An Exploratory Bioinformatic Investigation of Cats’ Susceptibility to Coronavirus-Deriving Epitopes
Abstract
:1. Introduction
2. Materials and Methods
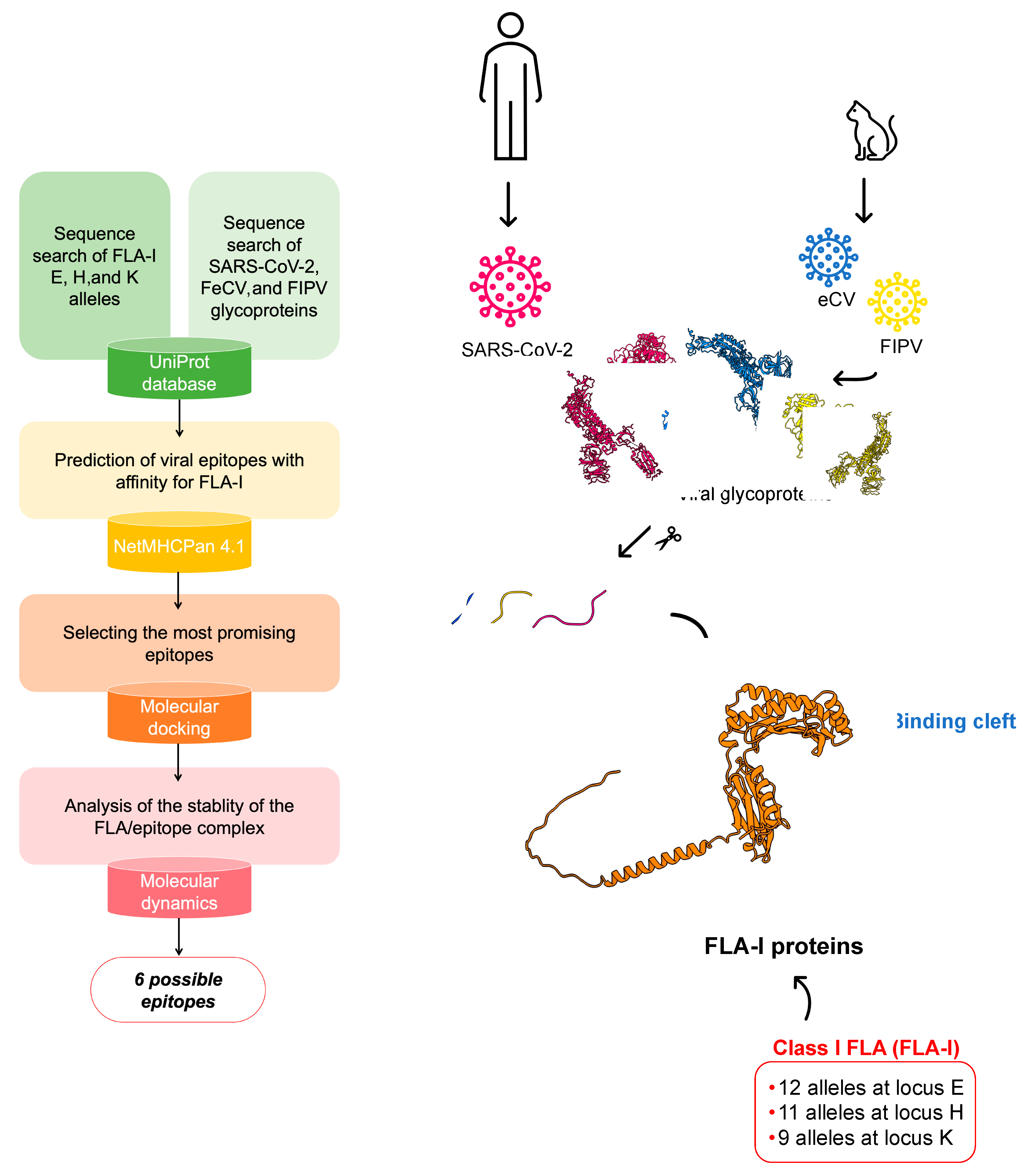
2.1. Epitope Mapping, Sequence Alignment, and Peptide Search
2.2. Molecular Docking
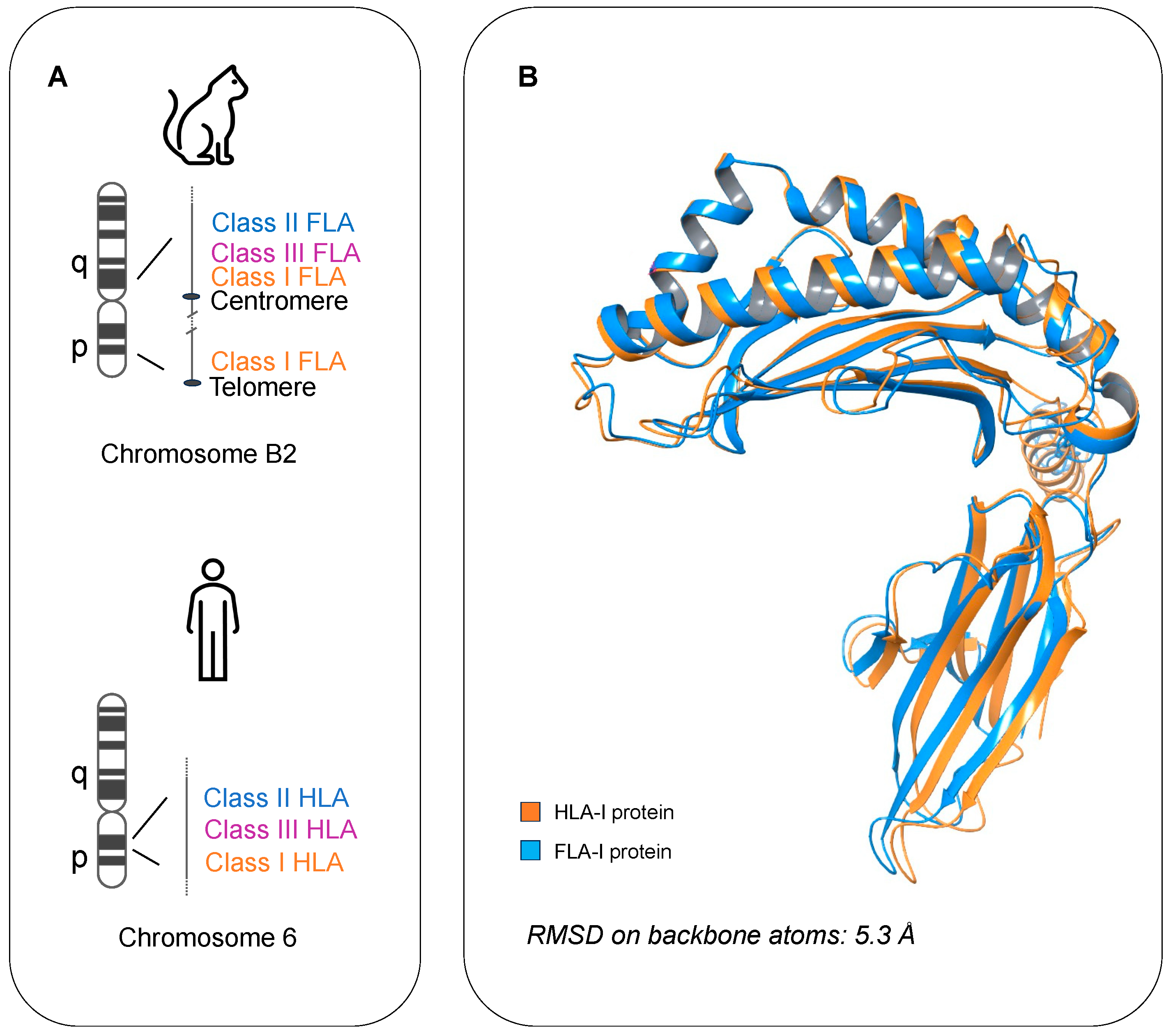
2.3. Protein Structure Prediction with AlphaFold2
2.4. Molecular Dynamics
3. Results
3.1. Epitope Mapping
3.2. Sequence Alignment
3.3. Molecular Docking
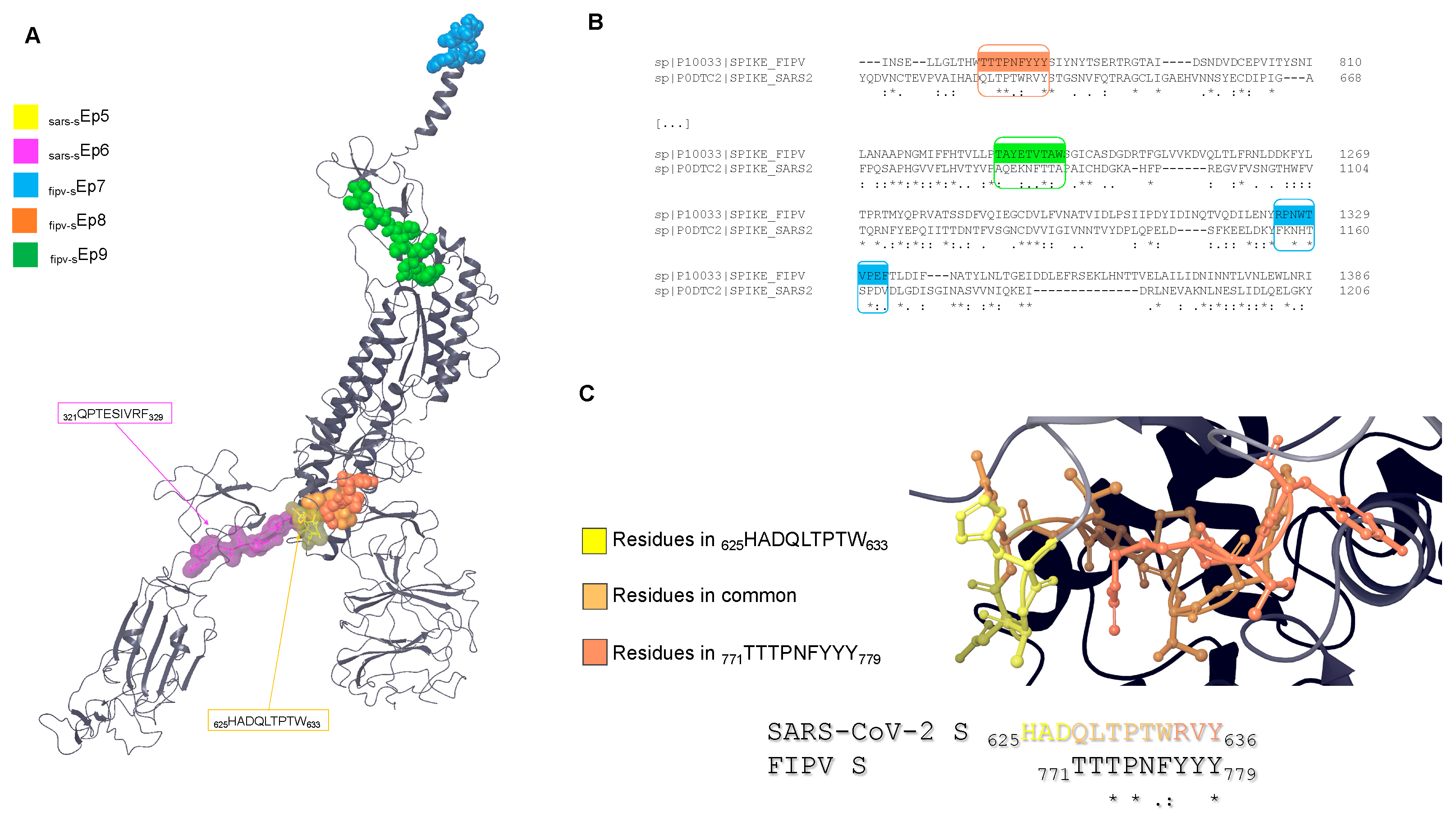
3.4. Analysis of Possible Surface Exposition
3.5. Molecular Dynamics
- sars-rEp1 with FLA-I H*00501 for the outstanding docking score (–22.6 extended (e) and –22.5 helix (h));
- fipv-rEp4 with FLA-I H*00501 for the docking score (–19.8 e and –20.2 h) and the possible localization on the viral glycoprotein surface;
- sars-sEp5 with FLA-I E*01001 and sars-sEp6 with FLA-I E*00701 for the docking scores (–16.3 e and –14.5 h; –15.6 e and –16.3 h) and the position on the viral glycoprotein surface;
- fipv-sEp8 with FLA-I H*00501 and fipv-sEp9 with H*00401 for the docking scores (–16.5 e and –16.3 h; –15.5 e and –15.5 h) and the possible localization on the viral glycoprotein surface.
3.6. Peptide Search
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kipar, A.; Meli, M.L. Feline infectious peritonitis: Still an enigma? Vet. Pathol. 2014, 51, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Coggins, S.J.; Norris, J.M.; Malik, R.; Govendir, M.; Hall, E.J.; Kimble, B.; Thompson, M.F. Outcomes of treatment of cats with feline infectious peritonitis using parenterally administered remdesivir, with or without transition to orally administered GS-441524. J. Veter Intern. Med. 2023, 37, 1772–1783. [Google Scholar] [CrossRef]
