tRNA Core Hypothesis for the Transition from the RNA World to the Ribonucleoprotein World
Abstract
:1. Introduction
The Hypothesis
2. Experimental Section
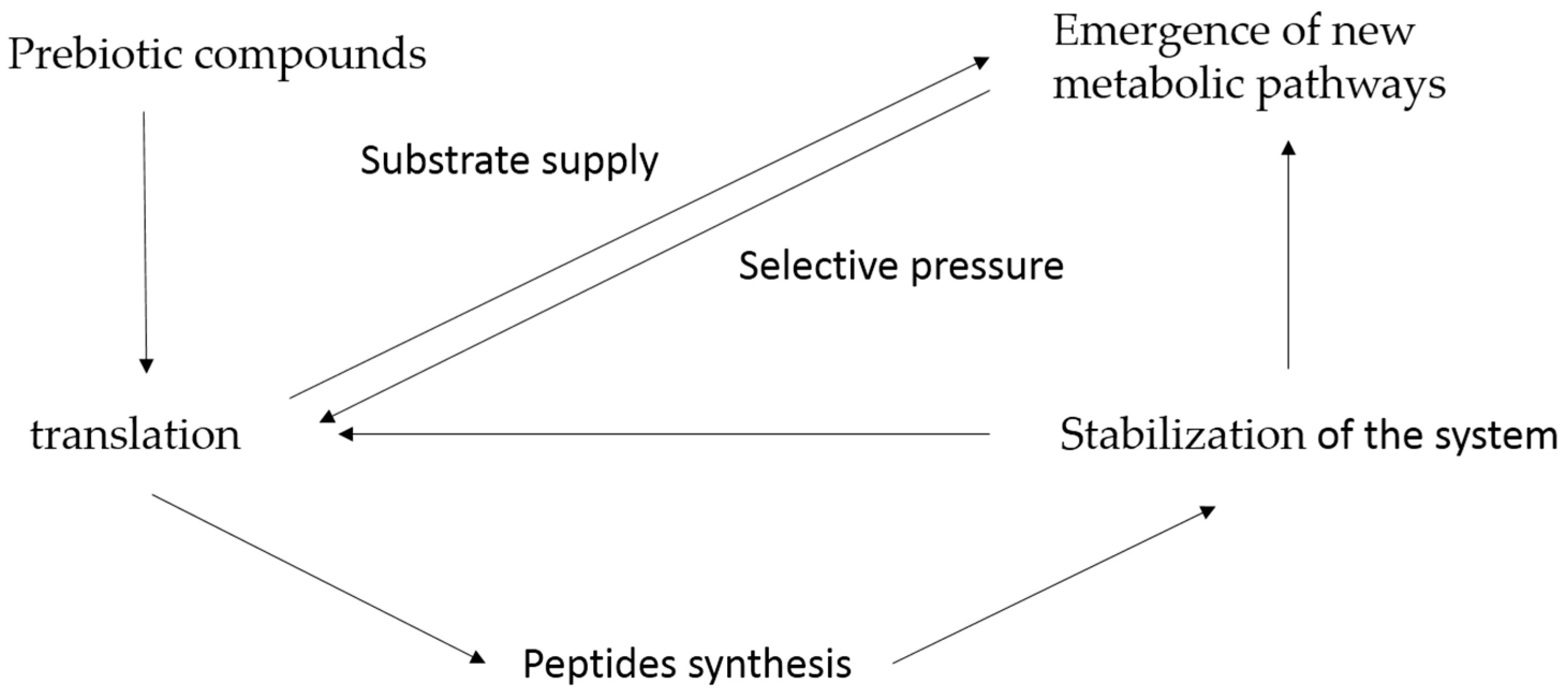
tRNA and the First Genes
3. Results and Discussion
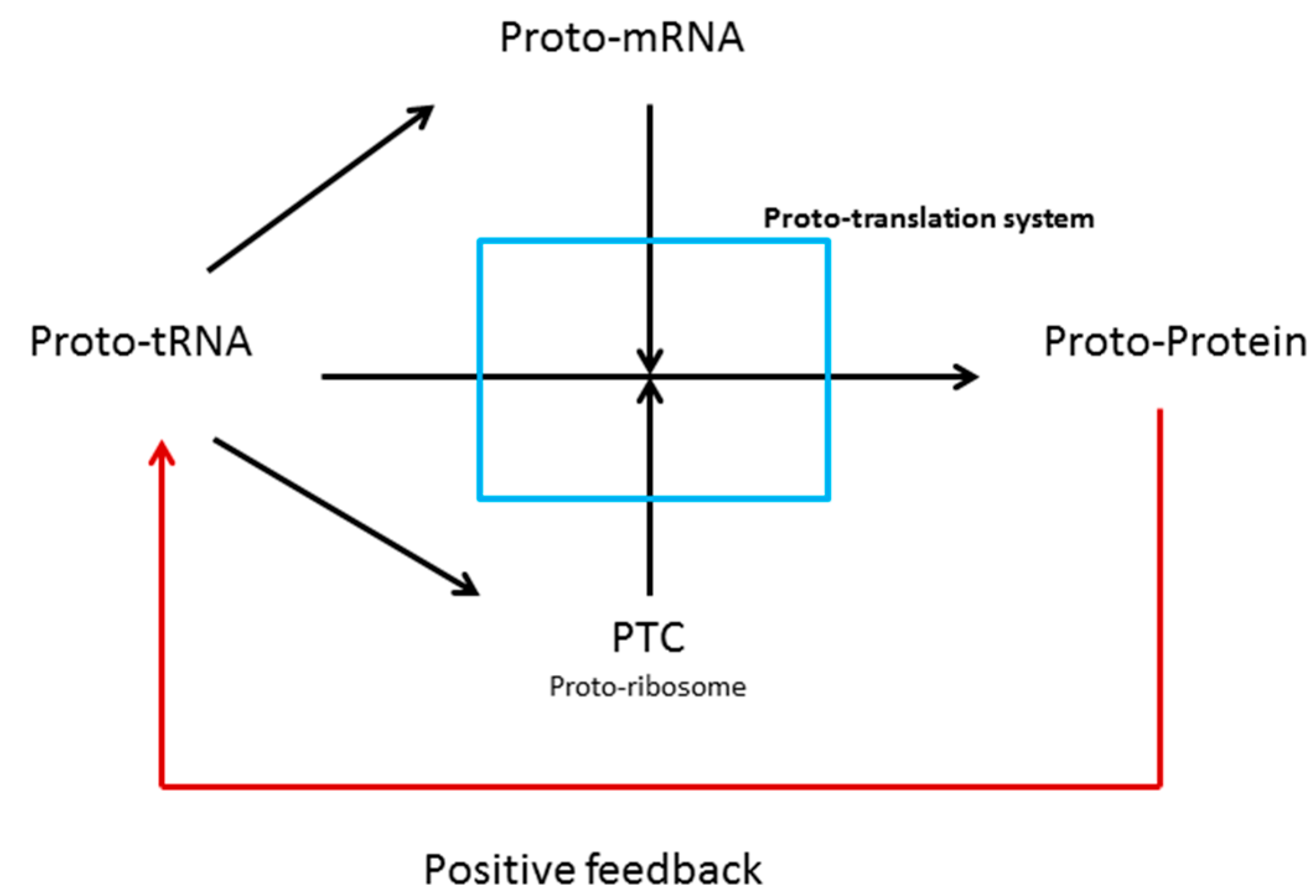
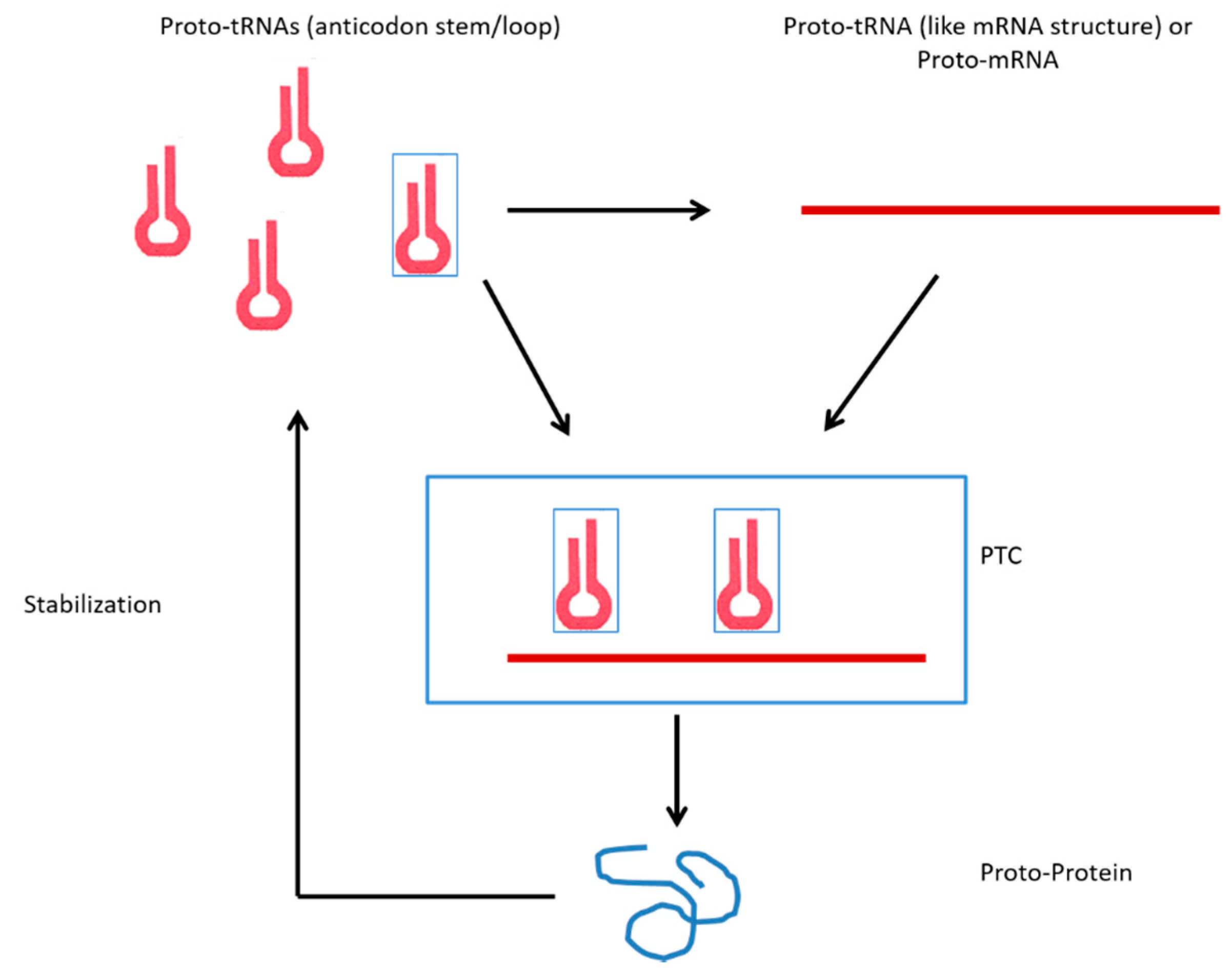
3.1. tRNAs and the Origin of Genes
3.2. tRNAs and the Origin of Ribosomes
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.T.; Cleaves, H.J.; Dworkin, J.P.; Glavin, D.P.; Callahan, M.; Aubrey, A.; Lazcano, A.; Bada, J.L. Primordial synthesis of amines and amino acids in a 1958 Miller H2S-rich spark discharge experiment. Proc. Natl. Acad. Sci. USA 2011, 108, 5526–5531. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.T.; Zhou, M.; Burton, A.S.; Glavin, D.P.; Dworkin, J.P.; Krishnamurthy, R.; Fernández, F.M.; Bada, J.L. A plausible simultaneous synthesis of amino acids and simple peptides on the primordial Earth. Angew. Chem. Int. Ed. Engl. 2014, 53, 8132–8136. [Google Scholar] [CrossRef] [PubMed]
- Bada, J.L. New insights into prebiotic chemistry from Stanley Miller’s spark discharge experiments. Chem. Soc. Rev. 2013, 42, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Kruger, K.; Grabowski, P.J.; Zaug, A.J.; Sands, J.; Gottschling, D.E.; Cech, T.R. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell 1982, 31, 147–157. [Google Scholar] [CrossRef]
- Guerrier-Takada, C.; Gardiner, K.; Marsh, T.; Pace, N.; Altman, S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 1983, 35, 849–857. [Google Scholar] [CrossRef]
- Poole, A.M.; Jeffares, D.C.; Penny, D. The path from the RNA world. J. Mol. Evol. 1998, 46, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jeffares, D.C.; Poole, A.M.; Penny, D. Relics from the RNA world. J. Mol. Evol. 1998, 46, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, J.P.; Lazcano, A.; Miller, S.L. The roads to and from the RNA world. J. Theor. Biol. 2003, 222, 127–134. [Google Scholar] [CrossRef]
