Prediction of the Maximum Temperature for Life Based on the Stability of Metabolites to Decomposition in Water
Abstract
:1. Introduction
2. Method
2.1. Literature Information
2.2. Exclusion Criteria
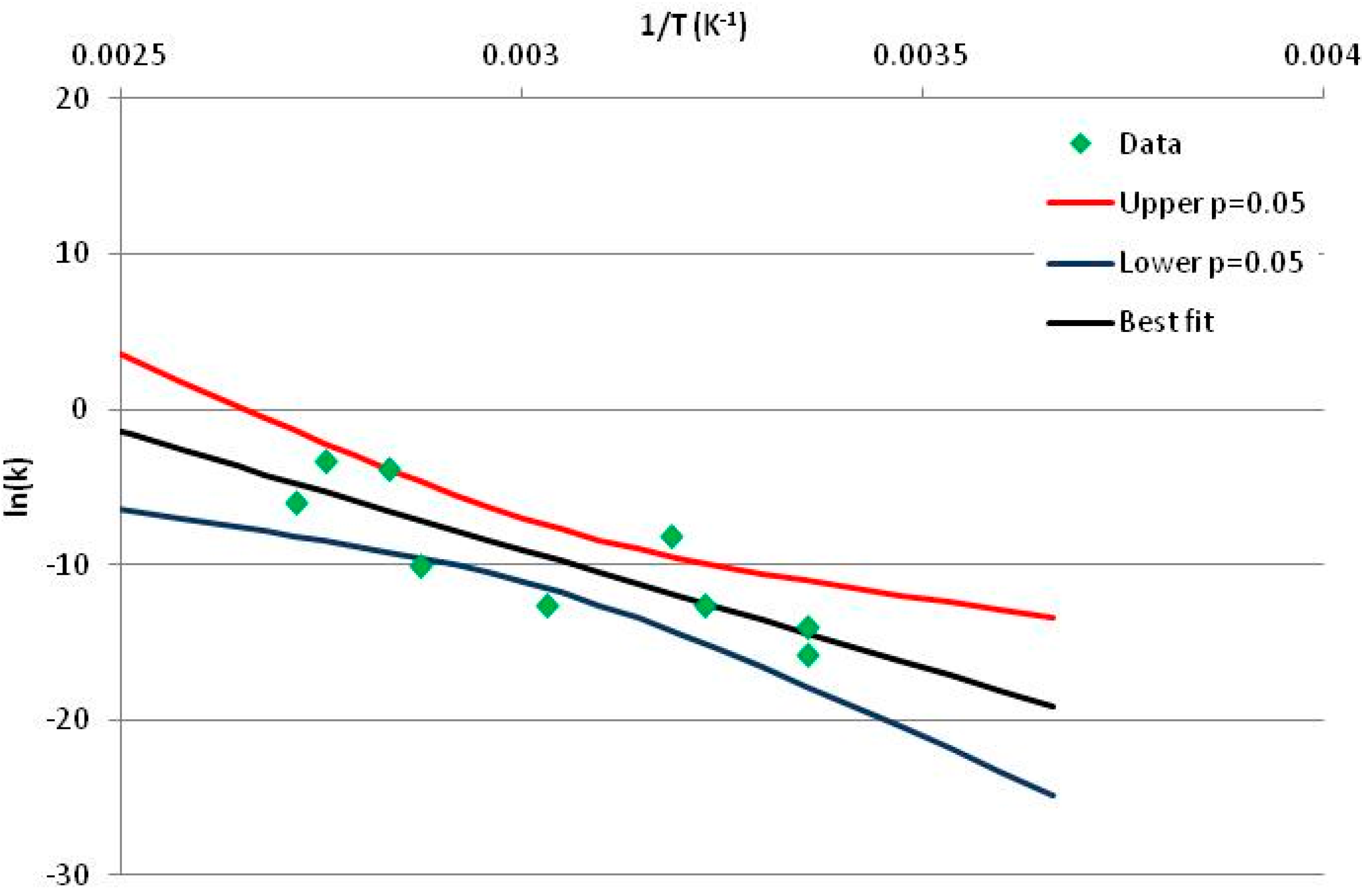
2.3. Kinetic Analysis
2.4. Limits of Temperature Explored
3. Results
3.1. Illustration of the Method: Fructose and Xylose
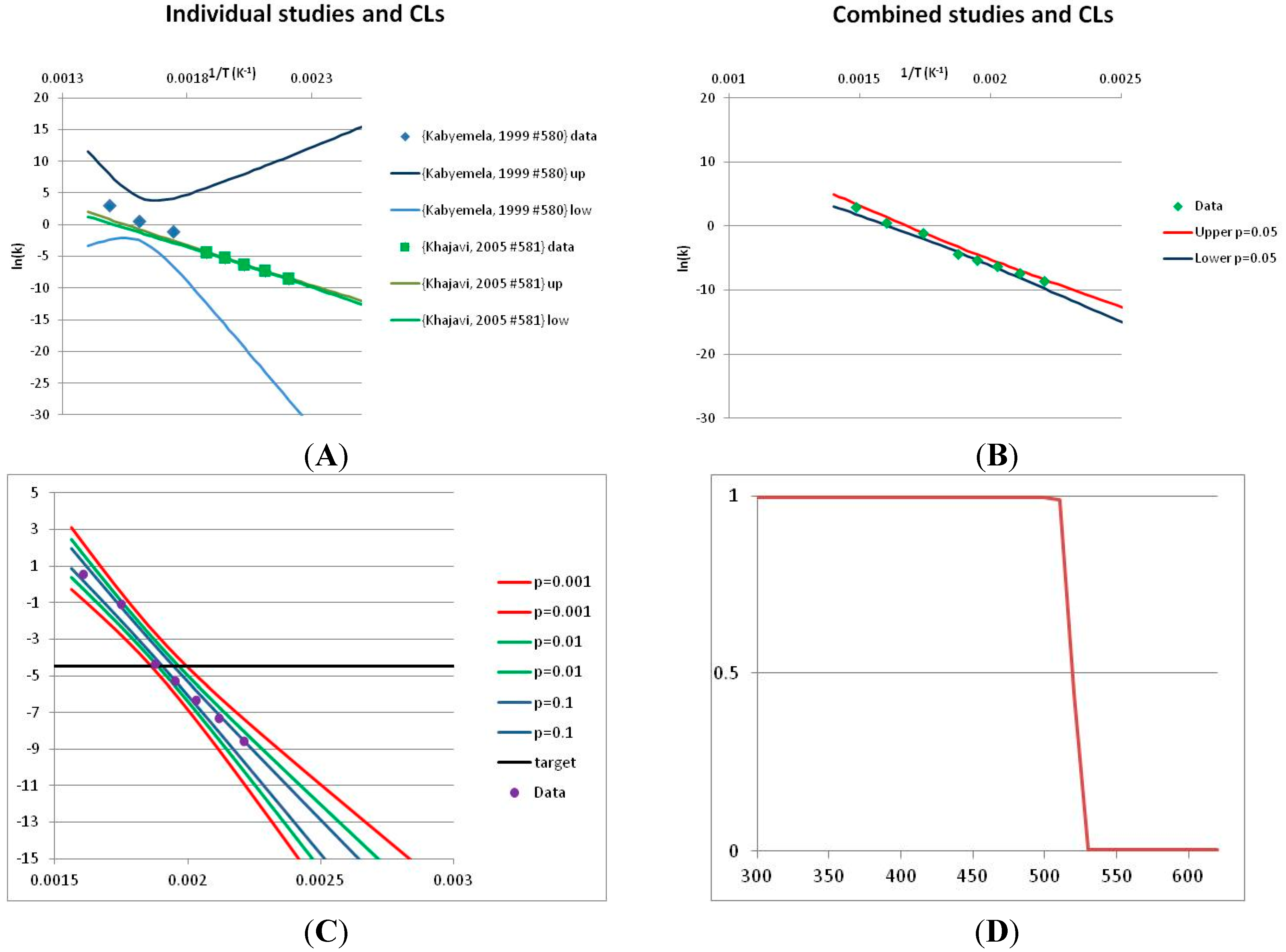
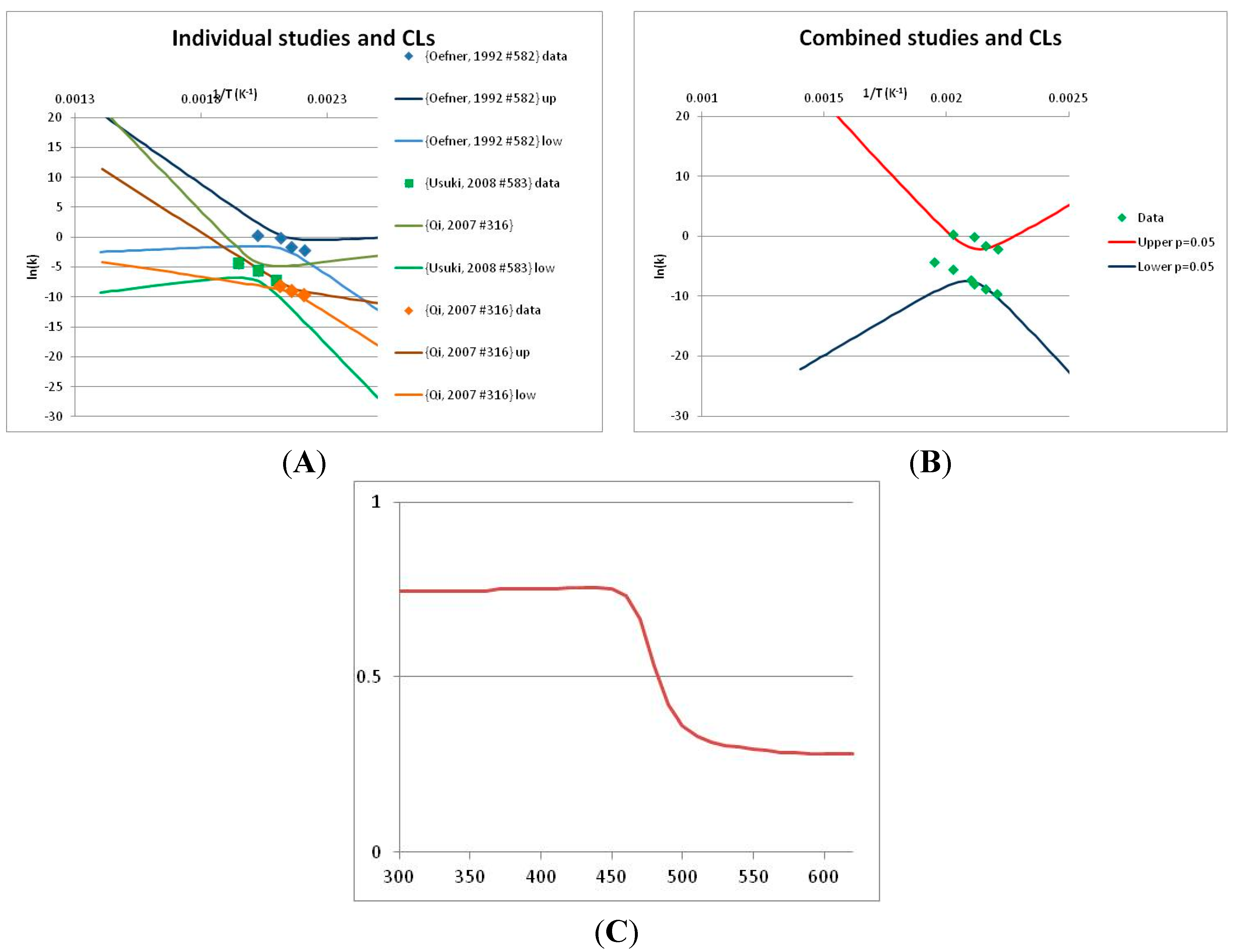
3.2. Definition of Stability
| Category | Code | Specific Metabolites Analysed in This Study | Half-Life | References for Half-Life | |
|---|---|---|---|---|---|
| Long | Short | ||||
| Amino acids | A | Alanine, Alpha-amino butyrate, Asparagine, Aspartate, Glycine, Histidine, Isoleucine, Leucine, Methionine, Phenylalanine, Proline, Serine, Threonine, Tyrosine, Valine | 200 | 20 | [56,57,58,59] |
| Sugars | S | Arabinose, Cellulobiose, Fructose, Galactose, Glucose, Isomaltose, Lactose, Lyxose, Maltose, Mannose, Melibiose, Palatinose, Ribose, Sucrose, Trehalose, Turanose, Xylose | 600 | 60 | [60] |
| Sugar derivatives | D | Glucosamine, N-acetyl glucosamine, | (a) | (a) | |
| Lipids | L | Long chain triglycerides, Phosphatidyl choline | 500 | 5000 | [61,62] |
| Nucleotides and their components | N | Adenine, Adenosine, Adenosine, Cytidine, Cytosine, Deoxyadenosine, Guanine, Thymidine, Uracil | 100 | 10 | [15] |
| Glycolytic intermediates | G | Dihydroxyacetone, Glyceraldehyde, Fructose-1,6-diphosphate, Pyruvate | 10 | 1 | [63,6465] |
| Tricarboxylic acid cycle intermediates | T | Fumarate, Oxaloacetate | 10 | 1 | [60,63] |
| Other intermediary metabolism components | I | 2-Dimethylaminoethanethiol Propionate (thioester analogue), Carbamoyl phosphate, Formic acid, Hypoxanthine, Malonate, Mandelic Acid, Orotic acid, Urea, Xanthine | (b) | (b) | |
| Energy carriers | E | AMP, ATP | 30 | 3 | [60,66] |
| Other carrier molecules | C | Coenzyme A, NADH | 500 (c) | 50 (c) | [67] |
3.3. Summary of Results
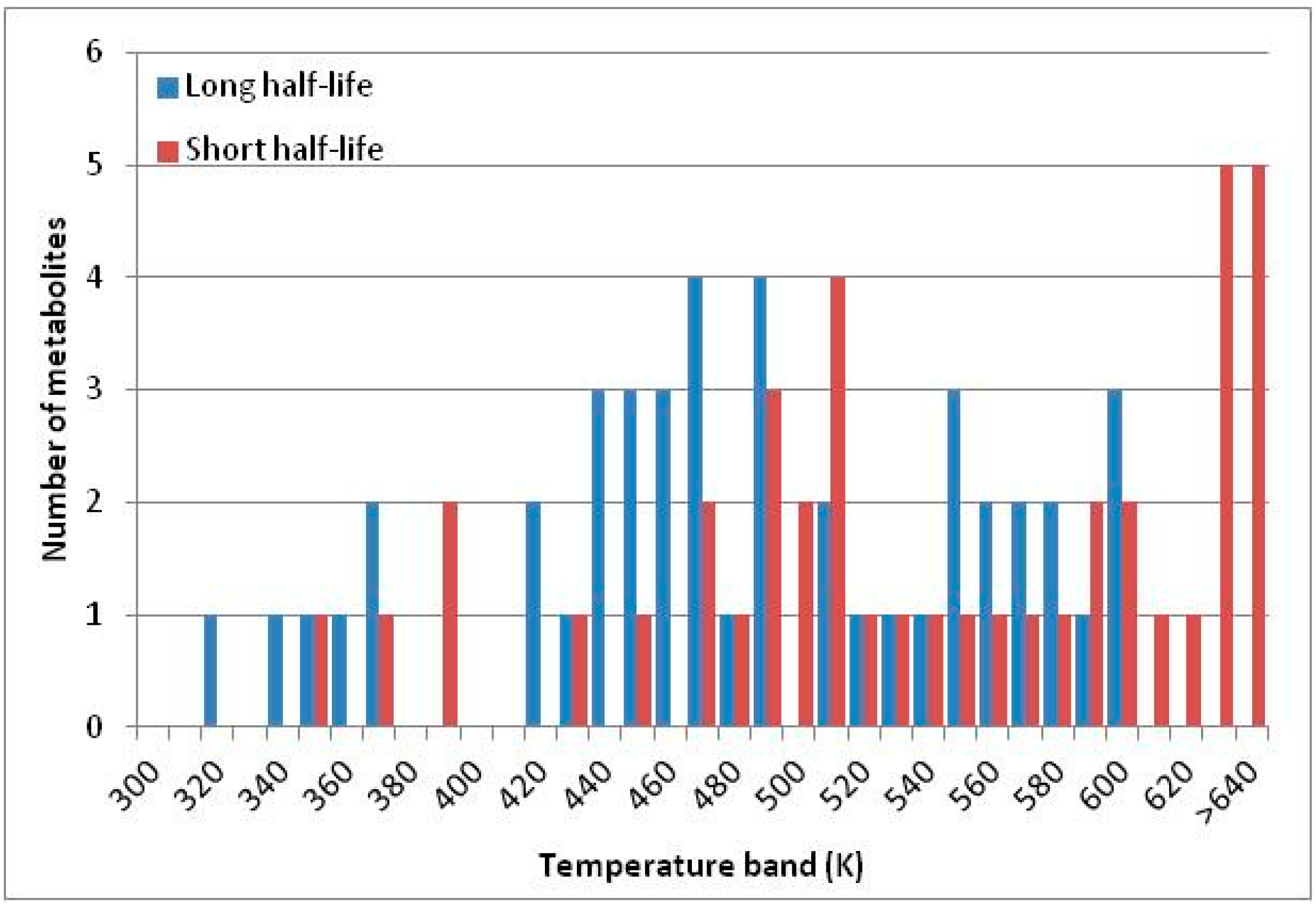

| Metabolite | Short Half-Life Vulnerable? | Category |
|---|---|---|
| 2-Dimethylaminoethanethiol Propionate (thio-ester analogue) | √ | Labile |
| Asparagine | - | Labile |
| ATP | - | Labile |
| Carbamoyl phosphate | √ | Labile |
| Deoxyadenosine | - | Labile |
| Glyceraldehyle | √ | Uncertain |
| Histidine | - | Uncertain |
| Long-chain triglycerides | √ | Uncertain |
| Lyxose | √ | Uncertain |
| NADH | √ | Labile |
| Oxaloacetate | √ | Uncertain |
| Palatinose | √ | Uncertain |
| Sucrose | √ | Uncertain |
| Xylose | √ | Uncertain |
4. Discussion
4.1. Pressure Effects
4.2. Limitations of This Study
5. Conclusions
Acknowledgments
Author Contributions
Appendix
| Compound | Metabolite Group Code | Degradation References | Combined Data | Degradation Notes | Metabolic Half-Life | Ref for Half-Life | Stability Confidence Curve | T(p = 0.5) – long | T(p = 0.5) – short |
|---|---|---|---|---|---|---|---|---|---|
| 2-Dimethylaminoethanethiol Propionate (thio-ester analogue) | I | [99] |  | Included as an analogue of Thioesters |  | 345.7 | 384.7 | ||
| Adenine | N | [100] |  |  | 586 | 668.8 | |||
| Adenosine | N | [101,102] |  | Includes deamination and cleavage of glycosidiv bond |  | 482.9 | 513.9 | ||
| Alanine | A | [103,104,105,106] |  | 20 | [56] |  | 568.4 | 597.8 | |
| Alpha-amino butyrate | A | [105] |  |  | 586.7 | 620 | |||
| AMP | E | [101] |  | 120 | [60] |  | 440.7 | 475.7 | |
| Arabinose | S | [49] |  | Marginal data quality, analysed anyway. |  | 480.6 | 528.5 | ||
| Asparagine (Side-chain) | A | [107] |  |  | 385.4 | 419.8 | |||
| Aspartate | A | [106,108,109] |  | 150 | [56] |  | 499.3 | 542.4 | |
| ATP | E | [25,101] |  | 45 1 | [60,66,110] |  | 414.5 | 442.3 | |
| Carbamoyl phosphate | I | [55] |  | 5 |  | 362 | 396.7 | ||
| Cellulobiose | S | [111] |  |  | 477.9 | 513.2 | |||
| Coenzyme A | C | [112] |  | 120 |  | 507.6 | 699 | ||
| Cytidine | N | [113] [skipped – inconsistent with three others] [114,115,116] |  | Data from Snider was inconsistent with other data sets, and so was excluded |  | 465.1 | 497 | ||
| Cytosine | N | [100,114,117] |  |  | 467.7 | 495.8 | |||
| Deoxyadenosine (deamination) | N | [118] |  |  | 377.5 | 463 | |||
| Dihydroxyacetone | G | [119] |  | 271 2.7 10 | [63] [64] [65] |  | 552.5 | 654.8 | |
