 | ++++ | ++ | + | - | ++ | ++++ | ++ | - |
| CBS 123046 | +++ | +++ | + | w | ++ | ++++ | +++ | - |
| CBS 126987 | +++ | ++ | + | w | - | ++++ | + | - |
| Strains from salterns | ||||||||
| CBS 100455 | +++ | ++ | + | w | - | ++ | + | - |
| CBS 100457 | ++++ | ++++ | + | w | ++ | +++ | + | - |
| CBS 120952 | ++++ | ++++ | ++ | + | - | ++++ | + | - |
| Strains from temperate and Mediterranean climatic zone | ||||||||
| CBS 100496 | +++ | ++ | + | w | ++ | ++++ | + | - |
| CBS 117931 | +++ | ++++ | ++ | w | - | + | + | - |
| CBS 123850 | ++++ | ++++ | ++++ | + | ++ | ++++ | +++ | - |
| CBS 132931 | +++ | ++ | + | + | + | ++ | + | - |
| CBS 132932 | ++ | ++ | + | w | w | + | + | - |
| CBS 255.96 | ++++ | ++++ | ++ | + | - | + | + | - |
| CBS 373.92 | ++++ | ++++ | ++++ | + | - | ++++ | +++ | - |
| CBS 410.51 | ++++ | ++++ | ++ | - | + | +++ | ++ | - |
| CBS 706.76 | +++ | ++++ | +++ | w | + | ++++ | ++ | - |
| Strains from tropical and arid climatic zone | ||||||||
| CBS 115.90 | +++ | +++ | + | w | - | + | + | - |
| CBS116.90 | +++ | ++++ | ++ | w | ++ | ++++ | ++ | - |
| CBS 117.90 | ++++ | ++++ | +++ | w | + | ++++ | ++ | - |
| CBS 110352 | ++++ | ++++ | +++ | - | - | ++++ | + | - |
| CBS 707.76 | +++ | ++++ | +++ | + | ++ | ++++ | ++ | - |
| CBS 132911 | ++++ | ++++ | +++ | + | - | ++++ | + | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetta, A.; Gerrits van den Ende, B.; Al-Hatmi, A.M.S.; Hagen, F.; Zalar, P.; Sudhadham, M.; Gunde-Cimerman, N.; Urzì, C.; De Hoog, S.; De Leo, F. Global Molecular Diversity of the Halotolerant Fungus Hortaea werneckii. Life 2018, 8, 31. https://doi.org/10.3390/life8030031
Marchetta A, Gerrits van den Ende B, Al-Hatmi AMS, Hagen F, Zalar P, Sudhadham M, Gunde-Cimerman N, Urzì C, De Hoog S, De Leo F. Global Molecular Diversity of the Halotolerant Fungus Hortaea werneckii. Life. 2018; 8(3):31. https://doi.org/10.3390/life8030031
Chicago/Turabian StyleMarchetta, Alessia, Bert Gerrits van den Ende, Abdullah M. S. Al-Hatmi, Ferry Hagen, Polona Zalar, Montarop Sudhadham, Nina Gunde-Cimerman, Clara Urzì, Sybren De Hoog, and Filomena De Leo. 2018. "Global Molecular Diversity of the Halotolerant Fungus Hortaea werneckii" Life 8, no. 3: 31. https://doi.org/10.3390/life8030031
APA StyleMarchetta, A., Gerrits van den Ende, B., Al-Hatmi, A. M. S., Hagen, F., Zalar, P., Sudhadham, M., Gunde-Cimerman, N., Urzì, C., De Hoog, S., & De Leo, F. (2018). Global Molecular Diversity of the Halotolerant Fungus Hortaea werneckii. Life, 8(3), 31. https://doi.org/10.3390/life8030031










