Improvement in the Stability and Enzymatic Activity of Pleurotus sapidus Lipoxygenase Dissolved in Natural Deep Eutectic Solvents (NADESs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NADESs
2.3. Protein Expression and Purification
2.4. Enzyme Activity Determination
2.5. LOXPSA Thermostability in Aqueous NADES Media
2.6. Biotransformation of Piperine to Piperonal with LOXPSA in NADES
2.6.1. UHPLC-DAD Measurements
2.6.2. GC-TDS-MS Measurements
3. Results
3.1. LOXPSA Activity Improvement Using NADESs
3.2. Enzyme Stability in NADES
3.3. Biotransformation of Piperine to Piperonal with LOX in NADESs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Feussner, I.; Kühn, H. Application of lipoxygenases and related enzymes for the preparation of oxygenated lipids. Enzym. Lipid Modif. 2000, 307–336. [Google Scholar] [CrossRef]
- Waldmann, D.; Schreier, P. Stereochemical studies of epoxides formed by lipoxygenase-catalyzed co-oxidation of retinol, .beta.-Ionone, and 4-Hydroxy-.beta.-ionone. J. Agric. Food Chem. 1995, 43, 626–630. [Google Scholar] [CrossRef]
- Arens, D.; Seilmeier, W.; Weber, F.; Kloos, G.; Grosch, W. Purification and properties of a carotene co-oxidizing lipoxygenase from peas. Biochim. Biophys. Acta (BBA)-Enzymol. 1973, 327, 295–305. [Google Scholar] [CrossRef]
- Krahe, N.K.; Berger, R.G.; Kahlert, L.; Ersoy, F. Co-Oxidative transformation of piperine to piperonal and 3,4-methylenedioxycinnamaldehyde by a lipoxygenase from Pleurotus sapidus. Chembiochem 2021, 22, 2857–2861. [Google Scholar] [CrossRef] [PubMed]
- Akash, J.; Dushyant, C.; Jasmine, C. Piperonal: The journey so far. Mini-Rev. Med. 2020, 20, 1846–1856. [Google Scholar] [CrossRef]
- Gnadinger, C. Piperonal in Vanilla extract. Ind. Eng. Chem. 1926, 18, 588–589. [Google Scholar] [CrossRef]
- Wen, P.; Wu, D.; Zheng, P.; Chen, P.; Liu, S.; Fu, Y. Highly efficient biosynthesis of heliotropin by engineered Escherichia coli coexpressing trans-anethole oxygenase and formate dehydrogenase. J. Agric. Food Chem. 2019, 67, 14121–14128. [Google Scholar] [CrossRef]
- Schwendenwein, D.; Fiume, G.; Weber, H.; Rudroff, F.; Winkler, M. Selective enzymatic transformation to aldehydes in vivo by fungal carboxylate reductase from Neurospora crassa. Adv. Synth. Catal. 2016, 358, 3414–3421. [Google Scholar] [CrossRef]
- Jankowski, N.; Koschorreck, K.; Urlacher, V.B. High-level expression of aryl-alcohol oxidase 2 from Pleurotus eryngii in Pichia pastoris for production of fragrances and bioactive precursors. Appl. Microbiol. Biotechnol. 2020, 104, 9205–9218. [Google Scholar] [CrossRef]
- Adams, T.; Cohen, S.M.; Doull, J.; Feron, V.; Goodman, J.; Marnett, L.; Munro, I.; Portoghese, P.; Smith, R.; Waddell, W. The FEMA GRAS assessment of hydroxy-and alkoxy-substituted benzyl derivatives used as flavor ingredients. Food Chem. Toxicol. 2005, 43, 1241–1271. [Google Scholar] [CrossRef]
- Thomas, F.; Kayser, O. Natural deep eutectic solvents enhance cannabinoid biotransformation. Biochem. Eng. J. 2022, 180, 108380. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A.; Holmes, S. New eutectic ionic liquids for lipase activation and enzymatic preparation of biodiesel. Org. Biomol. Chem. 2011, 9, 1908–1916. [Google Scholar] [CrossRef]
