Base Pairing Promoted the Self-Organization of Genetic Coding, Catalysis, and Free-Energy Transduction
Abstract
:1. Introduction
2. Creating the First Bit of Genetic Information from Scratch
2.1. AARS/tRNA Cognate Pairs Function as Mutually Exclusive Molecular AND Gates
2.2. Bidirectional Genetic Coding Projected Duality into the Proteome
2.3. AARS Protozymes Are Amino Acid-Activating Catalysts That Can Be Coded by a Bidirectional Gene
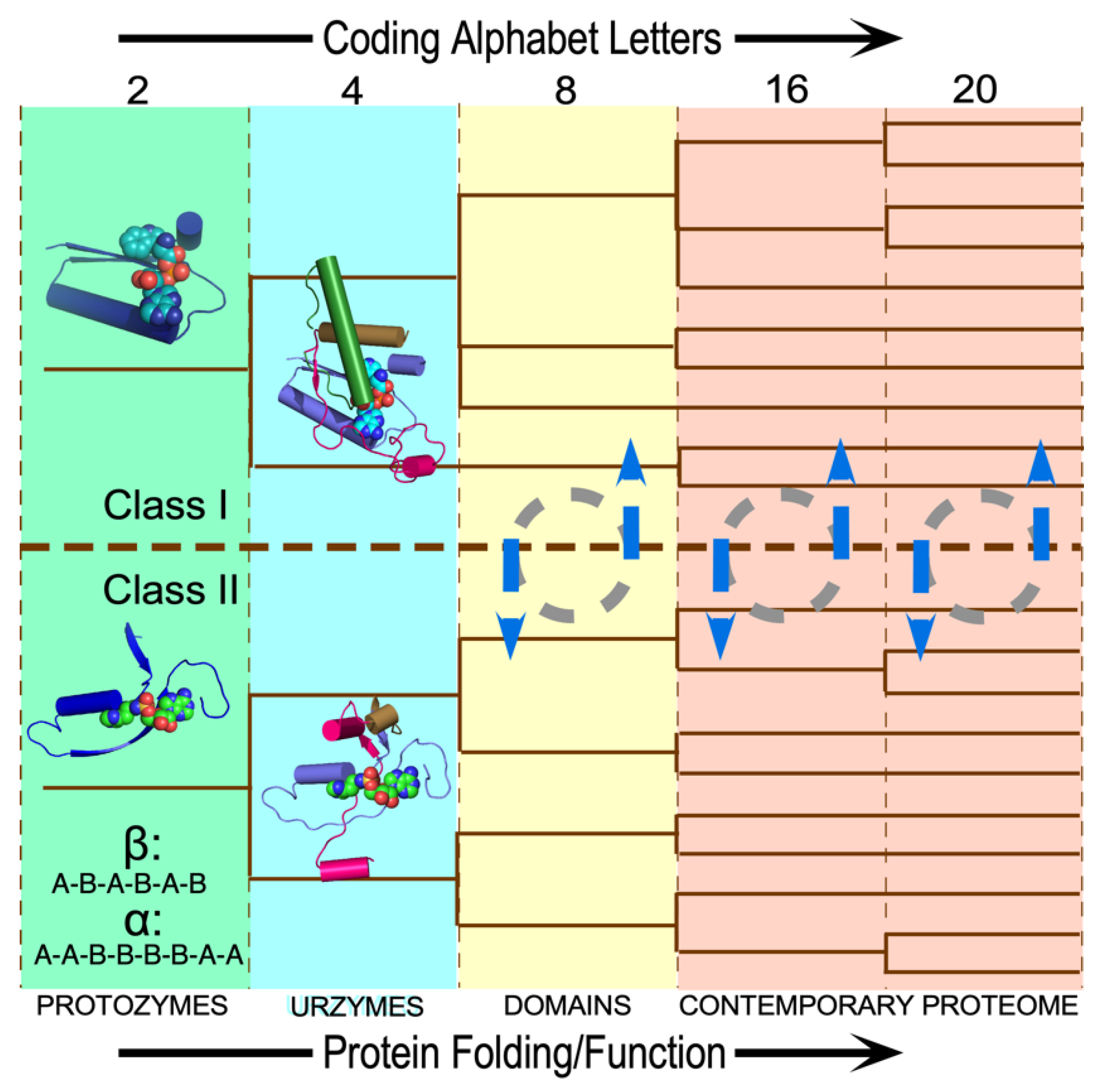
2.4. Consequences of the Inverse Complementarity of Nucleic Acid Base-Pairing Duality Project Deeply into the Proteome
2.5. The Projected Duality Creates Rudimentary Nanomachinery for Chemical Free-Energy Transduction
2.6. The Projected Duality Constrains Substrate Recognition by AARS Urzymes, Dividing Amino Acids and tRNA Acceptor Stems into Parallel Groups
3. Phylogenetics
4. Constraints: Impedance Matching and Reciprocally Coupled Gating
5. Experimental Challenges
5.1. Validating the Role of Bidirectional Coding
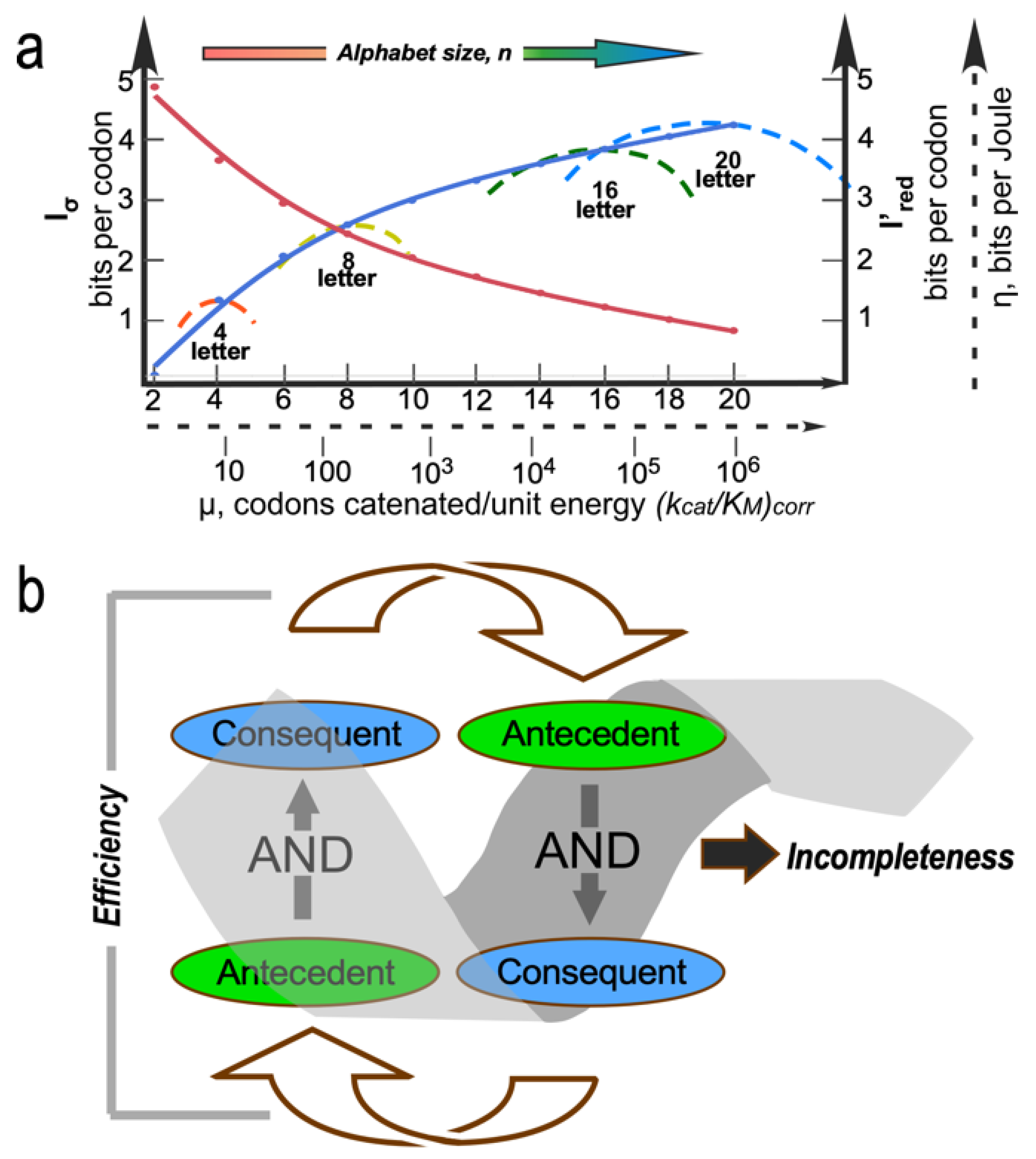
- How many bits (pairs of coding letters) were necessary to make bidirectional gene products sufficiently specific to achieve reflexivity? Experimental validation of reflexivity calls for designing a self-consistent alphabet and set of implementing genes. We must design functional bidirectional genes for Class I and II AARS precursors using a reduced alphabet. Then, those gene products must exhibit the experimental capability to discriminating between appropriate subsets of amino acids and tRNAs well enough to implement the corresponding alphabet.
- 2.
