Simulations of Prebiotic Chemistry under Post-Impact Conditions on Titan
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Apparatus and Cleaning
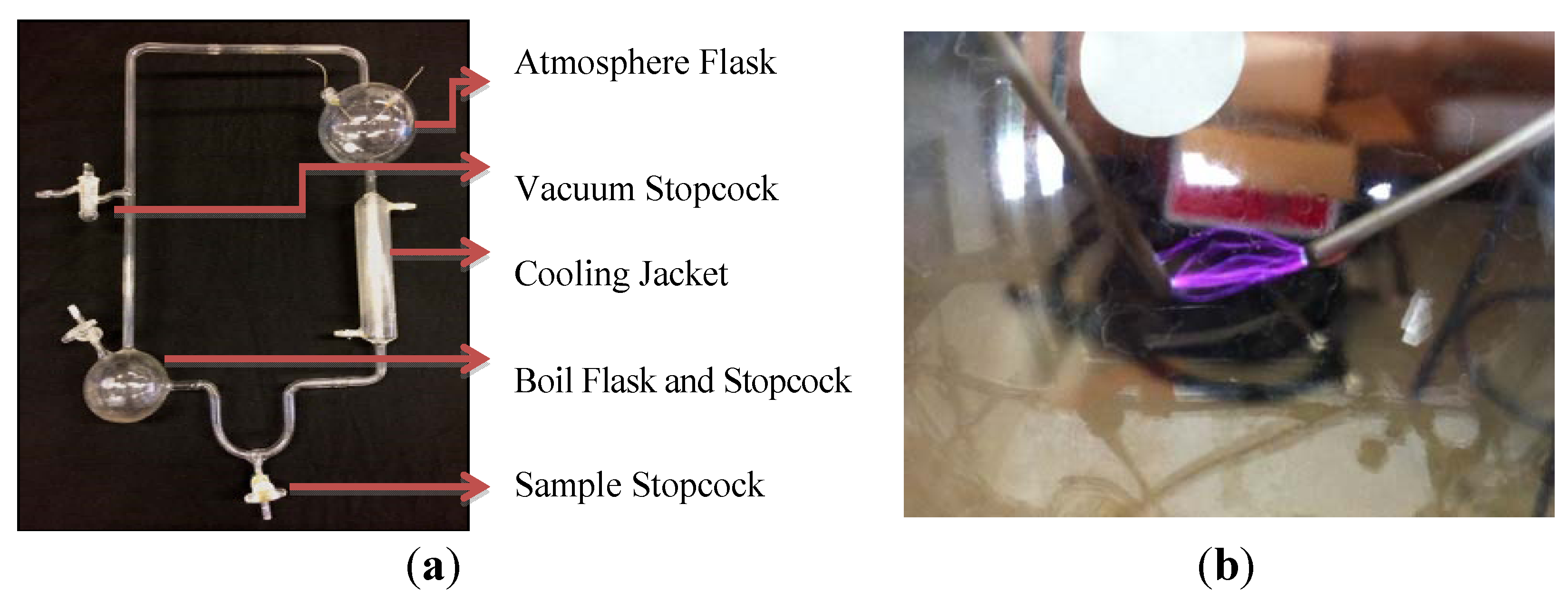
2.2. Experimental Conditions: Early Earth
2.3. Experimental Conditions: Titan Post-Impact, Time Course Experiment
| Sample | Identification | Sample Location |
|---|---|---|
| Sample 1 | Starting mixture of 30% aqueous ammonia | Sample Stopcock |
| Sample 2 | 2 days post-spark application | Vacuum Stopcock |
| Sample 3 | 4 days post-spark application | Sample Stopcock |
| Sample 4 | 7 days post-spark application | Sample Stopcock |
2.4. Sample Analysis
3. Results and Discussion
3.1. Early Earth Conditions
| Residue Mass (m/z) (monoisotopic, MH+1) | Peak Identification | Chemical Formula |
|---|---|---|
| 72.03712 | Alanine | C3H5NO |
| 58.02147 | Glycine | C2H3NO |
| 116.02695 | Aspartic Acid | C4H5NO3 |
| 114.08407 | Isoleucine | C6H11NO |
| 88.03203 | Serine | C3H5NO2 |
| 102.04768 | Threonine | C4H7NO2 |
| 113.08407 | Leucine | C6H11NO |
| 115.04293 | Asparagine | C4H6N2O2 |
| 129.09497 | Lysine | C6H12N2O |
| 100.06842 | Valine | C5H9NO |
| 130.0426 | Glutamic Acid | C5H7NO3 |
| 148.06842 | Phenylalanine | C9H9NO |
| 98.05277 | Proline | C5H7NO |
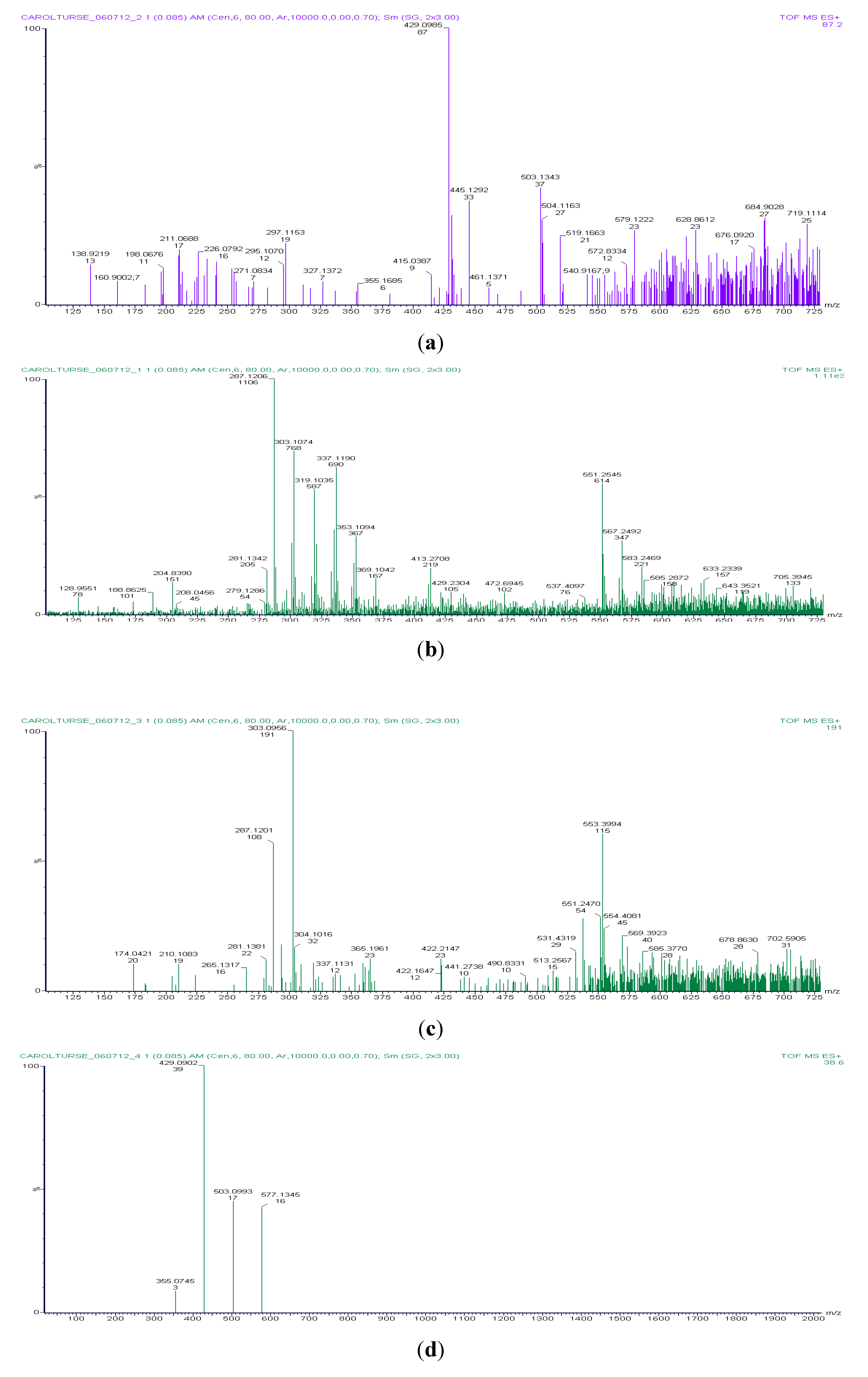
3.2. Titan Post-Impact Conditions, Time Course Experiment
| Time Course Sample Number | 1 | 2 | 3 | 4 | Early Earth Conditions | |
|---|---|---|---|---|---|---|
| Compound Identification, relative abundance (%) | Ammonia (30% aqueous) | Glutamine, <10% | Histidine, 50% | Larger ring structures only, no single amino acids | Glycine | |
| Alanine, 25% | Aspartic Acid, <10% | Alanine | ||||
| Tyrosine, <10% | Tryptophan, <10% | Aspartic Acid | ||||
| Serine, <10% | Serine, <10% | Serine | ||||
| Phenylalanine, 50% | Phenylalanine, 25% | Phenylalanine | ||||
| Glutamic acid, <10% | Glutamic acid, <10% | Glutamic acid | ||||
| Arganine, <10% | Arganine, <10% | Asparagine | ||||
| Leucine, <10% | Leucine, <10% | Leucine | ||||
