New Estimates of Nitrogen Fixation on Early Earth
Abstract
:1. Introduction
2. Methods
2.1. Thermochemical and Energetic Particle Deposition Modeling
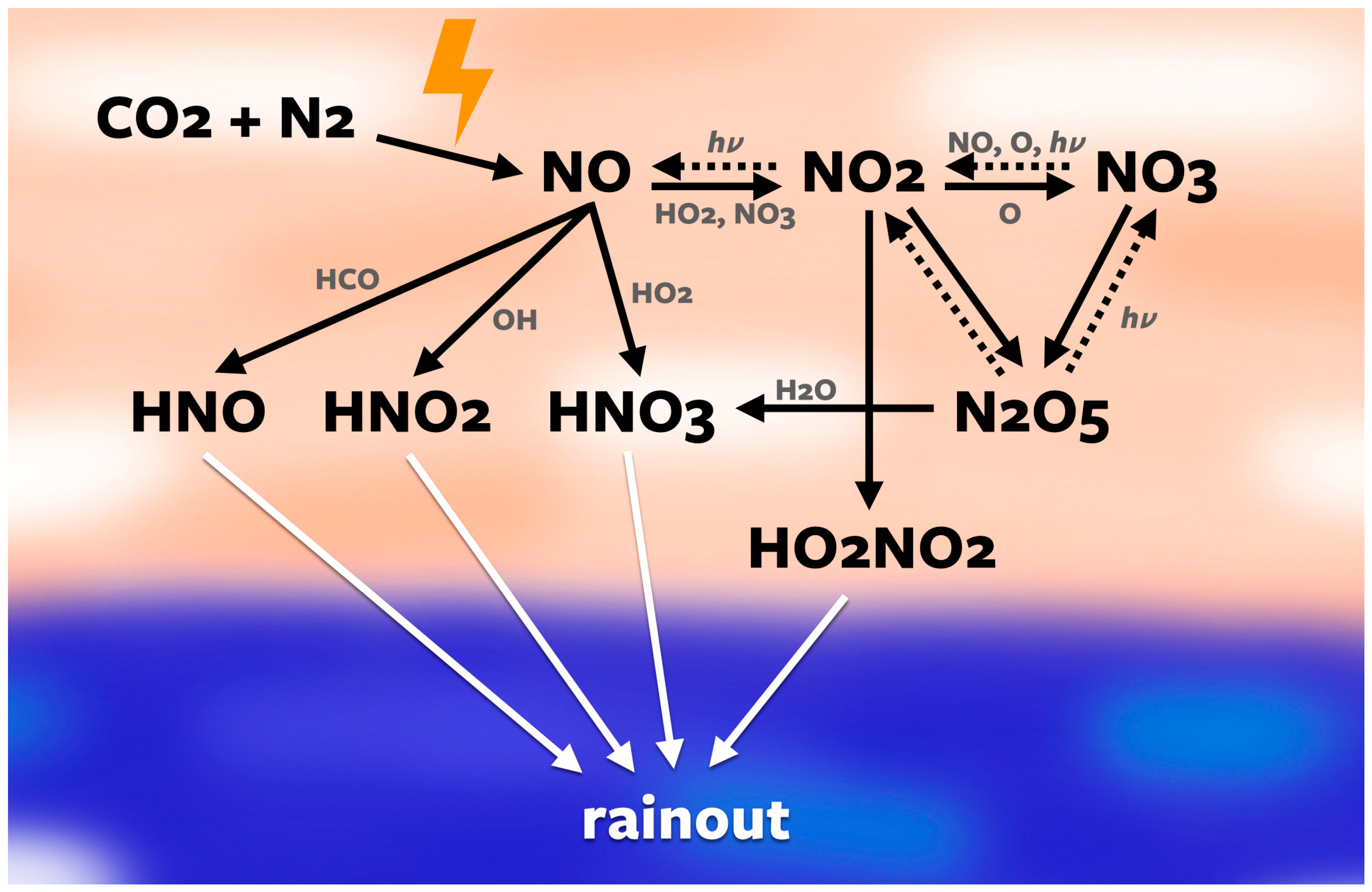
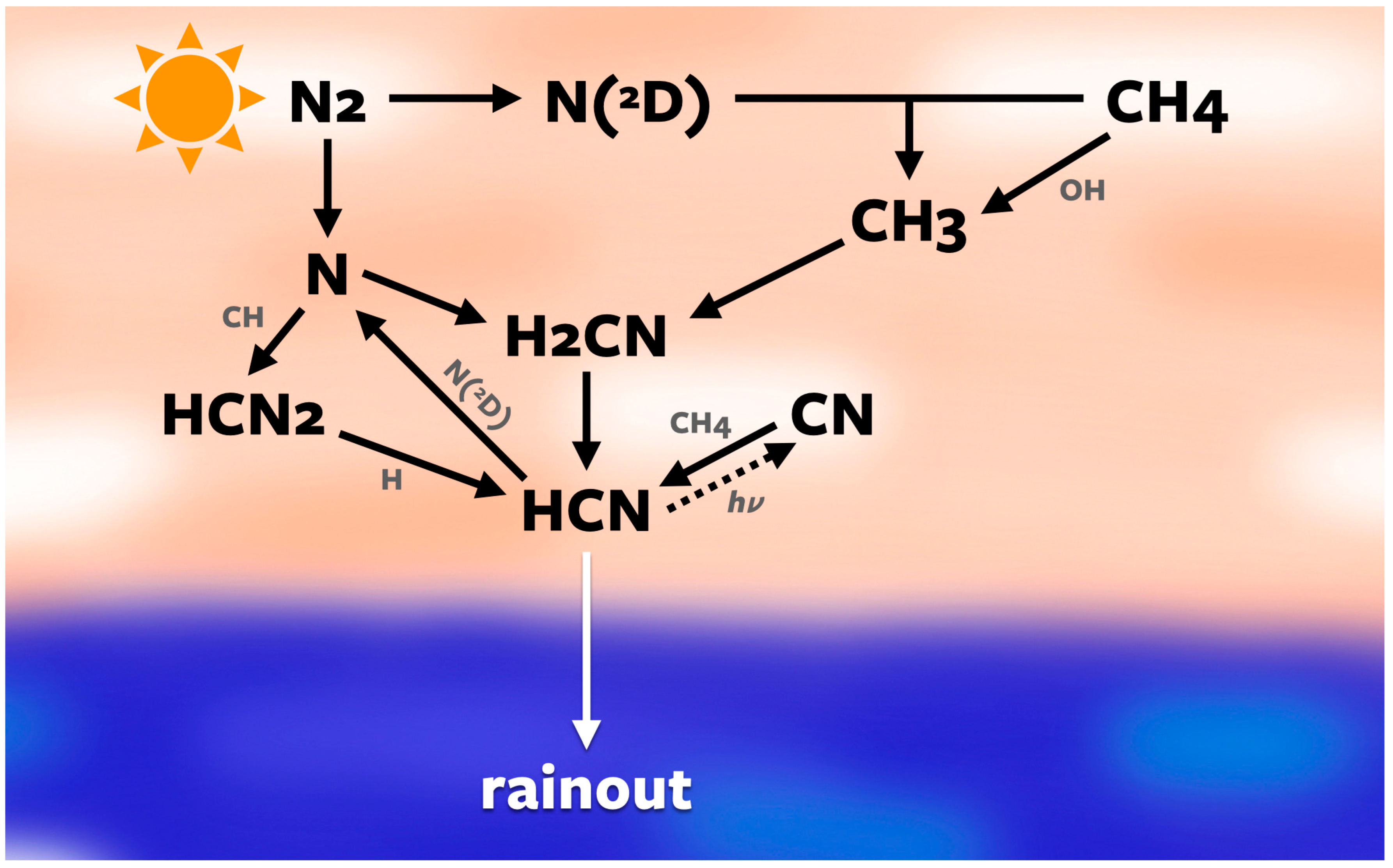
2.2. Photochemical Modeling
2.3. New Reactions Involving HCN2
2.4. Oceanic Concentrations
3. Results
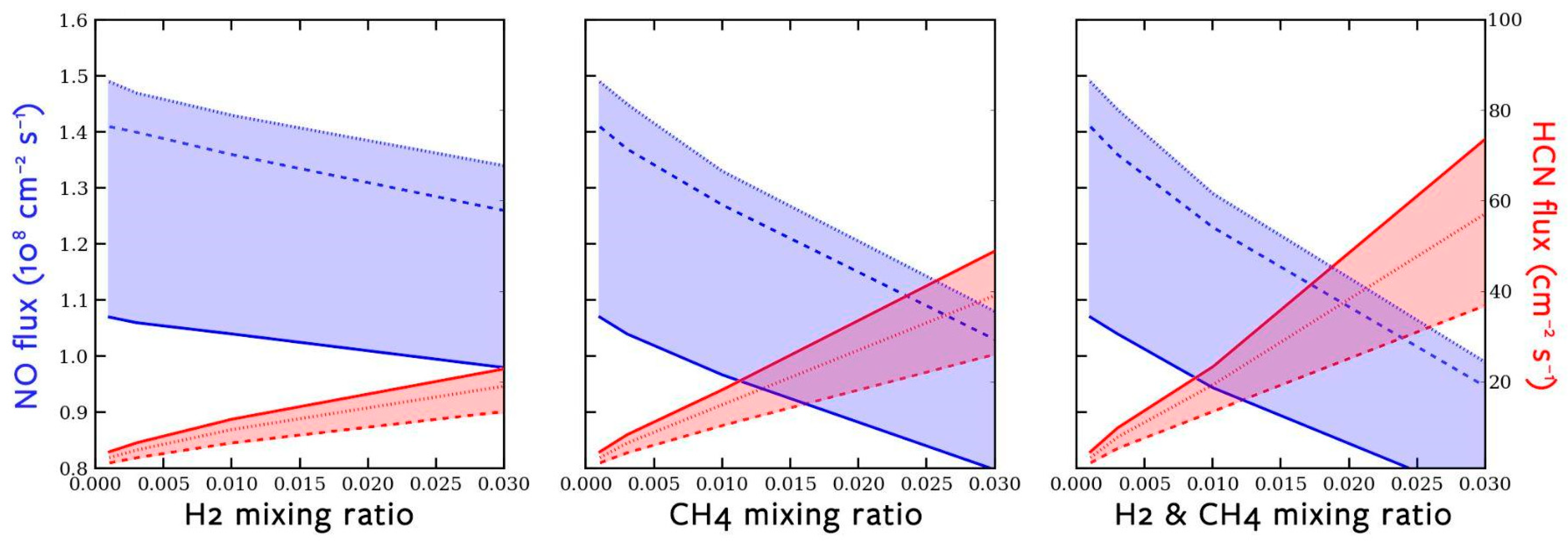
3.1. Lightning-Induced HCN and NO
3.2. Photochemistry
3.3. The Effect of Adding HCN2 Reactions
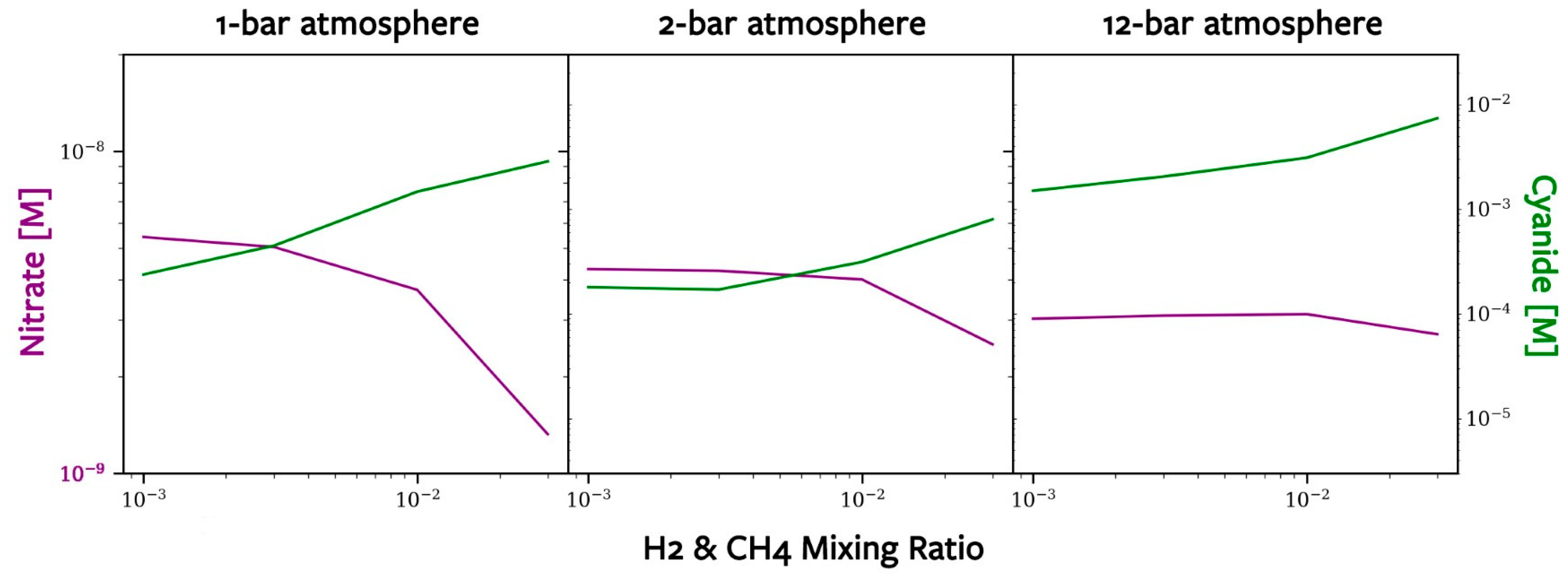
3.4. Oceanic Concentrations
4. Discussion
4.1. The Relevance of NOx and HCN
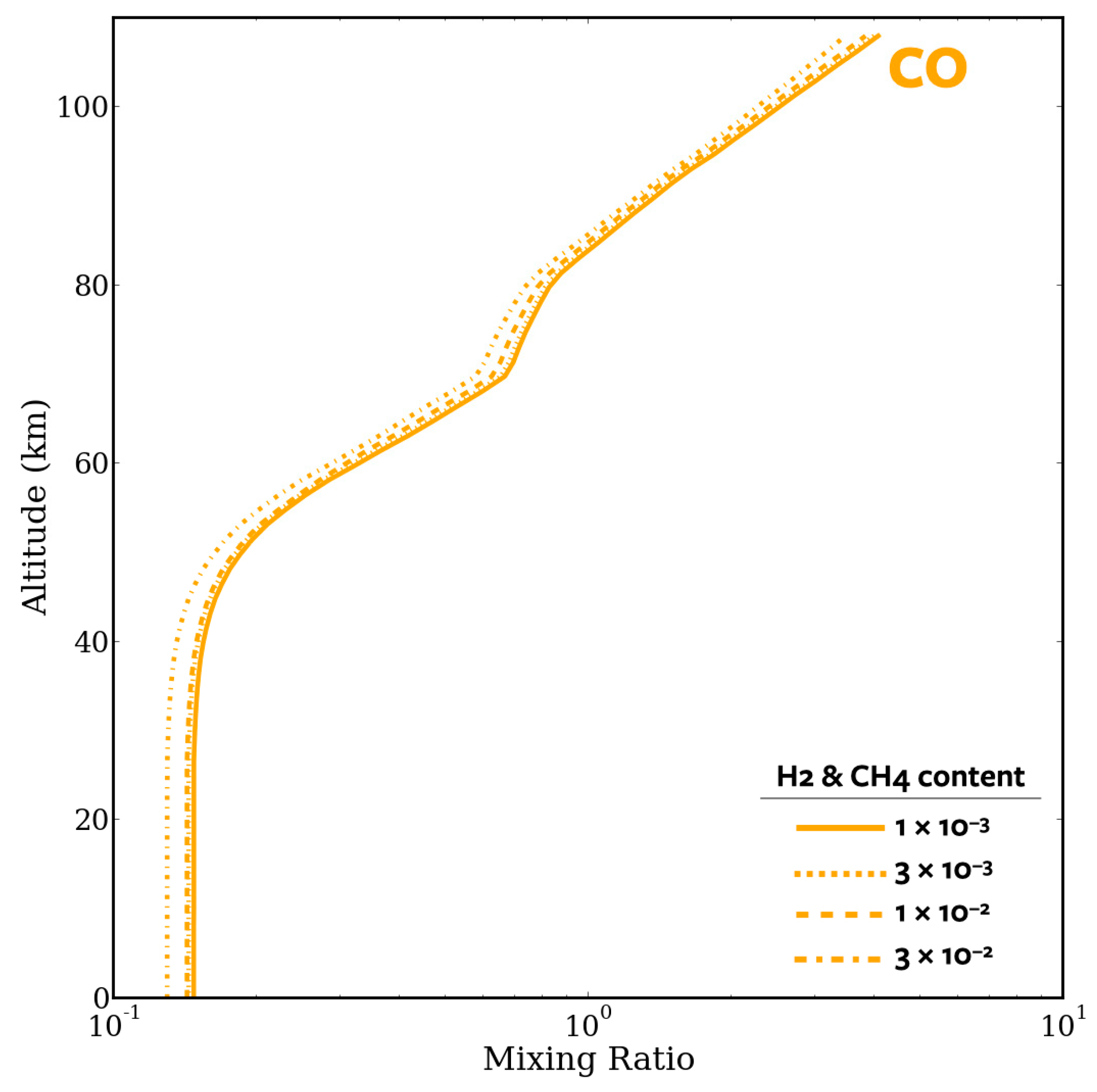
4.2. CO Runaway
4.3. Other Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sojo, V.; Herschy, B.; Whicher, A.; Camprubi, E.; Lane, N. The Origin of Life in Alkaline Hydrothermal Vents. Astrobiology 2016, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 2016, 1, 16116. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Baross, J.A.; Hoffman, S.E. Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig. Life Evol. Biosph. 1985, 15, 327–345. [Google Scholar] [CrossRef]
