Neuronal Activity in the Subthalamic Cerebrovasodilator Area under Partial-Gravity Conditions in Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals Selection and Preparation
2.2. Parabolic Flight
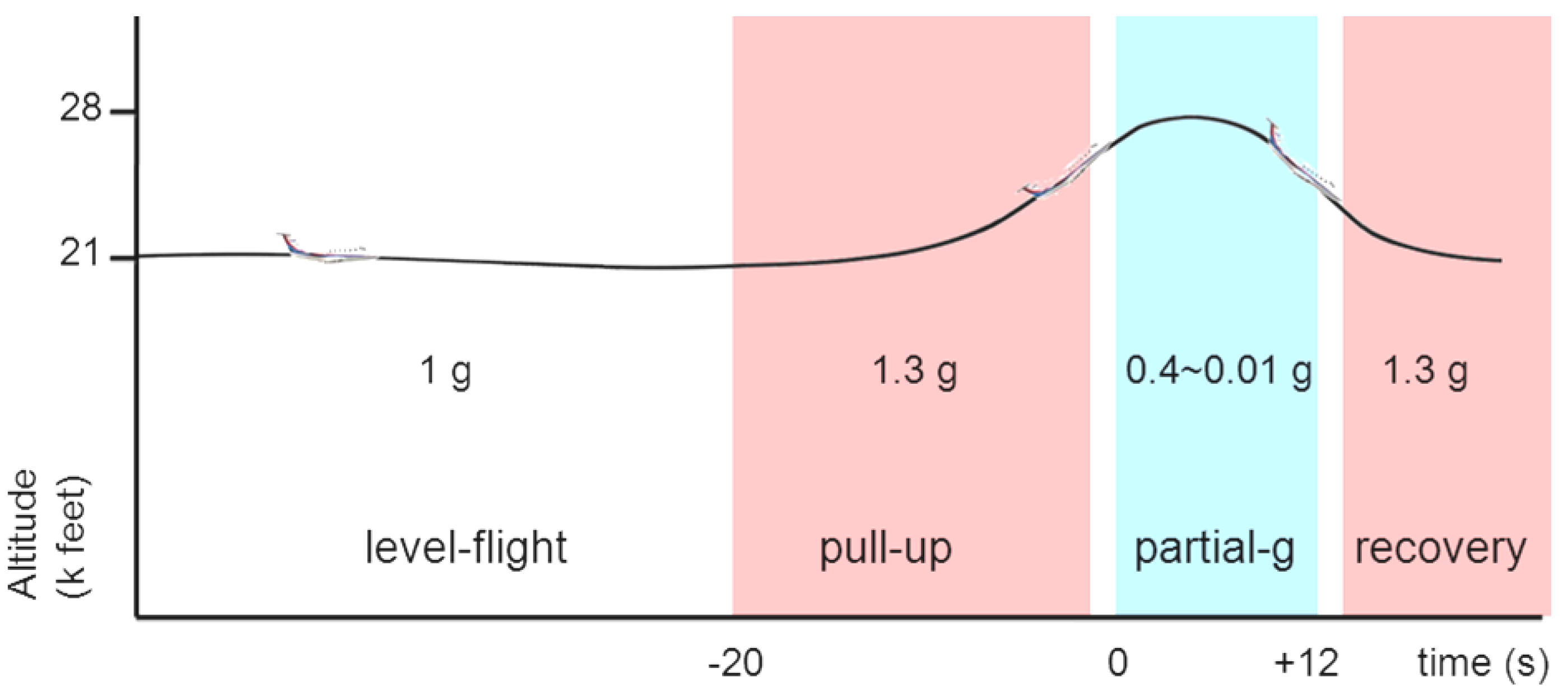
2.3. Data Collection
2.4. Data Analysis
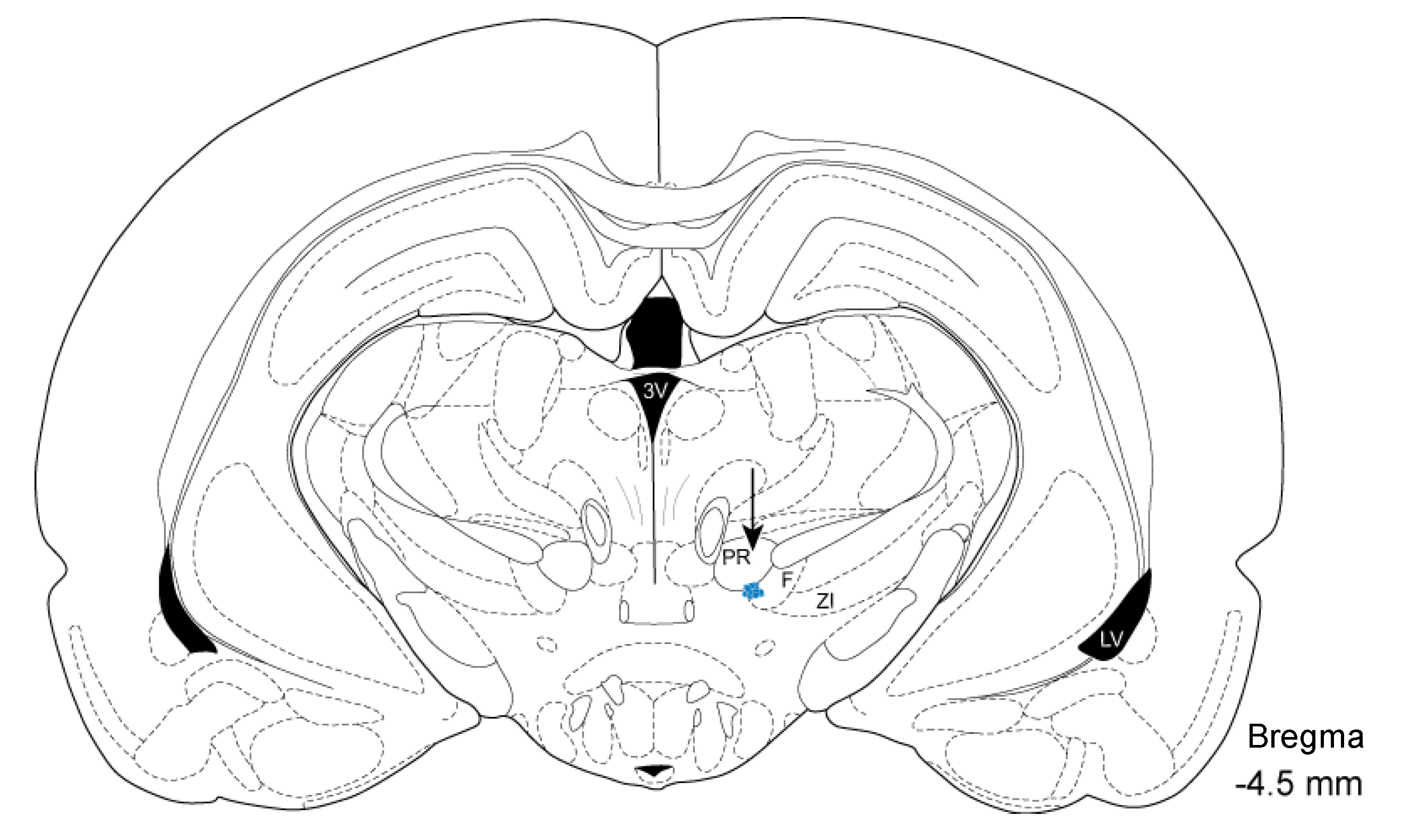
2.5. Histology and Localization of Electrode Insertion Site
3. Results and Discussion
| Partial gravity (g) | Number of recorded samples | Number of rats | Firing frequency during 1 g (Hz) | Firing frequency at each partial g (Hz) | Average difference (Hz) | Value of p |
|---|---|---|---|---|---|---|
| 0.40 | 9 | 3 | 1.57 ± 0.64 | 1.56 ± 0.75 | 0.00 ± 0.76 | 0.981 |
| 0.30 | 11 | 3 | 1.31 ± 0.53 | 1.36 ± 0.73 | 0.30 ± 1.32 | 0.798 |
| 0.20 | 25 | 8 | 1.86 ± 0.88 | 1.47 ± 0.96 | −0.39 ± 1.21 | 0.078 |
| 0.15 | 20 | 8 | 3.14 ± 2.56 | 2.09 ± 1.23 | −1.05 ± 1.65 | 0.001 ** |
| 0.10 | 37 | 12 | 2.15 ± 1.49 | 1.62 ± 0.88 | −0.53 ± 1.18 | 0.003 ** |
| 0.05 | 29 | 12 | 2.46 ± 1.69 | 1.67 ± 0.97 | −0.79 ± 1.13 | 0.001 ** |
| 0.01 | 9 | 3 | 3.68 ± 1.96 | 2.52 ± 0.98 | −1.16 ± 1.29 | 0.013 * |
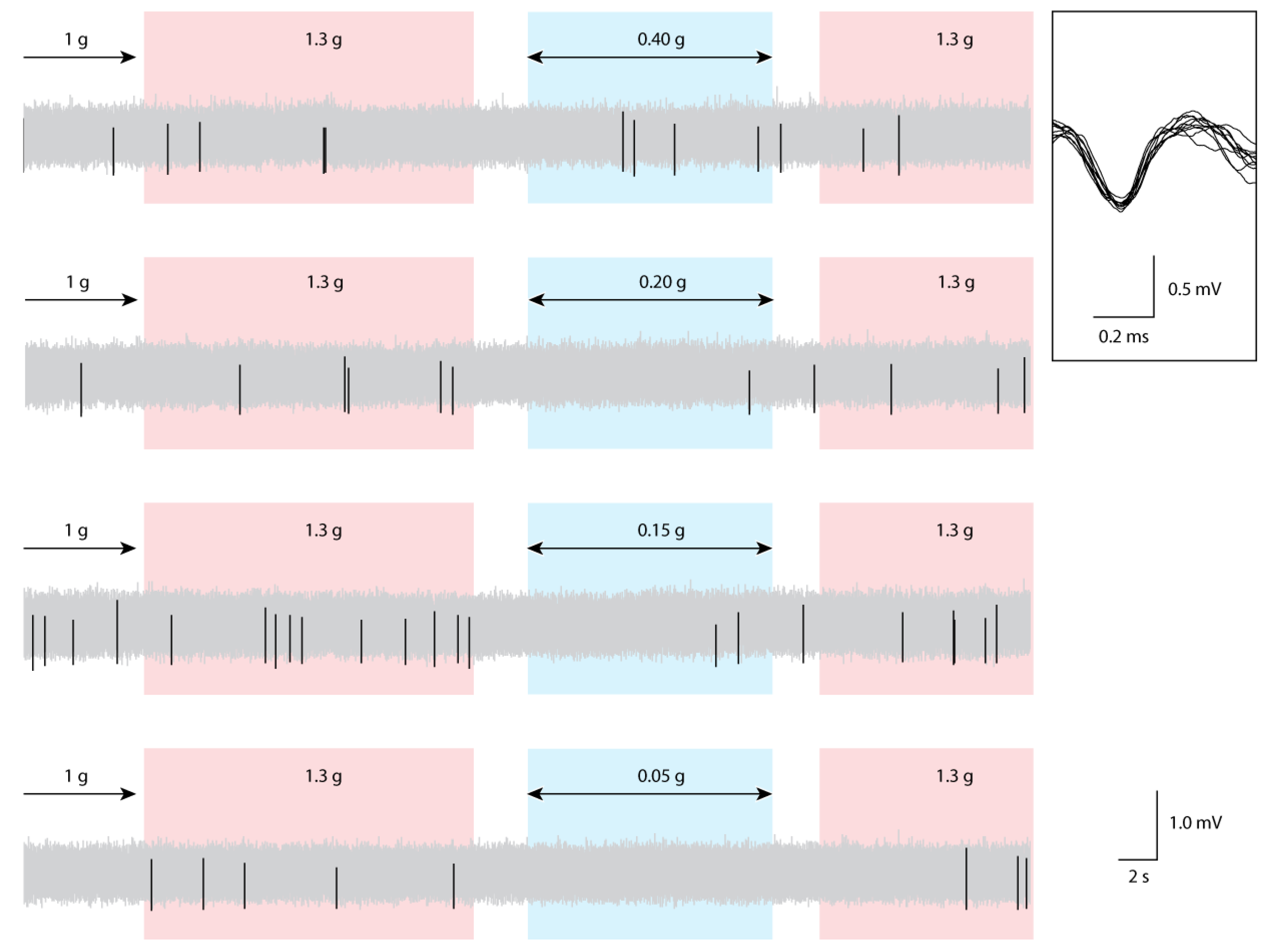
Limitations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Williams, D.; Kuipers, A.; Mukai, C.; Thirsk, R. Acclimation during space flight: Effects on human physiology. Can. Med. Assoc. J. 2009, 180, 1317–1323. [Google Scholar] [CrossRef]