- Sweet, A.; Andre, N.; Whittaker, G. RNA in-situ hybridization for pathology-based diagnosis of feline infectious peritonitis (FIP): Current diagnostics for FIP and comparison to the current gold standard. Qeios 2022. [Google Scholar] [CrossRef]
- Olsen, C.W.; Corapi, W.V.; Ngichabe, C.K.; Baines, J.D.; Scott, F.W. Monoclonal antibodies to the spike protein of feline infectious peritonitis virus mediate antibody-dependent enhancement of infection of feline macrophages. J. Virol. 1992, 66, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C. An overview of feline enteric coronavirus and infectious peritonitis virus infections. Feline Pract. 1995, 23, 7–20. [Google Scholar]
- Hayashi, T.; Doi, K.; Fujiwara, K. Role of circulating antibodies and thymus-dependent lymphocytes in production of effusive type feline infectious peritonitis after oral infection. Mol. Biol. Pathog. Coronaviruses 1984, 45, 383–384. [Google Scholar]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef]
- Sit, T.H.C.; Brackman, C.J.; Ip, S.M.; Tam, K.W.S.; Law, P.Y.T.; To, E.M.W.; Yu, V.Y.T.; Sims, L.D.; Tsang, D.N.C.; Chu, D.K.W.; et al. Infection of dogs with SARS-CoV-2. Nature 2020, 586, 776–778. [Google Scholar] [CrossRef]
- Buonocore, M.; Marino, C.; Grimaldi, M.; Santoro, A.; Firoznezhad, M.; Paciello, O.; Prisco, F.; D’Ursi, A.M. New putative animal reservoirs of SARS-CoV-2 in Italian fauna: A bioinformatic approach. Heliyon 2020, 6, e05430. [Google Scholar] [CrossRef]
- O’Brien, S.J.; Yuhki, N. Comparative genome organization of the major histocompatibility complex: Lessons from the Felidae. Immunol. Rev. 1999, 167, 133–144. [Google Scholar] [CrossRef]
- Sutmuller, R.P.; Offringa, R.; Melief, C.J. Revival of the regulatory T cell: New targets for drug development. Drug Discov. Today 2004, 9, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V.; Gonzales-Portillo, B.M.; Lee, J.