| Isoleucine, <10% | Isoleucine, <10% | Isoleucine | ||||
| Valine, <10% | Valine, <10% | Valine | ||||
| Proline, <10% | Proline | |||||
| Lysine | ||||||
| Threonine |
3.2.1. Titan Sample 1, Starting Solution of 30% Aqueous Ammonia
3.2.2. Titan Sample Solution 2
3.2.3. Titan Sample Solution 3
3.2.4. Titan Sample Solution 4: Sample Taken from the Boil Flask
3.3. Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar]
- Oparin, A.I.; Lorenz, R.D.; Lunine, J.I. The Origin of Life; U.S. Government Printing Office: Washington, DC, USA, 1968. [Google Scholar]
- Lob, W. Uber des Verhalten des Formamids Unter der Wirkung der stillen Entladung, Ein Beitrag zur Stickstoff-Assimilation. Berichte der Deutschen Chemischen Gesellschaft 1913, 46, 684–697. (in German). [Google Scholar]
- Garrison, W.M.; Morrison, J.G.; Hamilton, A.A.; Benson, M.; Calvin, M. The reduction of carbon dioxide by ionizing radiation. Science 1951, 114, 416–418. [Google Scholar]
- Kasting, J.F.; Pollack, J.B.; Crisp, D. Effects of high CO2 levels on surface temperature and atmospheric oxidation state of the early Earth. J. Atmos. Chem. 1984, 1, 403–428. [Google Scholar] [CrossRef]
- Kasting, J.F. Early Earth’s atmosphere. Science 1993, 259, 920–926. [Google Scholar] [CrossRef]
- Anderson, R.; Gathman, S.; Hughes, J.; Björnsson, S.; Jónasson, S.; Blanchard, D.C.; Moore, C.B.; Survilas, H.J.; Vonnegut, B. Electricity in volcanic clouds. Science 1965, 148, 1179–1189. [Google Scholar]
- Cleaves, H.J.; Chalmers, J.H.; Lazcano, A.; Miller, S.L.; Bada, J.L. A reassessment of prebiotic organic synthesis in neutral planetary atmospheres. Orig. Life Evol. Biosph. 2008, 38, 105–115. [Google Scholar] [CrossRef]
- Bada, J.L. New insights into prebiotic chemistry from Stanley Miller’s spark discharge experiments. Chem. Soc. Rev. 2013, 42, 2186–2196. [Google Scholar] [CrossRef]
- Schulze-Makuch, D.; Grinspoon, D.H. Biologically enhanced energy and carbon cycling on Titan? Astrobiology 2005, 5, 560–567. [Google Scholar] [CrossRef]