| Formic acid | I | [120,121] |  |  | 614.4 | 692.6 | |||
| Fructose | S | [46,47] |  |  | 484 | 519.6 | |||
| Fructose-1,6-diphosphate | G | [122] |  | 0.6 10 | [64] [65] |  | 467.1 | 511.8 | |
| Fumarate | T | [123] |  | 310 0.9 4 | [63] [64] [124] |  | 643 | 720.1 | |
| Galactose | S | [47] |  |  | 495.6 | 520.6 | |||
| Glucosamine | D | [125] |  |  | 492.1 | 551.3 | |||
| Glucose | S | [46,47,50] |  | 600 | [60] |  | 499.6 | 537.3 | |
| Glyceraldehyde | G | [119] |  | 2.7 10 | [64] [65] |  | 420.9 | 685.3 | |
| Glycine | A | [103,105,106,126,127] |  | 30 | [56] |  | 533.9 | 608.8 | |
| Guanine | N | [100] |  |  | 551.5 | 605.7 | |||
| Histidine | A | [105,128] |  |  | 873.6 | 7626.1 | |||
| Hypoxanthine | I | [100] |  |  | 516.9 | 572.2 | |||
| Isoleucine | A | [103,105,128] |  |  | 582.3 | 620.8 | |||
| Isomaltose | S | [111] |  |  | 478.4 | 509.1 | |||
| Lactose | S | [111] |  |  | 450.9 | 499.4 | |||
| Leucine | A | [103,105,128,129,130] |  | 45 | [57,58] |  | 563.8 | 601.8 | |
| Long chain triglycerides | L | [131] |  |  | 340.1 | 397.8 | |||
| Lyxose | S | [49] |  |  | 474.9 | 518.2 | |||
| Malonate | I | [132,133] |  |  | 456.4 | 515.9 | |||
| Maltose | S | [47,111] |  |  | 470.3 | 509.7 | |||
| Mandelic Acid (racemization) | I |  |  | 605 | 665.2 | ||||
| Mannose | S | [47] |  |  | 463.9 | 515.7 | |||
| Melibiose | S | [111] |  |  | 462 | 494 | |||
| Methionine | A | [103,105,128] |  |  | 546.5 | 581.3 | |||
| N-acetyl glucosamine | D | [125] |  |  | 429.5 | 458.8 | |||
| NADH | C | [27,28,29,30,31] |  |  | 352.3 | 372.2 | |||
| Orotic acid | I | [134] |  |  | 605 | 665.2 | |||
| Oxaloacetate | T | [135] |  | 319 1000 | 2.7 |  | 328.5 | 355.8 | |
| Palatinose | S | [111] |  |  | 459.1 | 493.3 | |||
| Phenylalanine | A | [103,105,128,129] |  | 180 | [59] |  | 569.1 | 613.6 | |
| Phosphatidyl choline | L | [136] |  | 3000 (phosphatidyl glycerol) 3600 | [61,62] |  | 472.3 | 544.3 | |
| Proline | A | [105] |  |  | 601.1 | 640.1 | |||
| Pyruvate | G | [137] |  | 294 | [63] |  | 570.9 | 620.6 | |
| Ribose | S | [49,138] |  |  | 440.4 | 516.3 | |||
| Serine | A | [103,105,106,128] |  |  | 525.7 | 560.9 | |||
| Sucrose | S | [139] |  |  | 375.5 | 430.3 | |||
| Threonine | A | [105] |  |  | 516 | 554.7 | |||
| Thymidine | N | [100] |  |  | 552.4 | 597.1 | |||
| Trehalose | S | [111] |  |  | 496.1 | 525.8 | |||
| Turanose | S | [111] |  |  | 449.3 | 478.1 | |||
| Tyrosine | A | [128] |  |  | 589.5 | 710.4 | |||
| Uracil | N | [100] |  |  | 552.4 | 597.1 | |||
| Urea | I | [140] |  |  | 446.3 | 475.5 | |||
| Valine | A | [103,105,130] |  | 40 | [56] |  | 577.4 | 600.6 | |
| Xanthine | I | [100] |  |  | 595 | 660.5 | |||
| Xylose | S | [48,49,50] |  |  | 434 | 482.3 |
Conflicts of Interest
References
- Clarke, A. The thermal limits to life on Earth. Int. J. Astrobiol. 2014, 13, 141–154. [Google Scholar] [Green Version]
- Bains, W. Hypotheses, limits, models and Life. Life 2015, 5, 1–3. [Google Scholar]
- Clarke, A.; Morris, G.J.; Fonseca, F.; Murray, B.J.; Acton, E.; Price, H.C. A Low Temperature Limit for Life on Earth. PLoS One 2013, 8, e66207. [Google Scholar]
- Takai, K.; Nakamura, K.; Toki, T.; Tsunogai, U.; Miyazaki, M.; Miyazaki, J.; Hirayama, H.; Nakagawa, S.; Nunoura, T.; Horikoshi, K. Cell proliferation at 122 °C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc. Natl. Acad. Sci. USA 2008, 105, 10949–10954. [Google Scholar]
- Kashefi, K.; Lovley, D.R. Extending the upper temperature limit for life. Science 2003, 301, 934. [Google Scholar]
- Deming, J.W.; Baross, J.A. Deep-sea smokers: Windows to a subsurface biosphere? Geochim. Cosmochim. Acta 1993, 57, 3219–3230. [Google Scholar]
- Cowan, D.A. The upper temperature for life—where do we draw the line? Trends Microbiol. 2004, 12, 58–60. [Google Scholar]
- Holden, J. Some like it hot: understanding the limits of life using hyperthermophilic microbes. In Proceedings of 37th COSPAR Scientific Assembly, Montreal, Canada, 13–20 July 2008; p. 1259.
- Kelley, D.S.; Girguis, P.R.; Wheat, G.; Cordes, E.; Schrenk, M.O.; Lin, M.; Baross, J.A.; Delaney, J.R. Towards Determining the Upper Temperature Limit to Life. Available online: http://adsabs.harvard.edu/abs/2007AGUFM.V23D..02K (accessed on 6 March 2015).