- Is a bidirectional urzyme gene feasible? Naïve analysis of the modular patchwork of Class I and II urzymes (see Figure 4A in [35]) has not resulted in an antiparallel alignment Class I and II urzyme sequences compatible with continuous bidirectional coding of their respective three-dimensional structures. That analysis likely cannot constrain protein design programs as hoped. Recently, a more suitable, alternatively threaded antiparallel alignment emerged. That alignment, also consistent with the high resolution modularity [26], may provide a template for bidirectionally coded urzymes. However, we have yet to test it.
- 3.
- What limitations of bidirectional coding forced its breakdown by providing new functionality? The CP1 insertion at the C-terminus of the protozyme interrupted all extant Class I urzymes, definitively ending bidirectional coding. Eventually, CP1 significantly enhanced amino acid specificity, but only when complemented by the anticodon-binding domain [89,90]. Simultaneous acquisition of both domains seems unlikely, so one might expect a more decisive selective advantage for so significant a modular acquisition. The LeuAC urzyme converts substantial amounts of ATP to ADP in single turnover experiments [46]. If a comparable analysis of the intact catalytic domain reduced ADP production, that would suggest that CP1 initially increased the efficiency of free-energy transduction. Modular deconstruction of Class II AARSs should also shed new light.
- 4.
- Can AARS urzyme acylation of TΨC minihelices confirm details of the operational code? Acylation of minihelices partially substantiated the “single-domain” model for the origin of coding [12]. Evidence that AARS urzymes catalyze acylation of full-length tRNAs [38] further strengthened that model. Recently, we showed that minihelixLeu is an even better substrate for LeuAC than tRNALeu [48]. That can now enable a detailed test of the operational code.
- 5.