-Y.; Vandenbark, A.; Offner, H. Major histocompatibility complex Class II-based therapy for stroke. Brain Circ. 2021, 7, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Castelli, V.; Bonsack, B.; Coats, A.B.; Navarro-Torres, L.; Garcia-Sanchez, J.; Kingsbury, C.; Nguyen, H.; Vandenbark, A.A.; Meza-Romero, R. A Novel Partial MHC Class II Construct, DRmQ, Inhibits Central and Peripheral Inflammatory Responses to Promote Neuroprotection in Experimental Stroke. Transl. Stroke Res. 2020, 11, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Kingsbury, C.; Lee, J.; Vandenbark, A.A.; Meza-Romero, R.; Offner, H.; Borlongan, C.V. Spleen participation in partial MHC class II construct neuroprotection in stroke. CNS Neurosci. Ther. 2020, 26, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Benedek, G.; Vandenbark, A.A.; Alkayed, N.J.; Offner, H. Partial MHC class II constructs as novel immunomodulatory therapy for stroke. Neurochem. Int. 2017, 107, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Benedek, G.; Zhu, W.; Libal, N.; Casper, A.; Yu, X.; Meza-Romero, R.; Vandenbark, A.A.; Alkayed, N.J.; Offner, H. A novel HLA-DRα1-MOG-35-55 construct treats experimental stroke. Metab. Brain Dis. 2014, 29, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Correale, P.; Mutti, L.; Pentimalli, F.; Baglio, G.; Saladino, R.E.; Sileri, P.; Giordano, A. HLA-B*44 and C*01 Prevalence Correlates with COVID-19 Spreading across Italy. Int. J. Mol. Sci. 2020, 21, 5205. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Sun, Y.; Liu, Y.; Wang, J.; Wu, Y.; Li, Z.; Ma, L.; Zhang, N.; Zhang, L.; Wei, X.; et al. Major Histocompatibility Complex Class I (FLA-E*01801) Molecular Structure in Domestic Cats Demonstrates Species-Specific Characteristics in Presenting Viral Antigen Peptides. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Beck, T.W.; Menninger, J.; Murphy, W.J.; Nash, W.G.; O’brien, S.J.; Yuhki, N. The feline major histocompatibility complex is rearranged by an inversion with a breakpoint in the distal class I region. Immunogenetics 2005, 56, 702–709. [Google Scholar] [CrossRef]