- Nitschke, W.; Schoepp-Cothenet, B.; Duval, S.; Zuchan, K.; Farr, O.; Baymann, F.; Panico, F.; Minguzzi, A.; Branscomb, E.; Russell, M.J. Aqueous electrochemistry: The toolbox for life’s emergence from redox disequilibria. Electrochem. Sci. Adv. 2022, 3, e2100192. [Google Scholar] [CrossRef]
- Nitschke, W.; Russell, M.J. Beating the acetyl coenzyme A-pathway to the origin of life. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120258. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Barge, L.M.; Bhartia, R.; Bocanegra, D.; Bracher, P.J.; Branscomb, E.; Kidd, R.; McGlynn, S.; Meier, D.H.; Nitschke, W.; et al. The Drive to Life on Wet and Icy Worlds. Astrobiology 2014, 14, 308–343. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.A.; Pollock, J.; Cole, K.A.; O’Connor, S.M.; Achenbach, L.A.; Coates, J.D. Anaerobic Nitrate-Dependent Iron(II) Bio-Oxidation by a Novel Lithoautotrophic Betaproteobacterium, Strain 2002. Appl. Env. Microbiol. 2006, 72, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Bryce, C.; Blackwell, N.; Schmidt, C.; Otte, J.; Huang, Y.M.; Kleindienst, S.; Tomaszewski, E.; Schad, M.; Warter, V.; Peng, C.; et al. Microbial anaerobic Fe(II) oxidation—Ecology, mechanisms and environmental implications. Environ. Microbiol. 2018, 20, 3462–3483. [Google Scholar] [CrossRef]