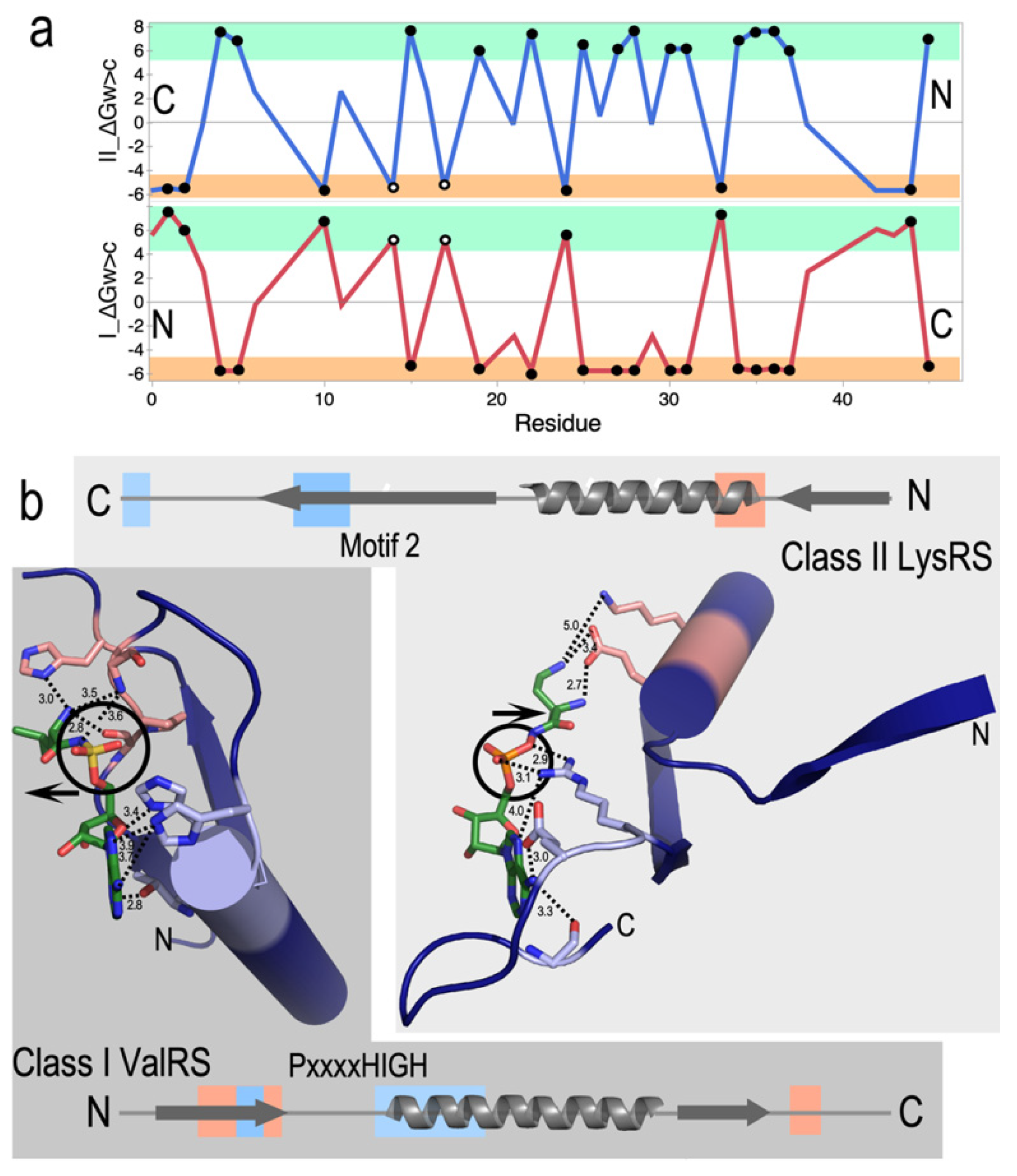
- Can AARS protozymes catalyze tRNA acylation? Polypeptide catalysis of aminoacylation must have appeared sometime between the ancestral bidirectional protozyme gene and the emergence of urzymes. Structures illustrated in Figure 4 are quite sophisticated, even though far simpler than contemporary AARSs. As AARS protozymes likely exemplify earlier ancestral catalytic polymers, it may be notable that the motif 2 loop Class II protozymes retains much of the tRNA-binding site [61], whereas the Class I tRNA-binding site is formed largely by a helix present only in the urzyme. That asymmetry, suggesting that Class II AARSs preceded Class I AARSs, raises the profound objection that functional polypeptides must have depended minimally at least on a binary code. Ribozymes similar to the flexizyme family [113,114] might have accelerated acyl transfer from aminoacyl-5′AMP produced by Class I protozymes to proto-tRNAs, assuring provision of aminoacylated RNAs for templated protein synthesis. That would have required an ad hoc mechanism to discriminate between two types of tRNA.
- 6.
- To what extent can the elements described in Section 2.6 account for the assignments of amino acids to codons in the coding table?There are two credible models attempting to establish how Nature assembled the contemporary coding table. One [28] is driven by the need to account for the AARS Class division. The other [115] has the advantage of preserving the ability of the coding table to optimize bidirectional coding because it preserves the codon–anticodon assignments to core and surface amino acids (Figure 3a; reference [49]). Satisfying both requirements is quite difficult. Neither model appears to be consistent with the goals of the other.Our hope is that progress in ancestral reconstruction can help resolve this important dilemma. Experimental characterization will augment the specificity spectra of both both amino acid [47] and RNA minihelix substrates [48]. Such data will inevitably be necessary to identify and address the underlying questions.
5.2. Beyond Genetic Coding
- (i)
- We can infer sequence/structure relationships from variations in both naturally occurring and designed sequence databases.
- (ii)
- Bioinformatic tools reducing tertiary structures to lower dimensions—conformational angles (φ, ψ); residue transfer free energies (ΔGvapor>chx, ΔGwater>chx) [27,117,118]; TetraDA one-dimensional strings derived from Delaunay tesselation [119]; SNAPP scoring [97,98]—provide alternative multidimensional windows into structural and evolutionary determinants.
- (iii)
- The highly sensitive Malachite Green assay for phosphates generated on amino acid activation [40] affords a five-fold increase in the rate at which assays can be performed on variants of this gene.
- (iv)
- Artificial intelligence has improved both protein design [95] and structure prediction [96], creating a dynamic virtual feedback loop capable of sampling substantially larger regions of the protein sequence space expected for early nodes in AARS speciation. Pruning those sequence distributions virtually, before committing to experimental construction, expression, and testing will greatly enhance the experimental tools described above.