- Yuhki, N.; Beck, T.; Stephens, R.; Neelam, B.; O’Brien, S.J. Comparative Genomic Structure of Human, Dog, and Cat MHC: HLA, DLA, and FLA. J. Hered. 2007, 98, 390–399. [Google Scholar] [CrossRef]
- Holmes, J.C.; Holmer, S.G.; Ross, P.; Buntzman, A.S.; Frelinger, J.A.; Hess, P.R. Polymorphisms and tissue expression of the feline leukocyte antigen class I loci FLAI-E, FLAI-H, and FLAI-K. Immunogenetics 2013, 65, 675–689. [Google Scholar] [CrossRef]
- Aranyos, A.M.; Roff, S.R.; Pu, R.; Owen, J.L.; Coleman, J.K.; Yamamoto, J.K. An initial examination of the potential role of T-cell immunity in protection against feline immunodeficiency virus (FIV) infection. Vaccine 2016, 34, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Omori, M.; Pu, R.; Tanabe, T.; Hou, W.; Coleman, J.K.; Arai, M.; Yamamoto, J.K. Cellular immune responses to feline immunodeficiency virus (FIV) induced by dual-subtype FIV vaccine. Vaccine 2004, 23, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Pu, R.; Omori, M.; Okada, S.; Rine, S.L.; Lewis, B.A.; Lipton, E.; Yamamoto, J.K. MHC-restricted protection of cats against FIV infection by adoptive transfer of immune cells from FIV-vaccinated donors. Cell Immunol. 1999, 198, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Reynisson, B.; Alvarez, B.; Paul, S.; Peters, B.; Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020, 48, W449–W454. [Google Scholar] [CrossRef] [PubMed]
- UniProt, C. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar]
- Rammensee, H.-G. Chemistry of peptides associated with MHC class I and class II molecules. Curr. Opin. Immunol. 1995, 7, 85–96. [Google Scholar] [CrossRef]
- Schrödinger, LLC. Maestro, version 2023-2; Schrödinger: New York, NY, USA, 2023.