- Price, A.; Pearson, V.K.; Schwenzer, S.P.; Miot, J.; Olsson-Francis, K. Nitrate-Dependent Iron Oxidation: A Potential Mars Metabolism. Front. Microbiol. 2018, 9, 513. [Google Scholar] [CrossRef]
- Xu, J.; Ritson, D.J.; Ranjan, S.; Todd, Z.R.; Sasselov, D.D.; Sutherland, J.D. Photochemical reductive homologation of hydrogen cyanide using sulfite and ferrocyanide. Chem. Commun. 2018, 54, 5566–5569. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Charnay, B.D.; Gao, P.; Yung, Y.L.; Russell, M.J. Nitrogen Oxides in Early Earth’s Atmosphere as Electron Acceptors for Life’s Emergence. Astrobiology 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mvondo, D.N.; Navarro-Gonzalez, R.; McKay, C.P.; Coll, P.; Raulin, F. Production of nitrogen oxides by lightning and coronae discharges in simulated early Earth, Venus and Mars environments. Adv. Space Res. 2001, 27, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Desch, S.J.; Borucki, W.J.; Russell, C.T.; Bar-Nun, A. Progress in planetary lightning. Rep. Progress. Phys. 2002, 65, 202. [Google Scholar] [CrossRef]
- Pearce, B.K.D.; Ayers, P.W.; Pudritz, R.E. A Consistent Reduced Network for HCN Chemistry in Early Earth and Titan Atmospheres: Quantum Calculations of Reaction Rate Coefficients. J. Phys. Chem. A 2019, 123, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Luo, Y.; Wong, M.L.; Dunn, P.; Christensen, M.; Dong, C.; Hu, R.; Yung, Y. Nitrogen Fixation at Early Mars. Astrobiology 2021, 21, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, S.; Todd, Z.R.; Rimmer, P.B.; Sasselov, D.D.; Babbin, A.R. Nitrogen Oxide Concentrations in Natural Waters on Early Earth. Geochem. Geophys. Geosystems 2019, 20, 2021–2039. [Google Scholar] [CrossRef]
- Isoda, K.; Zang, X.; Ueno, Y. Glyoxylate from CO atmosphere via UV photochemistry. In Proceedings of the Japan Geoscience Union Meeting 2019, Chiba, Japan, 26–30 May 2019. [Google Scholar]
- Eugene, A.J.; Xia, S.S.; Guzman, M.I. Aqueous Photochemistry of Glyoxylic Acid. J. Phys. Chem. A 2016, 120, 3817–3826. [Google Scholar] [CrossRef]
- Berry, J.L.; Ugelow, M.S.; Tolbert, M.A.; Browne, E.C. The Influence of Gas-phase Chemistry on Organic Haze Formation. Astrophys. J. Lett. 2019, 885, L6. [Google Scholar] [CrossRef]