- Koh, Q.Q.; Kua, Y.L.; Gan, S.; Tan, K.W.; Lee, T.Z.E.; Cheng, W.K.; Lau, H.L.N. Sugar-based natural deep eutectic solvent (NADES): Physicochemical properties, antimicrobial activity, toxicity, biodegradability and potential use as green extraction media for phytonutrients. Sustain. Chem. Pharm. 2023, 35, 101218. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdian, S.; Dabirmanesh, B.; Heydari, A.; Dashtban-Moghadam, E.; Khajeh, K.; Ghazi, F. Activity, stability and structure of laccase in betaine based natural deep eutectic solvents. Int. J. Biol. Macromol. 2018, 107, 2574–2579. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.E.; Andanson, J.-M.; Verney, V. Improving laccase thermostability with aqueous natural deep eutectic solvents. Int. J. Biol. Macromol. 2020, 163, 919–926. [Google Scholar] [CrossRef]
- Vrbka, L.; Jungwirth, P.; Bauduin, P.; Touraud, D.; Kunz, W. Specific ion effects at protein surfaces: A molecular dynamics study of bovine pancreatic trypsin inhibitor and horseradish peroxidase in selected salt solutions. J. Phys. Chem. 2006, 110, 7036–7043. [Google Scholar] [CrossRef]
- Amoroso, R. Chemical stability and catalytic activity of redox enzymes in NADES. Chem. Proc. 2021, 6, 8. [Google Scholar]
- Zelena, K.; Krings, U.; Berger, R.G. Functional expression of a valencene dioxygenase from Pleurotus sapidus in E. coli. Bioresour. Technol. 2012, 108, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, R.-H.; Plagemann, I.; Linke, D.; Zelena, K.; Berger, R.G. Orthologous lipoxygenases of Pleurotus spp.–A comparison of substrate specificity and sequence homology. J. Mol. Catal. B Enzym. 2013, 97, 189–195. [Google Scholar] [CrossRef]
- Plagemann, I.; Zelena, K.; Arendt, P.; Ringel, P.D.; Krings, U.; Berger, R.G. LOXPsa1, the first recombinant lipoxygenase from a basidiomycete fungus. J. Mol. Catal. B Enzym. 2013, 87, 99–104. [Google Scholar] [CrossRef]
- Harifi-Mood, A.R.; Ghobadi, R.; Divsalar, A. The effect of deep eutectic solvents on catalytic function and structure of bovine liver catalase. Int. J. Biol. Macromol. 2017, 95, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Taklimi, S.M.; Divsalar, A.; Ghalandari, B.; Ding, X.; Di Gioia, M.L.; Omar, K.A.; Saboury, A.A. Effects of deep eutectic solvents on the activity and stability of enzymes. J. Mol. Liq. 2023, 377, 121562. [Google Scholar] [CrossRef]
- Alam, M.A.; Muhammad, G.; Khan, M.N.; Mofijur, M.; Lv, Y.; Xiong, W.; Xu, J. Choline chloride-based deep eutectic solvents as green extractants for the isolation of phenolic compounds from biomass. J. Clean. Prod. 2021, 309, 127445. [Google Scholar] [CrossRef]
- Maimulyanti, A.; Nurhidayati, I.; Mellisani, B.; Amelia Rachmawati Putri, F.; Puspita, F.; Restu Prihadi, A. Development of natural deep eutectic solvent (NADES) based on choline chloride as a green solvent to extract phenolic compound from coffee husk waste. Arab. J. Chem. 2023, 16, 104634. [Google Scholar] [CrossRef]
- Cicci, A.; Sed, G.; Bravi, M. Potential of choline chloride-based natural deep eutectic solvents (NaDES) in the extraction of microalgal metabolites. Chem. Eng. Trans. 2017, 57, 61–66. [Google Scholar] [CrossRef]
- Weingärtner, H.; Cabrele, C.; Herrmann, C. How ionic liquids can help to stabilize native proteins. Phys. Chem. Chem. Phys. 2012, 14, 415–426. [Google Scholar] [CrossRef]
- Jha, I.; Rani, A.; Venkatesu, P. Sustained stability and activity of lysozyme in choline chloride against pH induced denaturation. ACS Sustain. Chem. Eng. 2017, 5, 8344–8355. [Google Scholar] [CrossRef]