- Danson, M.J.; Hough, D.W. The enzymology of archebacterial pathways of central metabolism. In Biochemical Society Symposium: The archebacteria: Biochemistry and Biotechnology; Danson, M.J., Hough, D.W., Lunt, G.G., Eds.; Portland Press: London, UK, 1992; pp. 7–22. [Google Scholar]
- Katritzky, A.R.; Allin, S.M.; Siskin, M. Aquathermolysis: Reactions of organic compounds with superheated water. Acc. Chem. Res. 1996, 29, 399–406. [Google Scholar]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant 2. Degradation reactions. J. Supercrit. Fluids 2007, 41, 361–379. [Google Scholar]
- Wolfenden, R. Degrees of Difficulty of Water-Consuming Reactions in the Absence of Enzymes. Chem. Rev. 2006, 106, 3379–3396. [Google Scholar]
- Savage, P.E. Organic chemical reactions in supercritical water. Chem. Rev. 1999, 99, 603–621. [Google Scholar]
- Neuhard, J.; Thomassen, E. Turnover of the Deoxyribonucleoside Triphosphates in Escherichia coli 15 T during Thymine Starvation. Eur. J. Biochem. 1971, 20, 36–43. [Google Scholar]
- Lang, E.W. Physical-chemical limits for the stability of biomolecules. Adv. Space Res. 1986, 6, 251–255. [Google Scholar]
- White, R.H. Hydrolytic stability of biomolecules at high temperatures and its implications for life at 250 °C. Nature 1984, 310, 430–432. [Google Scholar]
- Bernhardt, G.; Lüdemann, H.D.; Jaenicke, R.; König, H.; Stetter, K.O. Biomolecules are unstable under “black smoker” conditions. Naturwissenschaften 1984, 71, 583–586. [Google Scholar]
- Trent, J.D.; Chastain, R.A.; Yayanos, A.A. Possible artefactual basis for apparent bacterial growth at 250 °C. Nature 1984, 307, 737–740. [Google Scholar]
- Jaenicke, R. Protein stability and molecular adaptation to extreme conditions. Eur. J. Biochem. 1991, 202, 715–728. [Google Scholar]
- Committee on the origins and Evolution of Life. The limits of Organic Life in Planetary Systems; National Research Council: Washington, DC, USA, 2007. [Google Scholar]
- Schofield, L.R.; Patchett, M.L.; Parker, E.J. Expression, purification, and characterization of 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase from Pyrococcus furiosus. Protein Expr. Purif. 2004, 34, 17–27. [Google Scholar]
- Daniel, R.M.; Danson, M.J. Assaying Activity and Assessing Thermostability of Hyperthermophilic Enzymes. Methods Enzymol. 2001, 334, 283–293. [Google Scholar]
- Daniel, R.M.; Cowan, D.A. Biomolecular stability and life at high temperatures. Cell. Mol. Life Sci. 2000, 57, 250–264. [Google Scholar]
- Daniel, R.M.; van Eckert, R. The stability of biomolecules and the implications for life at high temperatures. In The Subseafloor Biosphere at Mid-Ocean Ridges: AGU Monograph 144; Wilcock, W.S.D., Delong, E.F., Kelley, D.S., Baross, J.A., Cary, S.C., Eds.; American Geophysical Union: Washington, DC, USA, 2004. [Google Scholar]
- Consalvi, V.; Chiaraluce, R.; Politi, L.; Vaccaro, R.; De Rosa, M.; Scandurra, R. Extremely thermostable glutamate dehydrogenase from the hyperthermophilic archaebacterium Pyrococcus furiosus. Eur. J. Biochem. 1991, 202, 1189–1196. [Google Scholar]
- McComb, R.B.; Bond, L.W.; Burnett, R.W.; Keech, R.C.; Bowers, G.N., Jr. Determination of the molar absorptivity of NADH. Clin. Chem. 1976, 22, 141–150. [Google Scholar]
- Wu, J.T.; Wu, L.H.; Knight, J.A. Stability of NADPH: Effect of various factors on the kinetics of degradation. Clin. Chem. 1986, 32, 314–319. [Google Scholar]
- Hudson, R.C.; Ruttersmith, L.D.; Daniel, R.M. Glutamate dehydrogenase from the extremely thermophilic archebacterial isolate AN1. Biochem. Biophys. Acta 1993, 1202, 244–250. [Google Scholar]
- Robb, F.T.; Park, J.-B.; Adams, M.W.W. Characterization of an extremely thermostable glutame dehydrogenase: A key enzyme in the primary metabolism of the hyperthermophilic archaebacterium Pyrococcus furiosus. Biochem. Biophys. Acta 1992, 1120, 267–272. [Google Scholar]
- Walsh, K.A.J.; Daniel, R.M.; Morgan, H.W. A soluble NADH dehydrogenase (NADH:ferricyanide oxireductase) from Thermus aquaticus strain T351. Biochem. J. 1983, 209, 427–433. [Google Scholar]
- Nicotri, M.E. Factors involved in herbivore food preference. J. Exp. Mar. Biol. Ecol. 1980, 42, 13–26. [Google Scholar]