- Krasnopolsky, V.A.; Cruikshank, D.P. Photochemistry of Pluto’s atmosphere and ionosphere near perihelion. J. Geophys. Res. Planets 1999, 104, 21979–21996. [Google Scholar] [CrossRef]
- McBride, B.J.; Gordon, S. Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications; NASA Lewis Research Center: Cleveland, OH, USA, 1996. [Google Scholar]
- Mewaldt, R.A.; Looper, M.D.; Cohen, C.M.S.; Haggerty, D.K.; Labrador, A.W.; Leske, R.A.; Mason, G.M.; Mazur, J.E.; von Rosenvinge, T.T. Energy Spectra, Composition, and Other Properties of Ground-Level Events During Solar Cycle 23. Space Sci. Rev. 2012, 171, 97–120. [Google Scholar] [CrossRef]
- Allen, M.; Yung, Y.L.; Waters, J.W. Vertical Transport and Photochemistry in the Terrestrial Mesosphere and Lower Thermosphere (50–120 km). J. Geophys. Res. 1981, 86, 3617–3627. [Google Scholar] [CrossRef]
- Yung, Y.L.; DeMore, W.B. Photochemistry of Planetary Atmospheres; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Trainer, M.G.; Jimenez, J.L.; Yung, Y.L.; Toon, O.B.; Tolbert, M.A. Nitrogen Incorporation in CH4-N2 Photochemical Aerosol Produced by Far Ultraviolet Irradiation. Astrobiology 2012, 12, 315–326. [Google Scholar] [CrossRef]
- Korenaga, J.; Planavsky, N.J.; Evans, D.A.D. Global water cycle and the coevolution of the Earth’s interior and surface environment. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20150393. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, S.; Cleaves, H.J.; Miller, S.L. The Cold Origin of Life: A. Implications Based On The Hydrolytic Stabilities Of Hydrogen Cyanide And Formamide. Orig. Life Evol. Biosph. 2002, 32, 195–208. [Google Scholar] [CrossRef]
- Ackerman, A.S.; Marley, M.S. Precipitating Condensation Clouds in Substellar Atmospheres. Astrophys. J. 2001, 556, 872–884. [Google Scholar] [CrossRef]
- Wogan, N.F.; Catling, D.C.; Zahnle, K.J.; Lupu, R. Origin-of-life Molecules in the Atmosphere after Big Impacts on the Early Earth. Planet. Sci. J. 2023, 4, 169. [Google Scholar] [CrossRef]
- Pearce, B.K.D.; He, C.; Hörst, S.M. An experimental and theoretical investigation of HCN production in the Hadean Earth atmosphere. 2022. Available online: https://doi.org/10.1021/acsearthspacechem.2c00138 (accessed on 5 February 2024).