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Jin, B.; Li, H.; Huang, S.-Y. Hpepdock: A web server for blind peptide–protein docking based on a hierarchical algorithm. Nucleic Acids Res. 2018, 46, W443–W450. [Google Scholar] [CrossRef]
- Zhang, Y.; Sanner, M.F. AutoDock CrankPep: Combining folding and docking to predict protein–peptide complexes. Bioinformatics 2019, 35, 5121–5127. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Huang, J.; Mackerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef]
- Gattiker, A.; Gasteiger, E.; Bairoch, A. ScanProsite: A reference implementation of a PROSITE scanning tool. Appl. Bioinform. 2002, 1, 107–108. [Google Scholar]
- NCBI Virus. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004—22 February 2024. Available online: www.ncbi.nlm.nih.gov/labs/virus/vssi/#/ (accessed on 22 February 2024).
- Kirchdoerfer, R.N.; Ward, A.B. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat. Commun. 2019, 10, 2342. [Google Scholar] [CrossRef]
- Wang, K.; Jia, Z.; Bao, L.; Wang, L.; Cao, L.; Chi, H.; Hu, Y.; Li, Q.; Zhou, Y.; Jiang, Y.; et al. Memory B cell repertoire from triple vaccinees against diverse SARS-CoV-2 variants. Nature 2022, 603, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, S.; Rahimova, R.; Sala, M.; Scala, M.C.; Vivenzio, G.; Musella, S.; Andrei, G.; Remans, K.; Mammri, L.; Snoeck, R. Rational design of the zonulin inhibitor AT1001 derivatives as potential anti SARS-CoV-2. Eur. J. Med. Chem. 2022, 244, 114857. [Google Scholar] [CrossRef] [PubMed]
- Weingarten-Gabbay, S.; Klaeger, S.; Sarkizova, S.; Pearlman, L.R.; Chen, D.Y.; Gallagher, K.M.E.; Bauer, M.R.; Taylor, H.B.; Dunn, W.A.; Tarr, C.J.; et al. Profiling SARS-CoV-2 HLA-I peptidome reveals T cell epitopes from out-of-frame ORFs. Cell 2021, 184, 3962–3980 e3917. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859 e811. [Google Scholar] [CrossRef] [PubMed]
- Gattinger, P.; Niespodziana, K.; Stiasny, K.; Sahanic, S.; Tulaeva, I.; Borochova, K.; Dorofeeva, Y.; Schlederer, T.; Sonnweber, T.; Hofer, G.; et al. Neutralization of SARS-CoV-2 requires antibodies against conformational receptor-binding domain epitopes. Allergy 2022, 77, 230–242. [Google Scholar] [CrossRef]
- Zhang, S.; Qiao, S.; Yu, J.; Zeng, J.; Shan, S.; Tian, L.; Lan, J.; Zhang, L.; Wang, X. Bat and pangolin coronavirus spike glycoprotein structures provide insights into SARS-CoV-2 evolution. Nat. Commun. 2021, 12, 1607. [Google Scholar] [CrossRef]
- Horzinek, M.C.; Lutz, H.; Pedersen, N.C. Antigenic relationships among homologous structural polypeptides of porcine, feline, and canine coronaviruses. Infect. Immun. 1982, 37, 1148–1155. [Google Scholar] [CrossRef]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef] [PubMed]