- Bratbak, G.; Dundas, I. Bacterial dry matter content and biomass estimations. Appl. Environ. Microbiol. 1984, 48, 755–757. [Google Scholar]
- Ricketts, T.R. On the chemical composition of some unicellular algae. Phytochemistry 1966, 5, 67–76. [Google Scholar]
- Bakken, L.R.; Olsen, R.A. Buoyant Densities and Dry-Matter Contents of Microorganisms: Conversion of a Measured Biovolume into Biomass. Appl. Environ. Microbiol. 1983, 45, 1188–1195. [Google Scholar]
- Lang, G.; Reiners, W.; Heier, R. Potential alteration of precipitation chemistry by epiphytic lichens. Oecologia 1976, 25, 229–241. [Google Scholar]
- Chandler, S.F.; Thorpe, T.A. Characterization of Growth, Water Relations, and Proline Accumulation in Sodium Sulfate Tolerant Callus of Brassica napus L. cv Westar (Canola). Plant Physiol. 1987, 84, 106–111. [Google Scholar]
- Black, M.; Corbineau, F.; Gee, H.; Côme, D. Water Content, Raffinose, and Dehydrins in the Induction of Desiccation Tolerance in Immature Wheat Embryos. Plant Physiol. 1999, 120, 463–472. [Google Scholar]
- Xiao, Y. Chemical Kinetic Estimate of the Maximum Temperature for Life; Department of Chemical Engineering And Biotechnology, University of Cambridge: Cambridge, UK, 2014. Available online: http://www.undergraduate.study.cam.ac.uk/courses/chemical-engineering (accessed on 19 March 2015).
- Yu, C. Chemical Kinetic Estimate of the Maximum Temperature for Life; Department of Chemical Engineering And Biotechnology, University of Cambridge: Cambridge, UK, 2014. Available online: http://www.undergraduate.study.cam.ac.uk/courses/chemical-engineering (accessed on 19 March 2015).
- Antal, M.J.; Allen, S.G.; Schulman, D.; Xu, X.; Divilio, R.J. Biomass gasification in supercritical water. Ind. Eng. Chem. Res. 2000, 39, 4040–4053. [Google Scholar]
- Chakinala, A.G.; Brilman, D.W.F.; van Swaaij, W.P.M.; Kersten, S.R.A. Catalytic and non-catalytic supercritical water gasification of microalgae and glycero. Ind. Eng. Chem. Res. 2010, 48, 1113–1122. [Google Scholar]
- Lu, Y.J.; Guo, L.J.; Ji, C.M.; Zhang, X.M.; Hao, X.H.; Yan, Q.H. Hydrogen production by biomass gasification in supercritical water: a parametric study. Intl. J. Hydrog. Energy 2006, 31, 822–831. [Google Scholar]
- Matsumura, Y.; Sasaki, M.; Okuda, K.; Takami, S.; Ohara, S.; Umetsu, M.; Adschiri, T. Supercritical water treatment of biomass for energy and material recovery. Combust. Sci. Technol. 2006, 178, 509–536. [Google Scholar]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Froling, M.; Antal, J.M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar]
- Kabyemela, B.M.; Adschiri, T.; Malaluan, R.M.; Arai, K. Glucose and Fructose Decomposition in Subcritical and Supercritical Water: Detailed Reaction Pathway, Mechanisms, and Kinetics. Ind. Eng. Chem. Res. 1999, 38, 2888–2895. [Google Scholar]
- Khajavi, S.H.; Kimura, Y.; Oomori, T.; Matsuno, R.; Adachi, S. Degradation kinetics of monosaccharides in subcritical water. J. Food Eng. 2005, 68, 309–313. [Google Scholar]
- Oefner, P.J.; Lanziner, A.H.; Bonn, G.; Bobleter, O. Quantitative studies on furfural and organic acid formation during hydrothermal, acidic and alkaline degradation of D-xylose. Monatshefte für Chemie 1992, 123, 547–556. [Google Scholar]
- Usuki, C.; Kimura, Y.; Adachi, S. Degradation of Pentoses and Hexouronic Acids in Subcritical Water. Chem. Eng. Technol. 2008, 31, 133–137. [Google Scholar]
- Qi, J.; Xiuyang, L. Kinetics of non-catalysed decomposition of D-xylose in high temperature liquid water. Chin. J. Chem. Eng. 2007, 15, 666–669. [Google Scholar]
- Jing, Q.; LÜ, X. Kinetics of Non-catalyzed Decomposition of D-xylose in High Temperature Liquid Water. Chin. J. Chem. Eng. 2007, 15, 666–669. [Google Scholar]
- Bundy, F.P.; Bassett, W.A.; Weathers, M.S.; Hemley, R.J.; Mao, H.U.; Goncharov, A.F. The pressure-temperature phase and transformation diagram for carbon, updated through 1994. Carbon 1996, 34, 141–153. [Google Scholar]
- Tateyama, Y.; Ogitsu, T.; Kusakabe, K.; Tsuneyuki, S. Constant-pressure first-principles studies on the transition states of the graphite-diamond transformation. Phys. Rev. B 1996, 54, 14954–15001. [Google Scholar]