- Holm, N.G.; Neubeck, A. Reduction of nitrogen compounds in oceanic basement and its implications for HCN formation and abiotic organic synthesis. Geochem. Trans. 2009, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.; Cary, F.; Damer, B. Urability: A Property of Planetary Bodies That Can Support an Origin of Life. Astrobiology 2022, 22, 889–900. [Google Scholar] [CrossRef]
- Wong, M.L.; Bartlett, S.; Chen, S.; Tierney, L. Searching for Life, Mindful of Lyfe’s Possibilities. Life 2022, 12, 783. [Google Scholar] [CrossRef]
- Zahnle, K.; Haberle, R.M.; Catling, D.C.; Kasting, J.F. Photochemical instability of the ancient Martian atmosphere. J. Geophys. Res. Planets 2008, 113, E11004. [Google Scholar] [CrossRef]
- Ranjan, S.; Schwieterman, E.W.; Harman, C.; Fateev, A.; Sousa-Silva, C.; Seager, S.; Hu, R. Photochemistry of Anoxic Abiotic Habitable Planet Atmospheres: Impact of New H2O Cross Sections. Astrophys. J. 2020, 896, 148. [Google Scholar] [CrossRef]
- Burkholder, J.B.; Sander, S.P.; Abbatt, J.P.D.; Barker, J.R.; Huie, R.E.; Kolb, C.E.; Kurylo, M.J.; Orkin, V.L.; Wilmouth, D.M.; Wine, P.H. Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies—Evaluation Number 18; Jet Propulsion Laboratory, National Aeronautics and Space Administration: Pasadena, CA, USA, 2015. [Google Scholar]
- Chimiak, L.M. Prebiotic Fingerprints; California Institute of Technology: Pasadena, CA, USA, 2021.