- Federico, L.; Malone, B.; Tennoe, S.; Chaban, V.; Osen, J.R.; Gainullin, M.; Smorodina, E.; Kared, H.; Akbar, R.; Greiff, V.; et al. Experimental validation of immunogenic SARS-CoV-2 T cell epitopes identified by artificial intelligence. Front Immunol. 2023, 14, 1265044. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.I.; Mateyka, L.M.; Jarosch, S.; Grass, V.; Weber, S.; Schober, K.; Hammel, M.; Burrell, T.; Kalali, B.; Poppert, H.; et al. Recruitment of highly cytotoxic CD8(+) T cell receptors in mild SARS-CoV-2 infection. Cell Rep. 2022, 38, 110214. [Google Scholar] [CrossRef] [PubMed]
- Lang-Meli, J.; Luxenburger, H.; Wild, K.; Karl, V.; Oberhardt, V.; Alizei, E.S.; Graeser, A.; Reinscheid, M.; Roehlen, N.; Reeg, D.B.; et al. SARS-CoV-2-specific T-cell epitope repertoire in convalescent and mRNA-vaccinated individuals. Nat. Microbiol. 2022, 7, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Sidney, J.; Kidd, C.K.; Dan, J.M.; Ramirez, S.I.; Yu, E.D.; Mateus, J.; da Silva Antunes, R.; Moore, E.; Rubiro, P.; et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep. Med. 2021, 2, 100204. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Oberhardt, V.; Luxenburger, H.; Kemming, J.; Schulien, I.; Ciminski, K.; Giese, S.; Csernalabics, B.; Lang-Meli, J.; Janowska, I.; Staniek, J.; et al. Rapid and stable mobilization of CD8(+) T cells by SARS-CoV-2 mRNA vaccine. Nature 2021, 597, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Nathan, A.; Rossin, E.J.; Kaseke, C.; Park, R.J.; Khatri, A.; Koundakjian, D.; Urbach, J.M.; Singh, N.K.; Bashirova, A.; Tano-Menka, R.; et al. Structure-guided T cell vaccine design for SARS-CoV-2 variants and sarbecoviruses. Cell 2021, 184, 4401–4413 e4410. [Google Scholar] [CrossRef] [PubMed]
- Satoh, R.; Furukawa, T.; Kotake, M.; Takano, T.; Motokawa, K.; Gemma, T.; Watanabe, R.; Arai, S.; Hohdatsu, T. Screening and identification of T helper 1 and linear immunodominant antibody-binding epitopes in the spike 2 domain and the nucleocapsid protein of feline infectious peritonitis virus. Vaccine 2011, 29, 1791–1800. [Google Scholar] [CrossRef] [PubMed]


| Extended Conformation | Helix Conformation | ||||||
|---|---|---|---|---|---|---|---|
| Organism | Viral Glycoprotein | Epitope | FLA-I Receptor | Docking Score (kcal/mol) | Number of Poses with RMSD < 6 Å | Docking Score (kcal/mol) | Number of Poses with RMSD < 6 Å |
| SARS-CoV-2 | R1a | RTIKGTHHW | FLA-I H*00501 | −22.6 | 41 | −22.5 | 40 |
| SARS-CoV-2 | R1ab | KQFDTYNLW | FLA-I K*00701 | −20.8 | 27 | −19.1 | 51 |
| FIPV | R1ab | AANELNITW | FLA-I H*00501 | −20.5 | 18 | −19 | 31 |
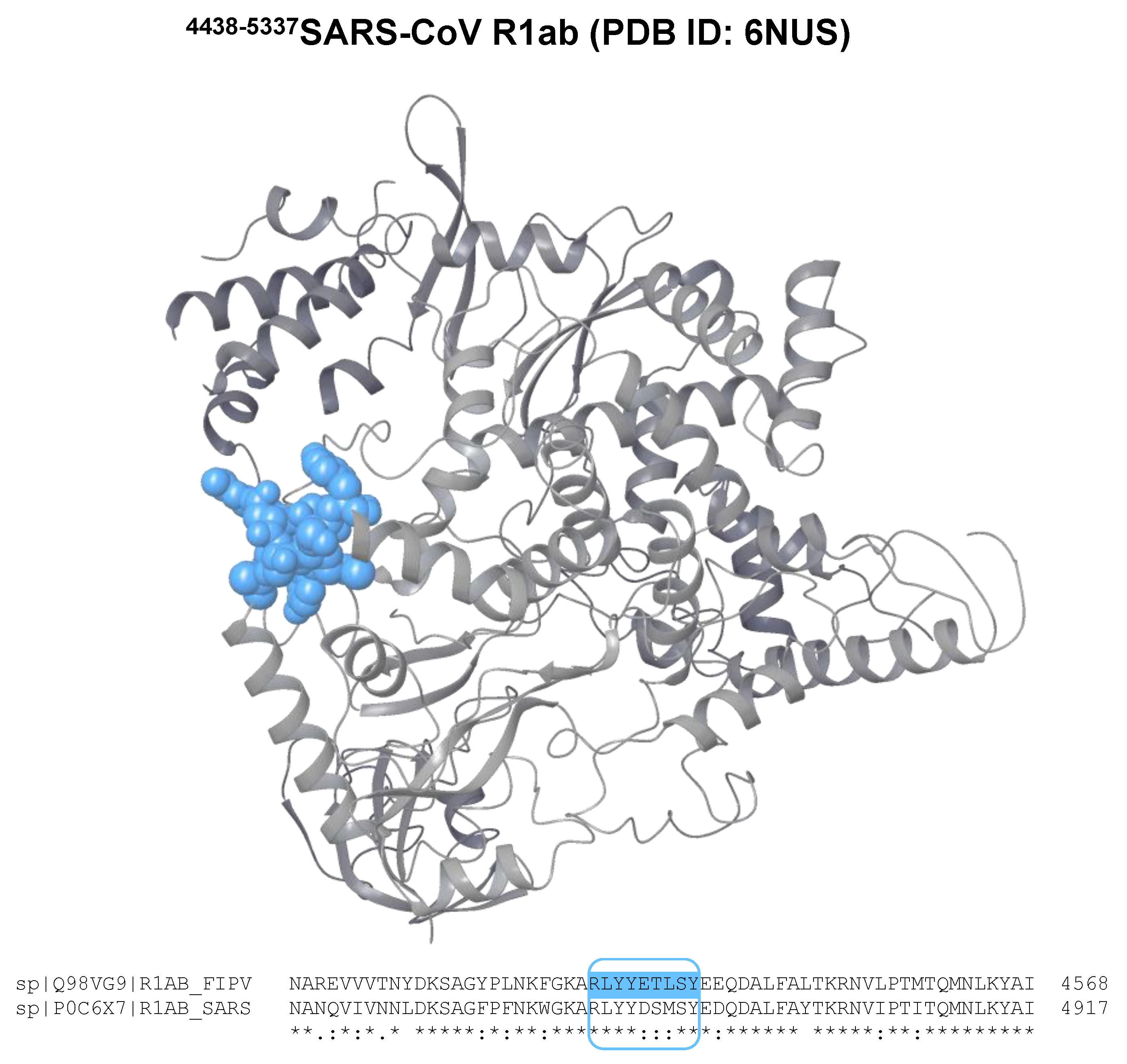
| FIPV | R1ab | RLYYETLSY | FLA-I H*00501 | −19.8 | 22 | −20.2 | 43 |
| SARS-CoV-2 | R1ab | KQFDTYNLW | FLA-I H*00501 | −19.3 | 22 | −17 | 32 |
| SARS-CoV-2 | R1a | RTIKGTHHW | FLA-I K*00701 | −19.2 | 38 | −18.7 | 35 |
| SARS-CoV-2 | R1a | RTIKGTHHW | FLA-I H*00401 | −18.7 | 15 | −19.6 | 7 |
| FIPV | R1ab | YNLDIPHKL | FLA-I K*00701 | −18.7 | 19 | −18.3 | 26 |
| SARS-CoV-2 | R1a | RTIKGTHHW | FLA-I H*008012 | −18.4 | 7 | −18.1 | 8 |