- Massant, J. How thermophiles cope with thermolabile metabolites. In Physiology and Biochemistry of Extremophiles; Gerday, G., Glansdorff, N., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 57–74. [Google Scholar]
- Marbaix, A.Y.; Noel, G.; Detroux, A.M.; Vertommen, D.; Van Schaftingen, E.; Linster, C.L. Extremely Conserved ATP- or ADP-dependent Enzymatic System for Nicotinamide Nucleotide Repair. J. Biol. Chem. 2011, 286, 41246–41252. [Google Scholar]
- Yuan, J.; Doucette, C.D.; Fowler, W.U.; Feng, X.J.; Piazza, M.; Rabitz, H.A.; Wingreen, N.S.; Rabinowitz, J.D. Metabolomics-driven quantitative analysis of ammonia assimilation in E. coli. Mol. Syst. Biol. 2009, 5. [Google Scholar] [CrossRef]
- Pine, M.J. Steady-state measurement of the turnover of amino acid in the cellular proteins of growing Escherichia coli: Existence of two kinetically distinct reactions. J. Bacteriol. 1970, 103, 207–215. [Google Scholar]
- Pine, M.J. Turnover of intracellular proteins. Ann. Rev. Microbiol. 1972, 26, 103–126. [Google Scholar]
- Brown, K.D. Maintenance and Exchange of the Aromatic Amino Acid Pool in Escherichia coli. J. Bacteriol. 1971, 106, 70–81. [Google Scholar]
- Enjalbert, B.; Letisse, F.; Portais, J.-C. Physiological and Molecular Timing of the Glucose to Acetate Transition in Escherichia coli. Metabolites 2013, 3, 820–837. [Google Scholar]
- Kanfer, J.; Kennedy, E.P. Metabolism and Function of Bacterial Lipids: I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J. Biol. Chem. 1963, 238, 2919–2922. [Google Scholar]
- Golden, N.G.; Powell, G.L. Stringent and Relaxed Control of Phospholipid Metabolism in Escherichia coli. J. Biol. Chem. 1972, 247, 6651–6658. [Google Scholar]
- Schaub, J.; Reuss, M. In vivo dynamics of glycolysis in Escherichia coli shows need for growth-rate dependent metabolome analysis. Biotechnol. Progress 2008, 24, 1402–1407. [Google Scholar]
- Taymaz-Nikerel, H.; de Mey, M.; Ras, C.; ten Pierick, A.; Seifar, R.M.; van Dam, J.C.; Heijnen, J.J.; van Gulik, W.M. Development and application of a differential method for reliable metabolome analysis in Escherichia coli. Anal. Biochem. 2009, 386, 9–19. [Google Scholar]
- Millard, P.; Massou, S.; Wittmann, C.; Portais, J.C.; Letisse, F. Sampling of intracellular metabolites for stationary and non-stationary 13C metabolic flux analysis in Escherichia coli. Anal. Biochem. 2014, 465, 38–49. [Google Scholar]
- Chapman, A.G.; Atkinson, D.E. Adenine Nucleotide Concentrations and Turnover Rates. Their Correlation with Biological Activity in Bacteria and Yeast. In Advances in Microbial Physiology; Wilkinson, J.F., Ed.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 253–306. [Google Scholar]
- Vallari, D.S.; Jackowski, S. Biosynthesis and degradation both contribute to the regulation of coenzyme A content in Escherichia coli. J. Bacteriol. 1988, 170, 3961–3966. [Google Scholar]
- Legrain, C.; Demarez, M.; Glansdorff, N.; Piérard, A. Ammonia-dependent synthesis and metabolic channelling of carbamoyl phosphate in the hyperthermophylic archaeon Pyrococcus furiosus. Microbiology 1995, 141, 1093–1099. [Google Scholar]
- Sterner, R.; Kleemann, G.R.; Szadkowski, H.; Lustig, A.; Kirschner, K.; Hennig, M. Phosphoribosyl anthranilate isomerase from Thermutuga maritima is an extremely stable and activehomodime. Protein Sci. 1996, 5, 2000–2008. [Google Scholar]
- Sanchez, R.; Baetens, M.; van De Casteele, M.; Roovers, M.; Legrain, C.; Glansdorff, N. Ornithine Carbamoyltransferase from the Extreme Thermophile Thermus Thermophilus Analysis of the Gene and Characterisation of the Protein. Eur. J. Biochem. 1997, 248, 466–474. [Google Scholar]
- Daniel, R.M.; Danson, M.J. Did primitive microorganisms use non-hem iron proteins in place of NAD/P? J. Mol. Evol. 1995, 40, 559–563. [Google Scholar]
- Kengen, S.W.M.; Tuininga, J.E.; de Bok, F.A.M.; Stams, A.J.M.; de Vos, W.M. Purification and Characterization of a Novel ADP-dependent Glucokinase from the Hyperthermophilic Archaeon Pyrococcus furiosus. J. Biol. Chem. 1995, 270, 30453–30457. [Google Scholar]
- Dörr, C.; Zaparty, M.; Tjaden, B.; Brinkmann, H.; Siebers, B. The Hexokinase of the Hyperthermophile Thermoproteus tenax: ATP-dependent hexokinases and ADP-dependent glucokinases, two alternatives for glucose phjosphorylation in Archaea. J. Biol. Chem. 2003, 278, 18744–18753. [Google Scholar]