- Lazcano, A. The biochemical roots of the RNA world: From zymonucleic acid to ribozymes. Hist. Philos. Life Sci. 2012, 34, 407–423. [Google Scholar] [PubMed]
- Reyes-Prieto, F.; Hernández-Morales, R.; Jácome, R.; Becerra, A.; Lazcano, A. Coenzymes, viruses and the RNA world. Biochimie 2012, 94, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Neveu, M.; Kim, H.J.; Benner, S.A. The “strong” RNA world hypothesis: Fifty years old. Astrobiology 2013, 13, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, K. Possible steps to the emergence of life: the [GADV] protein world hypothesis. Chem. Rec. 2005, 5, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Anollés, D.; Kim, K.M.; Mittenthal, J.E.; Caetano-Anollés, G. Proteome evolution and the metabolic origins of translation and cellular life. J. Mol. Evol. 2011, 72, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Anollés, G.; Wang, M.; Caetano-Anollés, D. Structural phylogenomics retrodicts the origin of the genetic code and uncovers the evolutionary impact of protein flexibility. PLoS ONE 2013, 8, e72225. [Google Scholar] [CrossRef]
- Farias, S.T.; Moreira, C.H.; Guimarães, R.C. Structure of the genetic code suggested by the hydropathy correlation between anticodons and amino acid residues. Orig. Life Evol. Biosph. 2007, 37, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.C. Metabolic basis for the self-referential genetic code. Orig. Life Evol. Biosph. 2011, 41, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M. On the RNA world: evidence in favor of an early ribonucleopeptide world. J. Mol Evol. 1997, 45, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Eigen, M. Self-organization of matter and the evolution of biological macromolecules. Naturwissenschaften 1971, 58, 465–523. [Google Scholar] [CrossRef] [PubMed]