6. Did Creating the Genetic Code Require New Physics?
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, J.D.; Crick, F.H.C. A Structure for Deoxyribose Nucleic Acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, L.; Jimenez-Rodriguez, M.; Gonzalez-Rivera, K.; Williams, T.; Li, L.; Weinreb, V.; Chandrasekaran, S.N.; Collier, M.; Ambroggio, X.; Kuhlman, B.; et al. Functional Class I and II Amino Acid Activating Enzymes Can Be Coded by Opposite Strands of the Same Gene. J. Biol. Chem. 2015, 290, 19710–19725. [Google Scholar] [CrossRef]
- Crick, F.H.C. Central Dogma of Molecular Biology. Nature 1970, 227, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Crick, F.H.C. The Origin of the Genetic Code. J. Mol. Biol. 1968, 38, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Crick, F.H.C. Codon-Anticodon Pairing: The Wobble Hypothesis. J. Mol. Biol. 1966, 19, 548–555. [Google Scholar] [CrossRef]
- Jones, O.W., Jr.; Nirenberg, M.W. Degeneracy in the amino acid code. Biochim. Et Biophys. Acta (BBA)-Nucleic Acids Protein Synth. 1966, 119, 400–406. [Google Scholar] [CrossRef]
- Trupin, J.S.; Rottman, F.M.; Brimacome, R.; Leder, P.; Bernfield, M.R.; Nirenberg, M. RNA Codewords and Protein Synthesis, VI. On the Nucleotide Sequences of Degenerate Codeword Sets for Isoleucine, Tyrosine, Asparagine, and Lysine. Proc. Natl. Acad. Sci. USA 1965, 53, 807–811. [Google Scholar] [CrossRef]
- Berg, P.; Ofengand, E.J. An Enzymatic Mechanism for Linking Amino Acids to RNA. Proc. Nat. Acad. Sci. USA 1958, 44, 78–85. [Google Scholar] [CrossRef]
- Hoagland, M.B.; Keller, E.B.; Zamecnik, P.C. Enzymatic Carboxyl Activation of Amino Acids. J. Biol. Chem. 1956, 21, 345–358. [Google Scholar] [CrossRef]
- Eriani, G.; Delarue, M.; Poch, O.; Gangloff, J.; Moras, D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature 1990, 347, 203–206. [Google Scholar] [CrossRef]
- Ribas de Pouplana, L.; Schimmel, P. Two Classes of tRNA Synthetases Suggested by Sterically Compatible Dockings on tRNA Acceptor Stem. Cell 2001, 104, 191–193. [Google Scholar] [CrossRef]
- Schimmel, P.; Giegé, R.; Moras, D.; Yokoyama, S. An operational RNA code for amino acids and possible relationship to genetic code. Proc. Nat. Acad. Sci. USA 1993, 90, 8763–8768. [Google Scholar] [CrossRef]
- Rodin, S.N.; Ohno, S. Two Types of Aminoacyl-tRNA Synthetases Could be Originally Encoded by Complementary Strands of the Same Nucleic Acid. Orig. Life Evol. Biosph. 1995, 25, 565–589. [Google Scholar] [CrossRef]
- Carter, C.W., Jr.; Wills, P.R. The Roots of Genetic Coding in Aminoacyl-tRNA Synthetase Duality. Annu. Rev. Biochem. 2021, 90, 349–373. [Google Scholar] [CrossRef]
- Carter, C.W., Jr.; Wills, P.R. Interdependence, Reflexivity, Fidelity, and Impedance Matching, and the Evolution of Genetic Coding. Mol. Biol. Evol. 2018, 35, 269–286. [Google Scholar] [CrossRef]
- Wills, P.R. Autocatalysis, information, and coding. BioSystems 2001, 50, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Nieselt-Struwe, K.; Wills, P.R. The Emergence of Genetic Coding in Physical Systems. J. Theor. Biol. 1997, 187, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Schmitt, C.; Liu, Z.; Roberts, S.J.; Liu, K.C.; Röder, K.; Jäschke, A.; Wales, D.J.; Sutherland, J.D. Triplet-Encoded Prebiotic RNA Aminoacylation. J. Am. Chem. Soc. 2023, 145, 15971–15980. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-F.; Su, M.; Liu, Z.; Bjork, S.J.; Sutherland, J.D. Interstrand Aminoacyl Transfer in a tRNA Acceptor Stem-Overhang Mimic. J. Am. Chem. Soc. 2021, 143, 11836–11842. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, L.-F.; Xu, J.; Bonfio, C.; Russell, D.A.; Sutherland, J.D. Harnessing chemical energy for the activation and joining of prebiotic building blocks. Nat. Chem. 2020, 12, 1023–1028. [Google Scholar] [CrossRef]
- Wu, L.-F.; Sutherland, J.D. Provisioning the origin and early evolution of life. Emerg. Top. Life Sci. 2019, 3, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.D. The Origin of Life—Out of the Blue. Angew. Chem. Int. Ed. 2016, 55, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.L.; Hordijk, W.; Steel, M.; Martin, W.F. Autocatalytic sets in E. coli metabolism. J. Syst. Chem. 2015, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Frenkel-Pinter, M.; Haynes, J.W.; Martin, C.; Petrova, A.S.; Burcar, B.T.; Krishnamurthy, R.; Hud, N.V.; Leman, L.J.; Williams, L.D. Selective incorporation of proteinaceous over nonproteinaceous cationic amino acids in model prebiotic oligomerization reactions. Proc. Nat. Acad. Sci. USA 2019, 116, 16338–16346. [Google Scholar] [CrossRef] [PubMed]
- Fournier, G.P.; Andam, C.P.; Alm, E.J.; Gogarten, J.P. Molecular Evolution of Aminoacyl tRNA Synthetase Proteins in the Early History of Life. Orig. Life Evol. Biosph. 2011, 41, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.W., Jr.; Popinga, A.; Bouckaert, R.; Wills, P.R. Multidimensional Phylogenetic Metrics Identify Class I Aminoacyl-tRNA Synthetase Evolutionary Mosaicity and Inter-modular Coupling. Int. J. Mol. Sci. 2022, 23, 1520. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.W., Jr.; Wolfenden, R. Acceptor-stem and anticodon bases embed amino acid chemistry into tRNA. RNA Biol. 2016, 13, 145–151. [Google Scholar] [CrossRef]