- Heer, M.; Paloski, W.H. Space motion sickness: Incidence, etiology, and countermeasures. Auton. Neurosci. 2006, 129, 77–79. [Google Scholar] [CrossRef]
- Charles, J.B.; Lathers, C.M. Cardiovascular adaptation to spaceflight. J. Clin. Pharmacol. 1991, 31, 1010–1023. [Google Scholar] [CrossRef]
- Stevens, S.A.; Lakin, W.D.; Penar, P.L. Modeling steady-state intracranial pressures in supine, head-down tilt and microgravity conditions. Aviat. Space Environ. Med. 2005, 76, 329–338. [Google Scholar]
- Lakin, W.D.; Stevens, S.A.; Penar, P.L. Modeling intracranial pressures in microgravity: The influence of the blood-brain barrier. Aviat. Space Environ. Med. 2007, 78, 932–936. [Google Scholar] [CrossRef]
- Guell, A.; Dupui, P.; Barrere, M.; Fanjaud, G.; Bes, A.; Kotowskaia, A. Changes in the loco-regional cerebral blood flow (r.C.B.F.) during a simulation of weightlessness. Acta Astronaut. 1982, 9, 689–690. [Google Scholar] [CrossRef]
- Wilson, M.H.; Imray, C.H.; Hargens, A.R. The headache of high altitude and microgravity—Similarities with clinical syndromes of cerebral venous hypertension. High Alt. Med. Biol. 2011, 12, 379–386. [Google Scholar] [CrossRef]