- Schönheit, P.; Brandis, A.; Thauer, R. Ferredoxin degradation in growing Clostridium pasteurianum during periods of iron deprivation. Arch. Microbiol. 1979, 120, 73–76. [Google Scholar]
- Kates, M. Biology of halophilic bacteria, Part II. Experientia 1993, 49, 1027–1036. [Google Scholar]
- Villanueva, L.; Damste, J.S.S.; Schouten, S. A re-evaluation of the archaeal membrane lipid biosynthetic pathway. Nat. Rev. Micro 2014, 12, 438–448. [Google Scholar]
- Le Noble, W.J.; Daka, M.R. Kinetics of reactions in solutions under pressure. 52. Effect of pressure on concerted and stepwise sigmatropic shifts. J. Am. Chem. Soc. 1978, 100, 5961–5962. [Google Scholar]
- Gonikberg, M.G. Chemical Equilibria and Reaction Rates at High Pressures (Tranls J. Schmorak); National Science Foundation: Washington, DC, USA, 1960. [Google Scholar]
- Eckert, C.A. High pressure kinetics in solution. Ann. Rev. Phys. Chem. 1972, 23, 239–264. [Google Scholar]
- Iyer, S.D.; Klein, M.T. Effect of pressure on the rate of butyronitrile hydrolysis in high-temperature water. J. Supercrit. Fluids 1997, 10, 191–200. [Google Scholar]
- Laidler, K.J.; Chen, D. The influence of pressure on the kinetics of the alkaline hydrolysis of esters and amides. Trans. Faraday Soc. 1958, 54, 1026–1033. [Google Scholar]
- Baliga, B.T.; Whalley, E. Pressure effect and mechanism in the acid-catalysed hydration of propylene and isobutylene. Can. J. Chem. 1964, 42, 1019–1026. [Google Scholar]
- Gay, D.L.; Whalley, E. Effect of pressure on the solvolysis of benzyl chloride in glycerol-water mixtures. Can. J. Chem. 1970, 48, 2021–2024. [Google Scholar]
- Mackinnon, M.; Lateef, A.B.; Hyne, J.B. Transition state volumes and solvolysis mechanisms. Can. J. Chem. 1970, 48, 2025–2030. [Google Scholar]
- Le Noble, W.J.; Srivastava, S.; Breslow, R.; Trainor, G. Effect of pressure on two cyclodextrin-promoted ester hydrolyses. J. Am. Chem. Soc. 1983, 105, 2745–2748. [Google Scholar]
- Taniguchi, Y.; Makimoto, S.; Suzuki, K.Z. Pressure effects on the hydrolysis of p-nitrophenyl and 2-naphthyl acetates catalysed by cyclodextrins. J. Phys. Chem. 1981, 85, 3469–3472. [Google Scholar]
- Koskikallio, J.; Pouli, D.; Whalley, E. Pressure effect and mechanism in acid catalysis. V. The hydrolysis of acetic anhydride. Can. J. Chem. 1959, 37, 1360–1366. [Google Scholar]
- Le Noble, W.J. Kinetics of reactions in solutions under pressure. In Progress in Physical Organic Chemistry v 5; Streitwieser, A.J., Taft, R.W., Eds.; Interscience Publishers: New York, NY, USA, 1967; pp. 207–330. [Google Scholar]
- Koskikallio, J.; Whalley, E. Effect of pressure on the spontaneous and the base-catalysed hydrolysis of epoxides. Trans. Faraday Soc. 1959, 37, 783–787. [Google Scholar]
- Osborn, A.R.; Whalley, E. Pressure effect and mechanism in acid catalysis. VII. Hydrolysis of methyl, ethyl and t-butyl acetates. Can. J. Chem. 1961, 39, 1101–1108. [Google Scholar]
- Farr, D. High pressure technology in the food industry. Trends Food Sci. Technol. 1990, 1, 14–16. [Google Scholar]
- Gross, M.; Jaenicke, R. Proteins under pressure. The influence of high hydrostatic pressure on structure, function and assembly of proteins and protein complexes. Eur. J. Biochem. 1994, 221, 617–630. [Google Scholar]
- Schmid, G.; Ludemann, H.-D.; Jaenicke, R. Oxidation of sulfhydryl groups in lactate dehydrogenase under high hydrostatic pressure. Eur. J. Biochem. 1978, 86, 219–224. [Google Scholar]
- Aoki, K.; Hiramatsu, K.; Tanaka, M.; Kaneshina, S. Bovine serum albumin exposed to high pressure. Biochem. Biophys. Acta 1968, 160, 368–377. [Google Scholar]
- Demazeau, G.; Rivalain, N. High hydrostatic pressure and biology: A brief review. Appl. Microbiol. Biotechnol. 2011, 89, 1305–1314. [Google Scholar]
- Shock, E.L. Do amino acids equilibrate in hydrothermal fluids? Geochim. Cosmochim. Acta 1990, 54, 1185–1189. [Google Scholar]
- Shock, E.L. Stability of peptides in high-temperature aqueous solutions. Geochim. Cosmochim. Acta 1992, 56, 3481–3491. [Google Scholar]
- Szwergold, B.S. Maillard Reactions in Hyperthermophilic Archaea: Implications for Better Understanding of Non-Enzymatic Glycation in Biology. Rejuvenation Res. 2013, 16, 259–272. [Google Scholar]