- Vetsigian, K.; Woese, C.R.; Goldenfeld, N. Collective Evolution and the Genetic Code. Proc. Nat. Acad. Sci. USA 2006, 103, 10696–10701. [Google Scholar] [CrossRef]
- de Duve, C. The Second Genetic Code. Nature 1988, 333, 117–118. [Google Scholar] [CrossRef]
- Cusack, S.; Berthet-Colominas, C.; Hartlein, M.; Nassar, N.; Leberman, R. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 A. Nature 1990, 347, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Ruff, M.; Krishnaswamy, S.; Boeglin, M.; Poterszman, A.; Mitschler, A.; Podjarny, A.; Rees, B.; Thierry, J.C.; Moras, D. Class II Aminoacyl Transfer RNA Synthetases: Crystal Structure of Yeast Aspartyl-tRNA Synthetase Complexed with tRNAAsp. Science 1991, 252, 1682–1689. [Google Scholar] [CrossRef]
- Carter, C.W., Jr. Cognition Mechanism and Evolutionary Relationships in Aminoacyl-tRNA Synthetases. Annu. Rev. Biochem. 1993, 62, 715–748. [Google Scholar] [CrossRef]
- Cusack, S. Evolutionary Implications. Nat. Struct. Mol. Biol. 1994, 1, 760. [Google Scholar] [CrossRef]
- Douglas, J.; Bouckaert, R.; Carter, C.W., Jr.; Wills, P. Enzymic recognition of amino acids drove the evolution of primordial genetic codes. Nucleic Acids Res. 2023, 52, 558–571. [Google Scholar] [CrossRef]
- Pham, Y.; Li, L.; Kim, A.; Erdogan, O.; Weinreb, V.; Butterfoss, G.; Kuhlman, B.; Carter, C.W., Jr. A Minimal TrpRS Catalytic Domain Supports Sense/Antisense Ancestry of Class I and II Aminoacyl-tRNA Synthetases. Mol. Cell 2007, 25, 851–862. [Google Scholar] [CrossRef]
- Pham, Y.; Kuhlman, B.; Butterfoss, G.L.; Hu, H.; Weinreb, V.; Carter, C.W., Jr. Tryptophanyl-tRNA synthetase Urzyme: A model to recapitulate molecular evolution and investigate intramolecular complementation. J. Biol. Chem. 2010, 285, 38590–38601. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Weinreb, V.; Francklyn, C.; Carter, C.W., Jr. Histidyl-tRNA Synthetase Urzymes: Class I and II Aminoacyl-tRNA Synthetase Urzymes have Comparable Catalytic Activities for Cognate Amino Acid Activation. J. Biol. Chem. 2011, 286, 10387–10395. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Francklyn, C.; Carter, C.W., Jr. Aminoacylating Urzymes Challenge the RNA World Hypothesis. J. Biol. Chem. 2013, 288, 26856–26863. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.W., Jr.; Li, L.; Weinreb, V.; Collier, M.; Gonzales-Rivera, K.; Jimenez-Rodriguez, M.; Erdogan, O.; Chandrasekharan, S.N. The Rodin-Ohno Hypothesis That Two Enzyme Superfamilies Descended from One Ancestral Gene: An Unlikely Scenario for the Origins of Translation That Will Not Be Dismissed. Biol. Direct 2014, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Onodera, K.; Suganuma, N.; Takano, H.; Sugita, Y.; Shoji, T.; Minobe, A.; Yamaki, N.; Otsuka, R.; Mutsuro-Aoki, H.; Umehara, T.; et al. Amino acid activation analysis of primitive aminoacyl-tRNA synthetases encoded by both strands of a single gene using the malachite green assay. BioSystems 2021, 208, 104481. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.W., Jr. Coding of Class I and II aminoacyl-tRNA synthetases. Adv. Exp. Med. Biol. Protein Rev. 2017, 18, 103–148. [Google Scholar] [CrossRef]
- Carter, C.W., Jr. What RNA World? Why a Peptide/RNA Partnership Merits Renewed Experimental Attention. Life 2015, 5, 294–320. [Google Scholar] [CrossRef]
- Carter, C.W., Jr. Urzymology: Experimental Access to a Key Transition in the Appearance of Enzymes. J. Biol. Chem. 2014, 289, 30213–30220. [Google Scholar] [CrossRef]
- Chandrasekaran, S.N.; Yardimci, G.; Erdogan, O.; Roach, J.M.; Carter, C.W., Jr. Statistical Evaluation of the Rodin-Ohno Hypothesis: Sense/Antisense Coding of Ancestral Class I and II Aminoacyl-tRNA Synthetases. Mol. Biol. Evol. 2013, 30, 1588–1604. [Google Scholar] [CrossRef]
- Tang, G.Q.; Hobson, J.J.; Carter, C.W.J. Domain Acquisition by Class I Aminoacyl-tRNA Synthetase Urzymes Coordinated the Catalytic Functions of HVGH and KMSKS Motifs. Nucleic Acids Res. 2023, 51, 8070–8084. [Google Scholar] [CrossRef] [PubMed]
- Hobson, J.J.; Li, Z.; Carter, C.W., Jr. A leucyl-tRNA synthetase urzyme: Authenticity of tRNA Synthetase urzyme catalytic activities and production of a non-canonical product. Int. J. Mol. Sci. 2022, 23, 4229. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.K.; Betts, L.; Tang, G.Q.; Douglas, J.; Wills, P.R.; Bouckeart, R.; Carter, C.W., Jr. Genomic databases furnish a spontaneous example of a functional Class II Glycyl-tRNA synthetase urzyme. 2024; in preparation. [Google Scholar]
- Tang, G.Q.; Carter, C.W., Jr. Primordial aminoacyl-tRNA synthetases preferred tRNA minihelix substrates over full-length tRNA. 2023; in preparation. [Google Scholar]