- Golanov, E.V.; Christensen, J.R.; Reis, D.J. Neurons of a limited subthalamic area mediate elevations in cortical cerebral blood flow evoked by hypoxia and excitation of neurons of the rostral ventrolateral medulla. J. Neurosci. 2001, 21, 4032–4041. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: New York, NY, USA, 1998. [Google Scholar]
- Zeredo, J.L.; Toda, K.; Matsuura, M.; Kumei, Y. Behavioral responses to partial-gravity conditions in rats. Neurosci. Lett. 2012, 529, 108–111. [Google Scholar] [CrossRef]
- Schmidt, E.M. Computer separation of multi-unit neuroelectric data: A review. J. Neurosci. Methods 1984, 12, 95–111. [Google Scholar] [CrossRef]
- Tatebayashi, K.; Asai, Y.; Maeda, T.; Shiraishi, Y.; Miyoshi, M.; Kawai, Y. Effects of head-down tilt on the intracranial pressure in conscious rabbits. Brain Res. 2003, 977, 55–61. [Google Scholar] [CrossRef]
- Tanaka, K.; Gotoh, T.M.; Awazu, C.; Morita, H. Regional difference of blood flow in anesthetized rats during reduced gravity induced by parabolic flight. J. Appl. Physiol. 2005, 99, 2144–2148. [Google Scholar] [CrossRef]
- Iwasaki, K.; Levine, B.D.; Zhang, R.; Zuckerman, J.H.; Pawelczyk, J.A.; Diedrich, A.; Ertl, A.C.; Cox, J.F.; Cooke, W.H.; Giller, C.A.; et al. Human cerebral autoregulation before, during and after spaceflight. J. Physiol. 2007, 579, 799–810. [Google Scholar] [CrossRef]