| SARS-CoV-2 | R1a | VPFWITIAY | FLA-I E*00701 | −17.1 | 6 | −18.1 | 16 |
| FIPV | R1ab | AANELNITW | FLA-I H*00401 | −17 | 19 | −18.1 | 25 |
| SARS-CoV-2 | R1a | VPFWITIAY | FLA-I K*00801 | −16.8 | 20 | −15.2 | 40 |
| FIPV | R1ab | RLYYETLSY | FLA-I H*00401 | −16.7 | 22 | −18 | 23 |
| FIPV | S | RPNWTVPEF | FLA-I E*00701 | −16.6 | 21 | −15.4 | 27 |
| FIPV | S | TTTPNFYYY | FLA-I H*00501 | −16.5 | 6 | −16.3 | 44 |
| SARS-CoV-2 | R1a | VPMEKLKTL | FLA-I E*00701 | −16.4 | 29 | −15.8 | 22 |
| SARS-CoV-2 | R1a | RTIKVFTTV | FLA-I E*01101 | −16.4 | 2 | −14.7 | 21 |
| SARS-CoV-2 | S | HADQLTPTW | FLA-I E*01001 | −16.3 | 55 | −14.5 | 19 |
| FIPV | S | RPNWTVPEF | FLA-I E*00101 | −16.2 | 29 | −13.2 | 21 |
| SARS-CoV-2 | R1a | VPFWITIAY | FLA-I E*00101 | −16.1 | 30 | −17.9 | 39 |
| FIPV | R1ab | QNFDTYMLW | FLA-I H*00501 | −16 | 12 | −19.2 | 33 |
| SARS-CoV-2 | S | HADQLTPTW | FLA-I H*008012 | −16 | 9 | −14.5 | 3 |
| SARS-CoV-2 | S | HADQLTPTW | FLA-I E*00101 | −15.9 | 17 | −14.5 | 19 |
| SARS-CoV-2 | S | HADQLTPTW | FLA-I H*00401 | −15.9 | 11 | −16 | 23 |
| SARS-CoV-2 | S | QPTESIVRF | FLA-I E*00701 | −15.6 | 14 | −16.3 | 17 |
| FIPV | S | TAYETVTAW | FLA-I H*00401 | −15.5 | 7 | −15.5 | 10 |
| SARS-CoV-2 | R1a | VPFWITIAY | FLA-I E*00501 | −15.2 | 3 | −15.2 | 7 |
| SARS-CoV-2 | R1a | VPMEKLKTL | FLA-I E*00101 | −15.1 | 16 | −14.5 | 20 |
| SARS-CoV-2 | R1a | VPFWITIAY | FLA-I H*008012 | −14.8 | 16 | −14.6 | 27 |
| SARS-CoV-2 | R1a | VPMEKLKTL | FLA-I K*00801 | −14.8 | 31 | −16.2 | 15 |
| SARS-CoV-2 | S | QPTESIVRF | FLA-I E*00501 | −14.5 | 16 | −14.5 | 2 |
| SARS-CoV-2 | R1a | VPMEKLKTL | FLA-I E*00501 | −14.4 | 9 | −14.2 | 23 |
| SARS-CoV-2 | S | QPTESIVRF | FLA-I E*00101 | −14.4 | 32 | −15.9 | 17 |
| SARS-CoV-2 | R1a | VPFWITIAY | FLA-I E*00501 | −14.3 | 7 | −14.2 | 24 |
| SARS-CoV-2 | R1a | LPSLATVAY | FLA-I E*00101 | −14.1 | 20 | −14.4 | 36 |
| FIPV | R1ab | YPYGSGMVV | FLA-I K*00801 | −13.8 | 35 | −14.8 | 39 |
| FIPV | S | RPNWTVPEF | FLA-I E*00501 | −13.1 | 1 | −11.7 | 5 |
| FIPV | S | TAYETVTAW | FLA-I H*008012 | −13.1 | 11 | −13.6 | 38 |
| FIPV | R1ab | RPIPDVPAY | FLA-I E*00501 | −13 | 16 | −11.3 | 26 |
| SARS-CoV-2 | S | QPTESIVRF | FLA-I E*00501 | −12.7 | 1 | −13.4 | 6 |
| FIPV | R1ab | RPIPDVPAY | FLA-I E*00701 | −12.5 | 11 | −13.7 | 34 |
| SARS-CoV-2 | R1a | LPSLATVAY | FLA-I E*00501 | −12.3 | 40 | −13.2 | 7 |
| FIPV | R1ab | RPIPDVPAY | FLA-I E*00101 | −12.2 | 23 | −10.5 | 20 |
| FIPV | S | TAYETVTAW | FLA-I H*00601 | −11.7 | 18 | −13.7 | 20 |
| SARS-CoV-2 | R1a | VPMEKLKTL | FLA-I E*00501 | −11.4 | 22 | −13.2 | 12 |
| FIPV | R1ab | RPIPDVPAY | FLA-I E*00501 | −11.1 | 5 | −10 | 15 |
| SARS-CoV-2 | R1a | LPSLATVAY | FLA-I E*00501 | −10.1 | 11 | −12.5 | 21 |
| Epitope | Alias |
| SARS-CoV-2 R1a 3574RTIKGTHHW3582 | sars-rEp1 |
| SARS-CoV-2 R1ab 6437KQFDTYNLW6445 | sars-rEp2 |
| FIPV R1ab 3756AANELNITW3764 | fipv-rEp3 |
| FIPV R1ab 4533RLYYETLSY4541 | fipv-rEp4 |