- Zull, J.E.; Smith, S.K. Is genetic code redundancy related to retention of structural information in both DNA strands? Trends Biochem. Sci. 1990, 15, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Opuu, V.; Silvert, M.; Simonson, T. Computational design of fully overlapping coding schemes for protein pairs and triplets. Sci. Rep. 2017, 7, 15873. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.W., Jr. Simultaneous codon usage, the origin of the proteome, and the emergence of de-novo proteins. Curr. Opin. Struct. Biol. 2021, 68, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.W., Jr. How did the proteome emerge from pre-biotic chemistry? In Pre-Biotic Chemistry and Life’s Origin; Fiore, M., Ed.; The Royal Society of Chemistry: London, UK, 2022; Chapter 11; pp. 317–346. [Google Scholar]
- Mullen, G.P.; Vaughn, J.B., Jr.; Mildvan, A.S. Sequential Proton NMR Resonance Assignments, Circular Dichroism, and Structural Properties of a 50-Residue Substrate-Binding Peptide from DNA Polymerase I. Arch. Biochem. Biophys. 1993, 301, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.-J.; Abeygunawardana, C.; Pedersen, P.L.; Mildvan, A.S. Two-Dimensional NMR, Circular Dichroism, and Fluorescence Studies of PP-50, a Synthetic ATP-Binding Peptide from the b-Subunit of Mitochondrial ATP Synthase. Biochemistry 1992, 31, 7915–7921. [Google Scholar] [CrossRef]
- Chuang, W.-J.; Abeygunawardana, C.; Gittis, A.G.; Pedersen, P.L.; Mildvan, A.S. Solution Structure and Function in Trifluoroethanol of PP-50, an ATP-Binding Peptide from F1ATPase. Arch. Biochem. Biophys. 1992, 319, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.C.; Byler, D.M.; Sisu, H.; Brown, E.M.; Kuby, S.A.; Mildvan, A.S. Solution Structure of the 45-Residue MgATP-Binding Peptide of Adenylate Kinase As Examined by 2-D NMR, FTIR, and CD Spectroscopy. Biochemistry 1988, 27, 3588–3598. [Google Scholar] [CrossRef]
- Fry, D.C.; Kuby, S.A.; Mildvan, A.S. NMR Studies of the MgATP Binding Site of Adenylate Kinase and of a 45-Residue Peptide Fragment of the Enzyme. Biochemistry 1985, 24, 4680–4694. [Google Scholar] [CrossRef]
- Kaiser, F.; Krautwurst, S.; Salentin, S.; Haupt, V.J.; Leberecht, C.; Bittrich, S.; Labudde, D.; Schroeder, M. The structural basis of the genetic code: Amino acid recognition by aminoacyl-tRNA synthetases. Sci. Rep. 2020, 10, 12647. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, F.; Bittrich, S.; Salentin, S.; Leberecht, C.; Haupt, V.J.; Krautwurst, S.; Schroeder, M.; Labudde, D. Backbone Brackets and Arginine Tweezers delineate Class I and Class II aminoacyl tRNA synthetases. PLoS Comput. Biol. 2018, 14, e1006101. [Google Scholar] [CrossRef]
- Carter, C.W., Jr.; Wills, P.R. Class I and II aminoacyl-tRNA synthetase tRNA groove discrimination created the first synthetase•tRNA cognate pairs and was therefore essential to the origin of genetic coding. IUBMB Life 2019, 71, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.W., Jr.; Wills, P.R. Hierarchical groove discrimination by Class I and II aminoacyl-tRNA synthetases reveals a palimpsest of the operational RNA code in the tRNA acceptor-stem bases. Nucleic Acids Res. 2018, 46, 9667–9683. [Google Scholar] [CrossRef]
- Carter, C.W., Jr.; Wills, P.R. Experimental Solutions to Problems Defining the Origin of Codon-Directed Protein Synthesis. BioSystems 2019, 183, 103979. [Google Scholar] [CrossRef]
- Mughal, F.; Caetano-Anollés, G. MANET 3.0: Hierarchy and modularity in evolving metabolic networks. PLoS ONE 2019, 14, e0224201. [Google Scholar] [CrossRef]
- Caetano-Anollés, G.; Aziz, M.F.; Mughal, F.M.; Gräter, F.; Koç, I.; Caetano-Anollés, K.; Caetano-Anollés, D. Emergence of Hierarchical Modularity in Evolving Networks Uncovered by Phylogenomic Analysis. Evol. Bioinform. 2019, 15, 1176934319872980. [Google Scholar] [CrossRef]
- Caetano-Anollés, G.; Nasir, A.; Kim, K.M.; Caetano-Anollés, D. Rooting Phylogenies and the Tree of Life While Minimizing Ad Hoc and Auxiliary Assumptions. Evol. Bioinform. 2018, 14, 11176934318805101. [Google Scholar] [CrossRef]
- Koç, I.; Caetano-Anollés, G. The natural history of molecular functions inferred from an extensive phylogenomic analysis of gene ontology data. PLoS ONE 2017, 12, e0176129. [Google Scholar] [CrossRef]
- Caetano-Anollés, D.; Caetano-Anollés, G. Piecemeal Buildup of the Genetic Code, Ribosomes, and Genomes from Primordial tRNA Building Blocks. Life 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.F.; Caetano-Anollés, K.; Caetano-Anollés, G. The early history and emergence of molecular functions and modular scale-free network behavior. Sci. Rep. 2016, 6, 25058. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Anollés, G.; Wang, M.; Caetano-Anollés, D. Structural Phylogenomics Retrodicts the Origin of the Genetic Code and Uncovers the Evolutionary Impact of Protein Flexibility. PLoS ONE 2013, 8, e72225. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Novozhilov, A.S. Origin and Evolution of the Universal Genetic Code. Annu. Rev. Genet. 2017, 51, 45–62. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, P.; Luthey-Schulten, Z. On the Evolution of Structure in Aminoacyl-tRNA Synthetases. Microbiol. Mol. Biol. Rev. 2003, 67, 550–573. [Google Scholar] [CrossRef]