- Hussain, A.; Schurman, P. Thiol Esters 11: A Kinetic Study of Hydrolysis and Aminolysis of Propionyl Thiocholine Iodide and 2-Dimethylaminoethanethiol Propionate. J. Pharm. Sci. 1969, 58, 687–693. [Google Scholar]
- Levy, M.; Miller, S.L. The stability of the RNA bases: Implications for the origin of life. Proc. Natl. Acad. Sci. USA 1998, 95, 7933–7938. [Google Scholar]
- Kawamura, K. Monitoring Hydrothermal Reactions on the Millisecond Time Scale Using a Micro-Tube Flow Reactor and Kinetics of ATP Hydrolysis for the RNA World Hypothesis. Bull. Chem. Soc. Jpn. 2000, 73, 1805–1811. [Google Scholar]
- Stockbridge, R.B.; Schroeder, G.K.; Wolfenden, R. The rate of spontaneous cleavage of the glycosidic bond of adenosine. Bioorganic Chem. 2010, 38, 224–228. [Google Scholar]
- Abdelmoez, W.; Yoshida, H.; Nakahasi, T. Pathways of Amino Acid Transformation and Decomposition in Saturated Subcritical Water Conditions. Int. J. Chem. Reactor Eng. 2010, 8. [Google Scholar] [CrossRef]
- Cox, J.S.; Seward, T.M. The reaction kinetics of alanine and glycine under hydrothermal conditions. Geochim. Cosmochim. Acta 2007, 71, 2264–2284. [Google Scholar]
- Li, J.; Brill, T.B. Spectroscopy of Hydrothermal Reactions 25: Kinetics of the Decarboxylation of Protein Amino Acids and the Effect of Side Chains on Hydrothermal Stability. J. Phys. Chem. A 2003, 107, 5987–5992. [Google Scholar]
- Sato, N.; Quitain, A.T.; Kang, K.; Daimon, H.; Fujie, K. Reaction Kinetics of Amino Acid Decomposition in High-Temperature and High-Pressure Water. Ind. Eng. Chem. Res. 2004, 43, 3217–3222. [Google Scholar]
- Geiger, T.; Steven, C. Deamidation, Isomerization, and Racemization at Asparaginyl and Aspartyl Residues in Peptides. J. Biol. Chem. 1987, 262, 785–794. [Google Scholar]
- Cox, J.S.; Seward, T.M. The hydrothermal reaction kinetics of aspartic acid. Geochim. Cosmochim. Acta 2007, 71, 797–820. [Google Scholar]
- Faisal, M.; Sato, N.; Quitain, A.T.; Daimon, H.; Fujie, K. Reaction kinetics and pathway of hydrothermal decomposition of aspartic acid. Int. J. Chem. Kinet. 2007, 39, 175–180. [Google Scholar]
- Holms, W.H.; Hamilton, I.D.; Robertson, A.G. The rate of turnover of the adenosine triphosphate pool of Escherichia coli growing aerobically in simple defined media. Archiv für Mikrobiologie 1972, 83, 95–109. [Google Scholar]
- Oomori, T.; Khajavi, S.H.; Kimura, Y.; Adachi, S.; Matsuno, R. Hydrolysis of disaccharides containing glucose residue in subcritical water. Biochem. Eng. J. 2004, 18, 143–147. [Google Scholar]
- Buyske, D.A.; Handschumacher, R.E.; Schilling, E.D.; Strong, F.M. The Stability of Coenzyme A1. J. Am. Chem. Soc. 1954, 76, 3575–3577. [Google Scholar]
- Snider, M.J.; Gaunitz, S.; Ridgway, C.; Short, S.A.; Wolfenden, R. Temperature Effects on the Catalytic Efficiency, Rate Enhancement, and Transition State Affinity of Cytidine Deaminase, and the Thermodynamic Consequences for Catalysis of Removing a Substrate “Anchor”. Biochemistry 2000, 39, 9746–9753. [Google Scholar]
- Garrett, E.R.; Tsau, J. Solvolysis of cytosine and cytidine. J. Pharm. Sci. 1972, 61, 1052–1061. [Google Scholar]
- Lindahl, T.; Nyberg, B. Heat-induced deamination of cytosine residues in deoxyribonucleic acid. Biochemistry 1974, 13, 3405–3410. [Google Scholar]
- Frederico, L.A.; Kunkel, T.A.; Shaw, B.R. Cytosine Deamination in Mismatched Base Pair. Biochemistry 1993, 32, 6523–6530. [Google Scholar]
- Shen, J.-C.; Rideout, W.M.I.; Jones, P.A. The rate of hydrolytic deamination of 5-methylcytosine in double-stranded DNA. Nucl. Acids Res. 1994, 22, 972–976. [Google Scholar]
- Garrett, E.R.; Mehta, P.J. Solvolysis of adenine nucleosides. II. Effects of sugars and ademine substituents on alkaline solvolysis. J. Am. Chem. Soc. 1972, 94, 8542–8547. [Google Scholar]
- Kabyemela, B.M.; Adschiri, T.; Malaluan, R.; Arai, K. Degradation Kinetics of Dihydroxyacetone and Glyceraldehyde in Subcritical and Supercritical Water. Ind. Eng. Chem. Res. 1997, 36, 2025–2030. [Google Scholar]
- Yu, J.; Savage, P.E. Decomposition of Formic Acid under Hydrothermal Conditions. Ind. Eng. Chem. Res. 1998, 37, 2–10. [Google Scholar]