- McHedlishvili, G. Physiological mechanisms controlling cerebral blood flow. Stroke 1980, 11, 240–248. [Google Scholar] [CrossRef]
- Wilkerson, M.K.; Colleran, P.N.; Delp, M.D. Acute and chronic head-down tail suspension diminishes cerebral perfusion in rats. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H328–H334. [Google Scholar]
- Wilkerson, M.K.; Lesniewski, L.A.; Golding, E.M.; Bryan, R.M., Jr.; Amin, A.; Wilson, E.; Delp, M.D. Simulated microgravity enhances cerebral artery vasoconstriction and vascular resistance through endothelial nitric oxide mechanism. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1652–H1661. [Google Scholar]
- Guyenet, P.G. Neural structures that mediate sympathoexcitation during hypoxia. Respir. Physiol. 2000, 121, 147–162. [Google Scholar] [CrossRef]
- Sun, M.K.; Reis, D.J. Central neural mechanisms mediating excitation of sympathetic neurons by hypoxia. Prog. Neurobiol. 1994, 44, 197–219. [Google Scholar] [CrossRef]
- Sun, M.K.; Reis, D.J. Hypoxia selectively excites vasomotor neurons of rostral ventrolateral medulla in rats. Am. J. Physiol. 1994, 266, R245–R256. [Google Scholar]