- Hohn, M.J.; Park, H.-S.; O’Donoghue, P.; Schnitzbauer, M.; Söll, D. Emergence of the universal genetic code imprinted in an RNA record. Proc. Nat. Acad. Sci. USA 2006, 103, 18095–18100. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Burton, Z.F. Evolution of Life on Earth: tRNA, Aminoacyl-tRNA Synthetases and the Genetic Code. Life 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Ibba, M.; Soll, D. Aminoacyl-tRNAs: Setting the limits of the genetic code. Genes Dev. 2004, 18, 731–738. [Google Scholar] [CrossRef]
- Woese, C.R.; Olsen, G.J.; Ibba, M.; Soll, D. Aminoacyl-tRNA Synthetases, the Genetic Code, and the Evolutionary Process. Microbiol. Mol. Biol. Rev. 2000, 64, 202–236. [Google Scholar] [CrossRef]
- Caetano-Anollés, G.; Sun, F.-J. The natural history of transfer RNA and its interactions with the ribosome. Front. Genet. 2014, 5, 127. [Google Scholar]
- Johnson, B.R.; Lam, S.K. Self-organization, Natural Selection, and Evolution: Cellular Hardware and Genetic Software. BioScience 2010, 60, 879–885. [Google Scholar] [CrossRef]
- Füchslin, R.M.; McCaskill, J.S. Evolutionary self-organization of cell-free genetic coding. Proc. Natl. Acad. Sci. USA 2001, 98, 9185–9190. [Google Scholar] [CrossRef]
- Eigen, M. Selforganization of Matter and the Evolution of Biological Macromolecules. Naturwissenschaften 1971, 58, 465–523. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A. New standards for collecting and fitting steady state kinetic data. Beilstein J. Org. Chem. 2019, 15, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, A.; Howorth, D.; Chandonia, J.-M.; Brenner, S.E.; Hubbard, T.J.P.; Chothia, C.; Murzin, A.G. Data growth and its impact on the SCOP database: New developments. Nucl. Acids Res. 2008, 36, D419–D425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chandonia, J.-M.; Ding, C.; Holbrook, S.R. Comparative mapping of sequence-based and structure-based protein domains. BMC Bioinform. 2005, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Hanson-Smith, V.; Kolaczkowski, B.; Thornton, J.W. Robustness of Ancestral Sequence Reconstruction to Phylogenetic Uncertainty. Mol. Biol. Evol. 2010, 27, 1988–1999. [Google Scholar] [CrossRef]
- Liberles, D.A. Ancestral Sequence Reconstruction; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Benner, S.A.; Sassi, S.O.; Gaucher, E.A. Molecular Paleoscience: Systems Biology from the Past. Adv. Enzymol. Relat. Areas Mol. Biol. 2007, 75, 9–140. [Google Scholar]
- Stackhouse, J.; Presnell, S.R.; McGeehan, G.M.; Nambiar, K.P.; Benner, S.A. The Ribonuclease from an extinct bovid ruminant. FEBS Lett. 1990, 262, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Praetorius-Ibba, M.; Stange-Thomann, N.; Kitabatake, M.; Ali, K.; Söll, I.; Carter, C.W., Jr.; Ibba, M.; Söll, D. Ancient Adaptation of the Active Site of Tryptophanyl-tRNA Synthetase for Tryptophan Binding. Biochemistry 2000, 39, 13136–13143. [Google Scholar] [CrossRef] [PubMed]
- Bullock, T.; Uter, N.; Nissan, T.A.; Perona, J.J. Amino Acid Discrimination by a class I aminoacyl-tRNA synthetase specified by negative determinants. J. Mol. Biol. 2003, 328, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Carter, C.W., Jr. Full Implementation of the Genetic Code by Tryptophanyl-tRNA Synthetase Requires Intermodular Coupling. J. Biol. Chem. 2013, 288, 34736–34745. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, V.; Li, L.; Chandrasekaran, S.N.; Koehl, P.; Delarue, M.; Carter, C.W., Jr. Enhanced Amino Acid Selection in Fully-Evolved Tryptophanyl-tRNA Synthetase, Relative to its Urzyme, Requires Domain Movement Sensed by the D1 Switch, a Remote, Dynamic Packing Motif. J. Biol. Chem. 2014, 289, 4367–4376. [Google Scholar] [CrossRef] [PubMed]
- Perona, J.J.; Gruic-Sovulj, I. Synthetic and Editing Mechanisms of Aminoacyl-tRNA Synthetases. Top. Curr. Chem. 2014, 344, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Shore, J.; Holland, B.R.; Sumner, J.G.; Nieselt, K.; Wills, P.R. The Ancient Operational Code is Embedded in the Amino Acid Substitution Matrix and aaRS Phylogenies. J. Mol. Evol. 2019, 88, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.C.; Minh, B.Q.; McShea, H.; Masel, J.; James, J.E.; Vinh, L.S.; Lanfear, R. nQMaker: Estimating Time Nonreversible Amino Acid Substitution Models. Syst. Biol. 2022, 71, 1110–1123. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Sottani, J.B.; Duchene, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An Advanced Software Platform for Bayesian Evolutionary Analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Dauparas, J.; Anishchenko, I.; Bennett, N.; Bai, H.; Ragotte, R.J.; Milles, L.F.; Wicky, B.I.M.; Courbet, A.; de Haas, R.J.; Bethel, N.; et al. Robust deep learning–based protein sequence design using ProteinMPNN. Science 2022, 378, 49–56. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–592. [Google Scholar] [CrossRef]
- Tropsha, A.; Carter, C.W.J.; Cammer, S.; Vaisman, I.I. Simplicial Neighborhood Analysis of Protein Packing (SNAPP): A Computational Geometry Approach to Studying Proteins. Methods Enzymol. 2003, 374, 509–544. [Google Scholar] [PubMed]