- Kreysing, M.; Keil, L.; Lanzmich, S.; Braun, D. Heat flux across an open pore enables the continuous replication and selection of oligonucleotides towards increasing length. Nat. Chem. 2015, 7, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Agmon, I.; Bashan, A.; Zarivach, R.; Yonath, A. Symmetry at the active site of the ribosome: Structural and functional implications. Biol. Chem. 2005, 386, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Agmon, I.; Bashan, A.; Yonath, A. On ribosome conservation and evolution. Isr. J. Ecol. Evol. 2006, 52, 359–374. [Google Scholar] [CrossRef]
- Agmon, I. The dimeric proto-ribosome: Structural details and possible implications on the origin of life. Int. J. Mol. Sci. 2009, 30, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Belousoff, M.J.; Davidovich, C.; Bashan, A.; Yonath, A. On the development towards the modern world: A plausible role of uncoded peptides in the RNA world. Orig. Life Evol. Biosph. 2010, 40, 415–419. [Google Scholar]
- Bashan, A.; Yonath, A. Ribosome crystallography: Catalysis and evolution of peptide-bond formation, nascent chain elongation and its co-translational folding. Biochem. Soc. Trans. 2005, 33, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Bashan, A.; Belousoff, M.J.; Davidovich, C.; Yonath, A. Linking the RNA world to modern life: The proto-ribosome conception. Orig. Life Evol. Biosph. 2010, 40, 425–429. [Google Scholar]
- Tamura, K. Ribosome evolution: Emergence of peptide synthesis machinery. J. Biosci. 2011, 36, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Eigen, M.; Winkler-Oswatitsch, R. Transfer-RNA, an early gene? Naturwissenschaften 1981, 68, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Bloch, D.; McArthur, B.; Widdowson, R.; Spector, D.; Guimaraes, R.C.; Smith, J. tRNA-rRNA sequence homologies: A model for the origin of a common ancestral molecule, and prospects for its reconstruction. Orig. Life 1984, 14, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Bloch, D.P.; McArthur, B.; Guimarães, R.C.; Smith, J.; Staves, M.P. tRNA-rRNA sequence matches from inter- and intraspecies comparisons suggest common origins for the two RNAs. Braz. J. Med. Biol. Res. 1989, 22, 931–944. [Google Scholar] [PubMed]