- Iwase, S.; Cui, J.; Kitazawa, H.; Miyazaki, S.; Sugiyama, Y.; Kohno, M.; Mukai, C.; Mano, T. Sympathetic nerve response to microgravity induced by parabolic flight. Environ. Med. 1997, 41, 141–144. [Google Scholar]
- Iwase, S.; Mano, T.; Cui, J.; Kitazawa, H.; Kamiya, A.; Miyazaki, S.; Sugiyama, Y.; Mukai, C.; Nagaoka, S. Sympathetic outflow to muscle in humans during short periods of microgravity produced by parabolic flight. Am. J. Physiol. 1999, 277, R419–R426. [Google Scholar]
- Morita, H.; Tanaka, K.; Tsuchiya, Y.; Miyahara, T.; Fujiki, N. Response of renal sympathetic nerve activity to parabolic flight-induced gravitational change in conscious rats. Neurosci. Lett. 2001, 310, 129–132. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zeredo, Z.L.; Toda, K.; Kumei, Y. Neuronal Activity in the Subthalamic Cerebrovasodilator Area under Partial-Gravity Conditions in Rats. Life 2014, 4, 107-116. https://doi.org/10.3390/life4010107
Zeredo ZL, Toda K, Kumei Y. Neuronal Activity in the Subthalamic Cerebrovasodilator Area under Partial-Gravity Conditions in Rats. Life. 2014; 4(1):107-116. https://doi.org/10.3390/life4010107
Chicago/Turabian StyleZeredo, Zeredo L, Kazuo Toda, and Yasuhiro Kumei. 2014. "Neuronal Activity in the Subthalamic Cerebrovasodilator Area under Partial-Gravity Conditions in Rats" Life 4, no. 1: 107-116. https://doi.org/10.3390/life4010107