- Baruah, I.; Borgohain, G. Temperature dependent molecular dynamics simulation study to understand the stabilizing effect of NADES on the protein β-Lactoglobulin. J. Mol. Graph. Model. 2023, 125, 108582. [Google Scholar] [CrossRef] [PubMed]
- Elgharbawy, A.A.; Hayyan, A.; Hayyan, M.; Rashid, S.N.; Nor, M.R.M.; Zulkifli, M.Y.; Alias, Y.; Mirghani, M.E.S. Shedding light on lipase stability in natural deep eutectic solvents. Chem. Biochem. Eng. Q. 2018, 32, 359–370. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Xie, G.; Timasheff, S.N. Mechanism of the stabilization of ribonuclease A by sorbitol: Preferential hydration is greater for the denatured than for the native protein. Protein Sci. 1997, 6, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, M.; Saraiva, J.; Lyssens, J.; Oliveira, J.; Tobback, P. The influence of water activity on thermal stability of horseradish peroxidase. Int. J. Food Sci. 1992, 27, 33–40. [Google Scholar] [CrossRef]
- Gao, W.-W.; Zhang, F.-X.; Zhang, G.-X.; Zhou, C.-H. Key factors affecting the activity and stability of enzymes in ionic liquids and novel applications in biocatalysis. Biochem. Eng. J. 2015, 99, 67–84. [Google Scholar] [CrossRef]
- Gajardo-Parra, N.F.; Meneses, L.; Duarte, A.R.C.; Paiva, A.; Held, C. Assessing the influence of betaine-based natural deep eutectic systems on horseradish peroxidase. ACS Sustain. Chem. Eng. 2022, 10, 12873–12881. [Google Scholar] [CrossRef]
- Gorke, J.T.; Srienc, F.; Kazlauskas, R.J. Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem. Commun. 2008, 1235–1237. [Google Scholar] [CrossRef]
- Nian, B.; Cao, C.; Liu, Y. How Candida antarctica lipase B can be activated in natural deep eutectic solvents: Experimental and molecular dynamics studies. J. Chem. Technol. Biotechnol. 2020, 95, 86–93. [Google Scholar] [CrossRef]
- Panić, M.; Elenkov, M.M.; Roje, M.; Bubalo, M.C.; Redovniković, I.R. Plant-mediated stereoselective biotransformations in natural deep eutectic solvents. Process Biochem. 2018, 66, 133–139. [Google Scholar] [CrossRef]

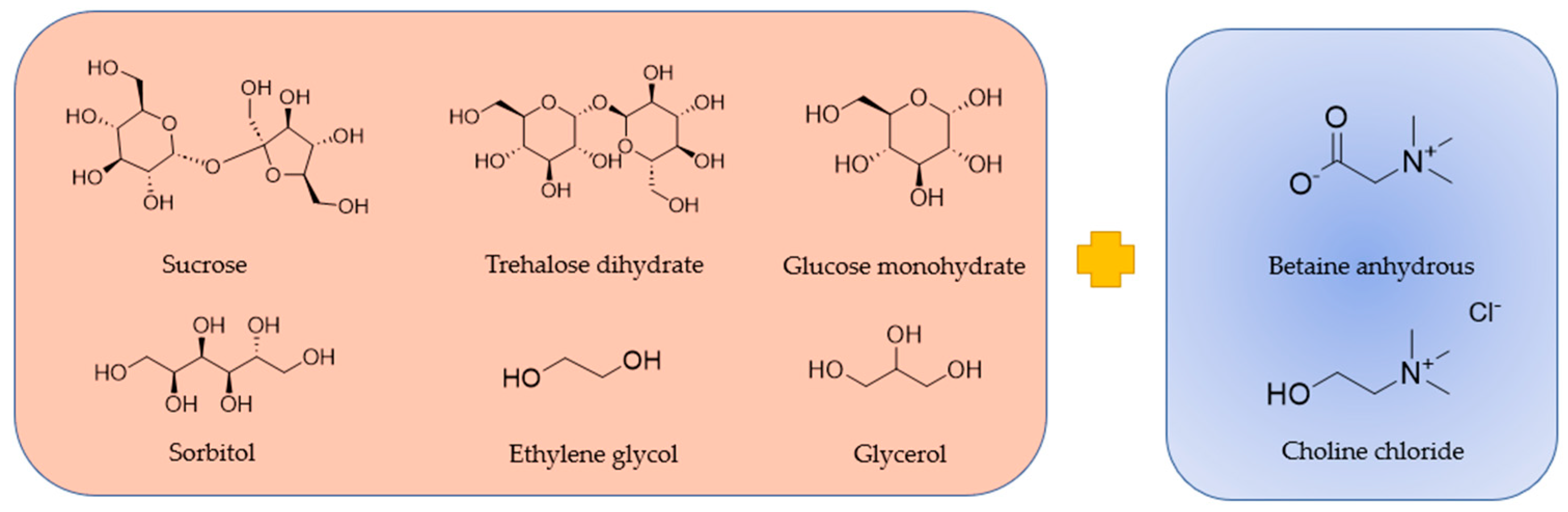



| Abbreviations | Component A | Component B | Component C | Molar Ratio |
|---|---|---|---|---|
| BTrehW | Betaine anhydrous | Trehalose dihydrate | Water | 4:1:14 |
| BSucW | Betaine anhydrous | Sucrose | Water | 2:1:10 |
| BGlu | Betaine anhydrous | Glucose monohydrate | - | 5:2 |
| BGly | Betaine anhydrous | Glycerol | - | 1:2 |
| BSorbW | Betaine anhydrous | Sorbitol | Water | 1:1:3 |
| BEtGly | Betaine anhydrous | Ethylene glycol | - | 1:3 |
| ChChGly | Choline chloride | Glycerol | - | 1:2 |
| ChChEtGly | Choline chloride | Ethylene glycol | - | 1:3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbe, M.; Lehmann, L.T.; Berger, R.G.; Ersoy, F. Improvement in the Stability and Enzymatic Activity of Pleurotus sapidus Lipoxygenase Dissolved in Natural Deep Eutectic Solvents (NADESs). Life 2024, 14, 271. https://doi.org/10.3390/life14020271
Garbe M, Lehmann LT, Berger RG, Ersoy F. Improvement in the Stability and Enzymatic Activity of Pleurotus sapidus Lipoxygenase Dissolved in Natural Deep Eutectic Solvents (NADESs). Life. 2024; 14(2):271. https://doi.org/10.3390/life14020271
Chicago/Turabian StyleGarbe, Maria, Leander Tom Lehmann, Ralf Günter Berger, and Franziska Ersoy. 2024. "Improvement in the Stability and Enzymatic Activity of Pleurotus sapidus Lipoxygenase Dissolved in Natural Deep Eutectic Solvents (NADESs)" Life 14, no. 2: 271. https://doi.org/10.3390/life14020271