- Root-Bernstein, M.; Root-Bernstein, R. The ribosome as a missing link in the evolution of life. J. Theor. Biol. 2015, 367, 130–158. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M. The non-monophyletic origin of the tRNA molecule and the origin of genes only after the evolutionary stage of the last universal common ancestor (LUCA). J. Theor Biol. 2006, 240, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Krupkin, M.; Matzov, D.; Tang, H.; Metz, M.; Kalaora, R.; Belousoff, M.J.; Zimmerman, E.; Bashan, A.; Yonath, A. A vestige of a prebiotic bonding machine is functioning within the contemporary ribosome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2972–2978. [Google Scholar] [CrossRef] [PubMed]
- Eigen, M.; Schuster, P. The hypercycle. A principle of natural self-organization. Part C: The realistic hypercycle. Naturwissenschaften 1978, 65, 341–369. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; et al. The COG database: An updated version includes eukaryotes. BMC Bioinformatics 2003, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Farias, S.T.; Rêgo, T.G.; José, M.V. Origin and evolution of the Peptidyl Transferase Center from proto-tRNAs. FEBS Open Bio. 2014, 4, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Szathmáry, E. The origin of the genetic code: amino acids as cofactors in an RNA world. Trends. Genet. 1999, 15, 223–229. [Google Scholar] [CrossRef]
- Da Silva, J.A. From the RNA world to the RNA/protein world: contribution of some riboswitch-binding species? J. Theor. Biol. 2015, 7, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M. A model for the origin of the first mRNAs. J Mol Evol. 2015, 81, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Noller, H.F. The driving force for molecular evolution of translation. RNA 2004, 10, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Noller, H.F. Evolution of protein synthesis from an RNA world. Cold Spring Harb. Perspect. Biol 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J. The eightfold path to non-enzymatic RNA replication. J. Syst. Chem. 2012, 3. [Google Scholar] [CrossRef]