- Maiella, P.G.; Brill, T.B. Spectroscopy of Hydrothermal Reactions. 10. Evidence of Wall Effects in Decarboxylation Kinetics of 1.00 m HCO2X (X = H, Na) at 280−330 °C and 275 bar. J. Phys. Chem. A 1998, 102, 5886–5891. [Google Scholar]
- Myung, S.; Wang, Y.; Zhang, Y.H.P. Fructose-1,6-bisphosphatase from a hyper-thermophilic bacterium Thermotoga maritima: Characterization, metabolite stability, and its implications. Process Biochem. 2010, 45, 1882–1887. [Google Scholar]
- Bearne, S.L.; Wolfenden, R. Enzymatic hydration of an olefin: The burden borne by fumarase. J. Am. Chem. Soc. 1995, 117, 9588–9589. [Google Scholar]
- De Mey, M.; Taymaz-Nikerel, H.; Baart, G.; Waegeman, H.; Maertens, J.; Heijnen, J.J.; van Gulik, W.M. Catching prompt metabolite dynamics in Escherichia coli with the BioScope at oxygen rich conditions. Metab. Eng. 2010, 12, 477–487. [Google Scholar]
- Wang, R.; Kobayashi, T.; Adachi, S. Degradation of N-Acetyl-D-glucosamine and D-Glucosamine in Subcritical Water and Properties of the Degradation Products. Food Sci. Technol. Res. 2011, 17, 273–278. [Google Scholar]
- Qian, Y.; Engel, M.H.; Macko, S.A.; Carpenter, S.; Deming, J.W. Kinetics of peptide hydrolysis and amino acid decomposition at high temperature. Geochim. Cosmochim. Acta 1993, 57, 3281–3293. [Google Scholar]
- Snider, M.J.; Wolfenden, R. The rate of spontaneous decarboxylation of amino acids. J. Am. Chem. Soc. 2000, 122, 11507–11508. [Google Scholar]
- Abdelmoez, W.; Nakahasi, T.; Yoshida, H. Amino acid transformation and decomposition in saturated subcritical water. Ind. Eng. Chem. Res. 2007, 46, 5286–5294. [Google Scholar]
- Kawamura, K.; Yukioka, M. Kinetics of the racemization of amino acids at 225–275 °C using a real-time monitoring method of hydrothermal reactions. Thermochim. Acta 2001, 375, 9–16. [Google Scholar]
- Kobayashi, T.; Takase, K.; Adachi, S. Degradation kinetics of branched chain amino acids in subcritical water. Biosci. Biotechnol. Biochem. 2010, 74, 649–651. [Google Scholar]
- Kocsisova, T.; Juhasz, J.; Cvengros, J. Hydrolysis of fatty acid esters in subcritical water. Eur. J. Lipid Sci. Technol. 2006, 108, 652–658. [Google Scholar]
- Maiella, P.G.; Brill, T.B. Spectroscopy of Hydrothermal Reactions. 5. Decarboxylation Kinetics of Malonic Acid and Monosodium Malonate. J. Phys. Chem. 1996, 100, 14352–14355. [Google Scholar]
- Hall, G.A. The Kinetics of the Decomposition of Malonic Acid in Aqueous Solution. J. Am. Chem. Soc. 1949, 71, 2691–2693. [Google Scholar]
- Radzicka, A.; Wolfenden, R. A proficient enzyme. Science 1995, 267, 90–93. [Google Scholar]
- Gelles, E. Kinetics of the decarboxylation of oxaloacetic acid. J. Chem. Soc. (Resumed) 1956, 4736–4739. [Google Scholar]
- Grit, M.; Underberg, W.J.M.; Crommelin, D.J.A. Hydrolysis of saturated soybean phosphatidylcholine in aqueous liposome dispersions. J. Pharm. Sci. 1993, 82, 362–366. [Google Scholar]
- Belsky, A.J.; Maiella, P.G.; Brill, T.B. Spectroscopy of Hydrothermal Reactions 13. Kinetics and Mechanisms of Decarboxylation of Acetic Acid Derivatives at 100−260 °C under 275 bar. J. Phys. Chem. A 1999, 103, 4253–4260. [Google Scholar]
- Larralde, R.; Robertson, M.P.; Miller, S.L. Rates of decomposition of ribose and other sugars: implications for chemical evolution. Proc. Natl. Acad. Sci. USA 1995, 92, 8158–8160. [Google Scholar]
- Haghighat Khajavi, S.; Kimura, Y.; Oomori, T.; Matsuno, R.; Adachi, S. Kinetics on sucrose decomposition in subcritical water. LWT–Food Sci. Technol. 2005, 38, 297–302. [Google Scholar]
- Shaw, W.H.R.; Bordeux, J.J. The decomposition of urea in aqueous media. J. Am. Chem. Soc. 1955, 77, 4729–4733. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bains, W.; Xiao, Y.; Yu, C. Prediction of the Maximum Temperature for Life Based on the Stability of Metabolites to Decomposition in Water. Life 2015, 5, 1054-1100. https://doi.org/10.3390/life5021054
Bains W, Xiao Y, Yu C. Prediction of the Maximum Temperature for Life Based on the Stability of Metabolites to Decomposition in Water. Life. 2015; 5(2):1054-1100. https://doi.org/10.3390/life5021054
Chicago/Turabian StyleBains, William, Yao Xiao, and Changyong Yu. 2015. "Prediction of the Maximum Temperature for Life Based on the Stability of Metabolites to Decomposition in Water" Life 5, no. 2: 1054-1100. https://doi.org/10.3390/life5021054