- Carter, C.W., Jr.; LeFebvre, B.; Cammer, S.A.; Tropsha, A.; Edgell, M.H. Four-body potentials reveal protein-specific correlations to stability changes caused by hydrophobic core mutations. J. Mol. Biol. 2001, 311, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zou, Q. Prediction of protein solubility based on sequence physicochemical patterns and distributed representation information with DeepSoluE. BMC Biol. 2023, 21, 12. [Google Scholar] [CrossRef]
- Douglas, J.; Carter, C.W., Jr.; Wills, P.R. HetMM: A Michaelis-Menten model for non-homogeneous enzyme mixtures. iScience, 2024; in press. [Google Scholar]
- Stubbs, R.T.; Yadav, M.; Krishnamurthy, R.; Springsteen, G.R. A plausible metal-free ancestral analogue of the Krebs cycle composed entirely of α-ketoacids. Nat. Chem. 2020, 12, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Frenkel-Pinter, M.; Haynes, J.W.; Mohyeldin, A.M.; Martin, C.; Sargon, A.B.; Petrov, A.S.; Krishnamurthy, R.; Hud, N.V.; Williams, L.D.; Leman, L.J. Mutually stabilizing interactions between proto-peptides and RNA. Nat. Commun. 2020, 11, 3137. [Google Scholar] [CrossRef] [PubMed]
- Ivankov, D.N.; Finkelstein, A.V. Solution of Levinthal’s Paradox and a Physical Theory of Protein Folding Times. Biomolecules 2020, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Dill, K.; Chan, H.S. From Levinthal to pathways to funnels. Nat. Struct. Biol. 1997, 4, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Levinthal, C. Are there pathways for protein folding? J. De Chim. Phys. 1968, 65, 44–45. [Google Scholar] [CrossRef]
- Mattenet, A.L.; Davidson, I.; Nijssen, S.; Schaus, P. Constraint Programming for an Efficient and Flexible Block Modeling Solver. AAAI Conf. Artif. Intell. 2020, 34, 13685–13688. [Google Scholar] [CrossRef]
- Wills, P.R.; Carter, C.W., Jr. Impedance matching and the choice between alternative pathways for the origin of genetic coding. Int. J. Mol. Sci. 2020, 21, 7392. [Google Scholar] [CrossRef]
- San Andrés, L. Impedance Matching. Available online: https://oaktrust.library.tamu.edu/handle/1969.1/188313 (accessed on 20 September 2023).
- Hofstadter, D.R. I Am A Strange Loop; Basic Books: Philadelphia, PA, USA, 2007. [Google Scholar]
- Hofstadter, D.R. Gödel, Escher, Bach: An Eternal Golden Braid; Basic Books, Inc.: New York, NY, USA, 1979; p. 777. [Google Scholar]
- Carter, C.W., Jr.; Wills, P.R. Reciprocally-coupled Gating: Strange Loops in Bioenergetics, Genetics, and Catalysis. Biomolecules 2021, 11, 265. [Google Scholar] [CrossRef]
- Carter, C.W., Jr. Escapement mechanisms: Efficient free energy transduction by reciprocally-coupled gating. Proteins Struct. Funct. Bioinform. 2019, 88, 710–717. [Google Scholar] [CrossRef]
- Niwa, N.; Yamagishi, Y.; Murakami, H.; Suga, H. A flexizyme that selectively charges amino acids activated by a water-friendly leaving group. Bioorg. Med. Chem. Lett. 2009, 19, 3892–3894. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Murakami, H.; Suga, H.; Ferre-D’Amare, A.R. Structural basis of specific tRNA aminoacylation by a small in vitro selected ribozyme. Nature 2008, 454, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Nesterov-Mueller, A.; Popov, R. The Combinatorial Fusion Cascade to Generate the Standard Genetic Code. Life 2021, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Buehner, M.; Ford, G.C.; Moras, D.; Olsen, K.W.; Rossmann, M.G. D-Glyceraldehyde 3-Phosphate Dehydrogenase: Three Dimensional Structure and Evolutionary Significance. Proc. Nat. Acad. Sci. USA 1973, 70, 3052–3054. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.W., Jr.; Wolfenden, R. tRNA Acceptor-Stem and Anticodon Bases Form Independent Codes Related to Protein Folding. Proc. Nat. Acad. Sci. USA 2015, 112, 7489–7494. [Google Scholar] [CrossRef] [PubMed]
- Wolfenden, R.; Lewis, C.A.; Yuan, Y.; Carter, C.W., Jr. Temperature dependence of amino acid hydrophobicities. Proc. Nat. Acad. Sci. USA 2015, 112, 7484–7488. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.M.; Sharma, S.; Kapustina, M.; Carter, C.W., Jr. Structure alignment via Delaunay tetrahedralization. Proteins Struct. Funct. Bioinform. 2005, 60, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Witten, E. What every physicist should know about String Theory. Phys. Today 2015, 68, 38–43. [Google Scholar] [CrossRef]
- Carter, C.W., Jr.; Kraut, J. A Proposed Model for Interaction of Polypeptides with RNA. Proc. Natl. Acad. Sci. USA 1974, 71, 283–287. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, C.W., Jr. Base Pairing Promoted the Self-Organization of Genetic Coding, Catalysis, and Free-Energy Transduction. Life 2024, 14, 199. https://doi.org/10.3390/life14020199
Carter CW Jr. Base Pairing Promoted the Self-Organization of Genetic Coding, Catalysis, and Free-Energy Transduction. Life. 2024; 14(2):199. https://doi.org/10.3390/life14020199
Chicago/Turabian StyleCarter, Charles W., Jr. 2024. "Base Pairing Promoted the Self-Organization of Genetic Coding, Catalysis, and Free-Energy Transduction" Life 14, no. 2: 199. https://doi.org/10.3390/life14020199