- Keller, M.A.; Turchyn, A.V.; Ralser, M. Non-enzymatic glycolysis and pentose phosphate pathway-like reactions in a plausible Archean ocean. Mol. Syst. Biol. 2014, 10, 725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaye, L.; Becerra, A.; Lazcano, A. The last common ancestor: What’s in a name? Orig. Life Evol. Biosph. 2005, 35, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, C.; Belousoff, M.; Bashan, A.; Yonath, A. The evolving ribosome: From non-coded peptide bond formation to sophisticated translation machinery. Res. Microbiol. 2009, 160, 487–492. [Google Scholar] [CrossRef] [PubMed]
| Protein | Process | Protein | Process |
|---|---|---|---|
| Elongation factor 1–alfa RNA transport | Translation | Putative ribose/galactose/methyl galactose import | Glycolysis/glycogenesis |
| Elongation factor 4–mRNA translation assisting | Translation | Glycerate kinase | Glycolysis/glycogenesis |
| tRNA uridine 5-carboxymethylaminomethyl modification | Translation | Triosephosphate isomerase | Glycolysis/glycogenesis |
| 60S ribosomal protein L3 | Translation | Beta-glucosidase A | Glycolysis/glycogenesis |
| 60S ribosomal protein L7a | Translation | Glucose-6-phosphate-1-dehydrogenase | Glycolysis/glycogenesis |
| 60S ribosomal protein L27a-1 | Translation | Glucose 6 phosphate isomerase | Glycolysis/glycogenesis |
| 60S ribosomal protein L27a-3 | Translation | Phosphoglycerate kinase | Glycolysis/glycogenesis |
| 60S ribosomal protein L27a-2/4 | Translation | Glycerol-3-phosphate dehydrogenase | Glycolysis/glycogenesis |
| 50S ribosomal protein L10 | Translation | Transketolase | Glycolysis/glycogenesis |
| Methionyl–tRNA formyl transferase | Translation | α-galactosidase | Glycolysis/glycogenesis |
| Lysys-tRNA synthetase | Translation | Diaminopimelate epimerase | Amino acids pathways |
| Asparaginyl-tRNA synthetase | Translation | l-asparaginase | Amino acids pathways |