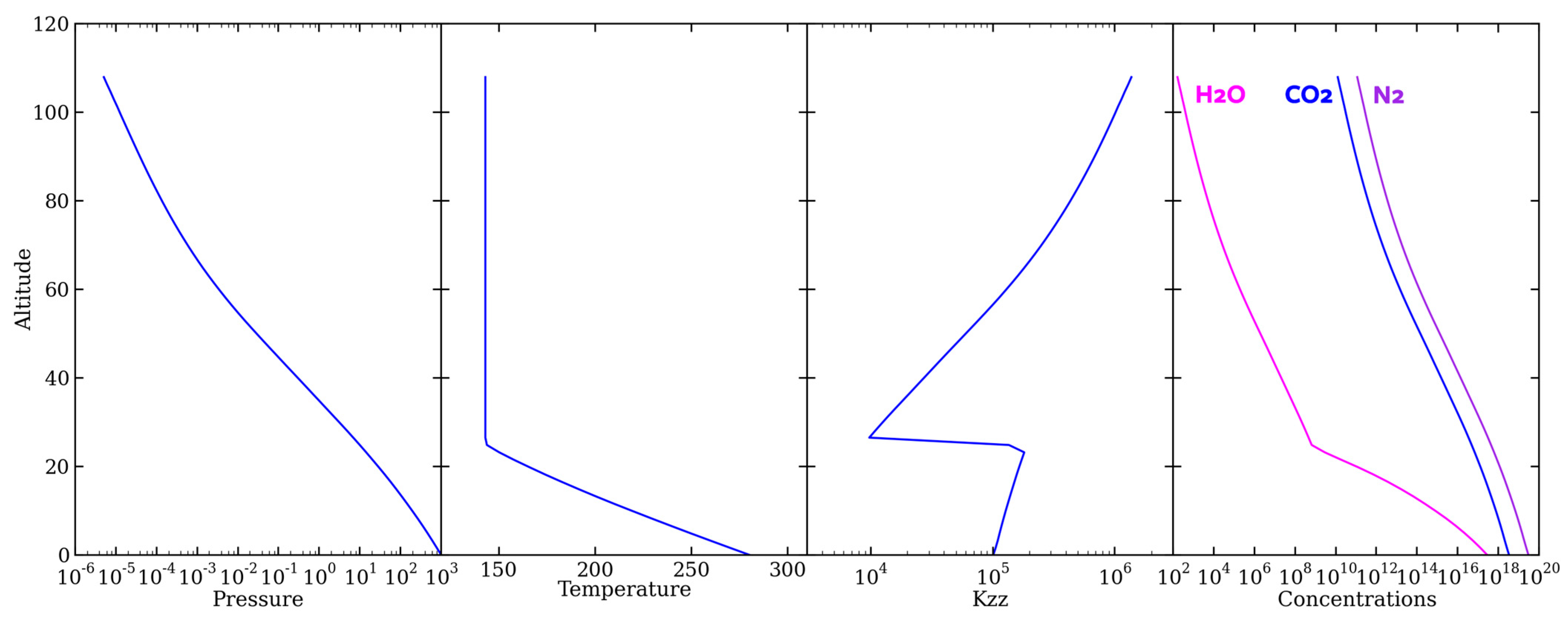


| Species | Lower Boundary | Upper Boundary |
|---|---|---|
| O | 0 | 0 |
| O2 | −1.00 × 10−6 | 0 |
| O3 | −1.00 × 10−2 | 0 |
| H2 | 1.00 × 10−3– 3.00 × 10−2 | 0 |
| HOx | 0 | 0 |
| NxHy | −1.00 × 10−2 | 0 |
| HNOx | −1.00 × 10−2 | 0 |
| NxOy | 0 | 0 |
| CHx (x ≠ 4) | 0 | 0 |
| CH4 | 1.00 × 10−3– 3.00 × 10−2 | 0 |
| CO | −1.00 × 10−6 | 0 |
| N | 0 | −1.60 × 109 |
| HxCyO | −1.00 × 10−2 | 0 |
| NO | 1.07 × 108– 1.49 × 108 | 0 |
| HCN | 3.43 × 100– 6.67 × 100 | 0 |
| CxHy (y = even) | −1.00 × 10−3 | 0 |
| CxHy (y = odd) | 0 | 0 |
| CN | −1.00 × 10−2 | 0 |
| CxN | −1.00 × 10−2 | 0 |
| HxCyNz | −1.00 × 10−2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, M.; Adams, D.; Wong, M.L.; Dunn, P.; Yung, Y.L. New Estimates of Nitrogen Fixation on Early Earth. Life 2024, 14, 601. https://doi.org/10.3390/life14050601
Christensen M, Adams D, Wong ML, Dunn P, Yung YL. New Estimates of Nitrogen Fixation on Early Earth. Life. 2024; 14(5):601. https://doi.org/10.3390/life14050601
Chicago/Turabian StyleChristensen, Madeline, Danica Adams, Michael L. Wong, Patrick Dunn, and Yuk L. Yung. 2024. "New Estimates of Nitrogen Fixation on Early Earth" Life 14, no. 5: 601. https://doi.org/10.3390/life14050601