- O’Brien, D.P.; Lorenz, R.D.; Lunine, J.I. Numerical calculations of the longevity of impact oases on Titan. Icarus 2005, 173, 243–253. [Google Scholar] [CrossRef]
- Fischer, G.; Gurnett, D.A. The search for Titan lightning radio emissions. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Horvath, G.; Skalny, J.D.; Mason, N.J.; Klas, M.; Zahoran, M.; Vladoiu, R.; Manole, M. Corona discharge experiments in admixtures of N2 and CH4: A laboratory simulation of Titan’s atmosphere. Plasma Sources Sci. Technol. 2009, 18. [Google Scholar] [CrossRef]
- Plankensteiner, K.; Reiner, H.; Rode, B.M.; Mikoviny, T.; Wisthaler, A.; Hansel, A.; Märk, T.D.; Fischer, G.; Lammer, H.; Rucker, H.O. Discharge experiments simulating chemical evolution on the surface of Titan. Icarus 2007, 187, 616–619. [Google Scholar] [CrossRef]
- Scripps Center for Metabolomics. Available online: http://metlin.scripps.edu/ (accessed on 4 November 2013).
- Lavvas, P.; Yelle, R.V.; Vuitton, V. The detached haze layer in Titan’s mesophere. Icarus 2009, 201, 626–633. [Google Scholar] [CrossRef]
- Horst, S.M.; Yelle, R.V.; Buch, A.; Carrasco, N.; Cernogora, G.; Dutuit, O.; Quirico, E.; Sciamma-O’Brien, E.; Smith, M.A.; Somogyi, A.; et al. Formation of amino acids and nucleotide bases in a Titan atmosphere simulation experiment. Astrobiology 2012, 12, 809–817. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Turse, C.; Leitner, J.; Firneis, M.; Schulze-Makuch, D. Simulations of Prebiotic Chemistry under Post-Impact Conditions on Titan. Life 2013, 3, 538-549. https://doi.org/10.3390/life3040538
Turse C, Leitner J, Firneis M, Schulze-Makuch D. Simulations of Prebiotic Chemistry under Post-Impact Conditions on Titan. Life. 2013; 3(4):538-549. https://doi.org/10.3390/life3040538
Chicago/Turabian StyleTurse, Carol, Johannes Leitner, Maria Firneis, and Dirk Schulze-Makuch. 2013. "Simulations of Prebiotic Chemistry under Post-Impact Conditions on Titan" Life 3, no. 4: 538-549. https://doi.org/10.3390/life3040538