| SARS-CoV-2 S 625HADQLTPTW633 | sars-sEp5 |
| SARS-CoV-2 S 321QPTESIVRF329 | sars-sEp6 |
| FIPV S 1325RPNWTVPEF1333 | fipv-sEp7 |
| FIPV S 771TTTPNFYYY779 | fipv-sEp8 |
| FIPV S 1228TAYETVTAW1236 | fipv-sEp9 |
| fipv-rEp4 (FIPV R1ab 4533RLYYETLSY4541—UniProt ID: Q98VG9) | ||
|---|---|---|
| Retrieved from | ||
| Organism | Protein | UniProt ID |
| Porcine transmissible gastroenteritis coronavirus (strain Purdue) (TGEV) | R1ab | P0C6Y5 |
| Canine coronavirus | R1ab | A0A0D5ZXX1 |
| Mink coronavirus strain WD1133 | R1ab | D9J202 |
| Swine enteric coronavirus | R1ab | A0A0U2LWJ9 |
| Transmissible gastroenteritis virus | R1ab | C8YR34 |
| fipv-sEp8 (FIPV S 771TTTPNFYYY779—UniProt ID: P10033) | ||
| Retrieved from | ||
| Organism | Protein | UniProt ID |
| Canine coronavirus (strain BGF10) | S | Q7T6T3 |
| Canine coronavirus K378 | S | Q65984 |
| Canine coronavirus strain Insavc-1 | S | P36300 |
| Porcine transmissible gastroenteritis coronavirus (strain Miller) (TGEV) | S | P33470 |
| Porcine transmissible gastroenteritis coronavirus (strain FS772/70) (TGEV) | S | P18450 |
| Porcine transmissible gastroenteritis coronavirus (strain NEB72-rt) (TGEV) | S | Q01977 |
| Porcine transmissible gastroenteritis coronavirus (strain Purdue) (TGEV) | S | P07946 |
| fipv-sEp9 (FIPV S 771TAYETVTAW779—UniProt ID: P10033) | ||
| Retrieved from | ||
| Organism | Protein | UniProt ID |
| Canine coronavirus (strain BGF10) | S | Q7T6T3 |
| Canine coronavirus K378 | S | Q65984 |
| Canine coronavirus strain Insavc-1 | S | P36300 |
| Porcine transmissible gastroenteritis coronavirus (strain Miller) (TGEV) | S | P33470 |
| Porcine transmissible gastroenteritis coronavirus (strain FS772/70) (TGEV) | S | P18450 |
| Porcine transmissible gastroenteritis coronavirus (strain NEB72-rt) (TGEV) | S | Q01977 |
| Porcine transmissible gastroenteritis coronavirus (strain Purdue) (TGEV) | S | P07946 |
| Mink coronavirus strain WD1133 | S | D9J204 |
| Porcine respiratory coronavirus (strain RM4) | S | P24413 |
| Porcine respiratory coronavirus (86/137004/isolate British) | S | P27655 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonocore, M.; De Biase, D.; Sorrentino, D.; Giordano, A.; Paciello, O.; D’Ursi, A.M. An Exploratory Bioinformatic Investigation of Cats’ Susceptibility to Coronavirus-Deriving Epitopes. Life 2024, 14, 334. https://doi.org/10.3390/life14030334
Buonocore M, De Biase D, Sorrentino D, Giordano A, Paciello O, D’Ursi AM. An Exploratory Bioinformatic Investigation of Cats’ Susceptibility to Coronavirus-Deriving Epitopes. Life. 2024; 14(3):334. https://doi.org/10.3390/life14030334
Chicago/Turabian StyleBuonocore, Michela, Davide De Biase, Domenico Sorrentino, Antonio Giordano, Orlando Paciello, and Anna Maria D’Ursi. 2024. "An Exploratory Bioinformatic Investigation of Cats’ Susceptibility to Coronavirus-Deriving Epitopes" Life 14, no. 3: 334. https://doi.org/10.3390/life14030334