| Glutamyl-tRNA synthetase | Translation | ATP phosphoribosyl transferase | Amino acids pathways |
| Leucyl-tRNA synthetase | Translation | Histidinol phosphate aminotransfarase | Amino acids pathways |
| Valyl-tRNA synthetase | Translation | 4-aminobutyrate aminotransferase | Amino acids pathways |
| Phenylalanine-tRNA synthetase | Translation | Ornithine carboxylase antizyme | Amino acids pathways |
| RNA methyltransferase—tRNA modification | Translation | N-acetyl-γ-glutamyl-phosphate redutase | Amino acids pathways |
| DNA-direct RNA polymerase or RNA-direct RNA polymerase | Transcription | Homoserine kinase | Amino acids pathways |
| Thymidylate kinase | Nucleotides pathways | Aromatic amino acid aminotransferase | Amino acids pathways |
| Cytidine deaminase | Nucleotides pathways | Ornithine carbomyltransferase | Amino acids pathways |
| Uridylate kinase | Nucleotides pathways | Tryptophan synthase α-chain | Amino acids pathways |
| Orotidine 5-phosphate decarboxilase | Nucleotides pathways | Fatty acid synthase | Lipids pathways |
| Dihydroorotate dehydrogenase | Nucleotides pathways | CoA mutase | Lipids pathways |
| Phosphoribosyl formyl glycinamidine cyclo-ligase | Nucleotides pathways | Phosphate acyltransferase | Lipids pathways |
| Phosphoribosyl glycinamidine synthase | Nucleotides pathways | Lycopene cyclase | Lipids pathways |
| 3-β-hydroxisteroid dehydrogenase | Lipids pathways |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Farias, S.T.; Rêgo, T.G.; José, M.V. tRNA Core Hypothesis for the Transition from the RNA World to the Ribonucleoprotein World. Life 2016, 6, 15. https://doi.org/10.3390/life6020015
De Farias ST, Rêgo TG, José MV. tRNA Core Hypothesis for the Transition from the RNA World to the Ribonucleoprotein World. Life. 2016; 6(2):15. https://doi.org/10.3390/life6020015
Chicago/Turabian StyleDe Farias, Savio T., Thais G. Rêgo, and Marco V. José. 2016. "tRNA Core Hypothesis for the Transition from the RNA World to the Ribonucleoprotein World" Life 6, no. 2: 15. https://doi.org/10.3390/life6020015






