Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Parameters Could Predict International Society of Urological Pathology Risk Groups of Prostate Cancers on Radical Prostatectomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
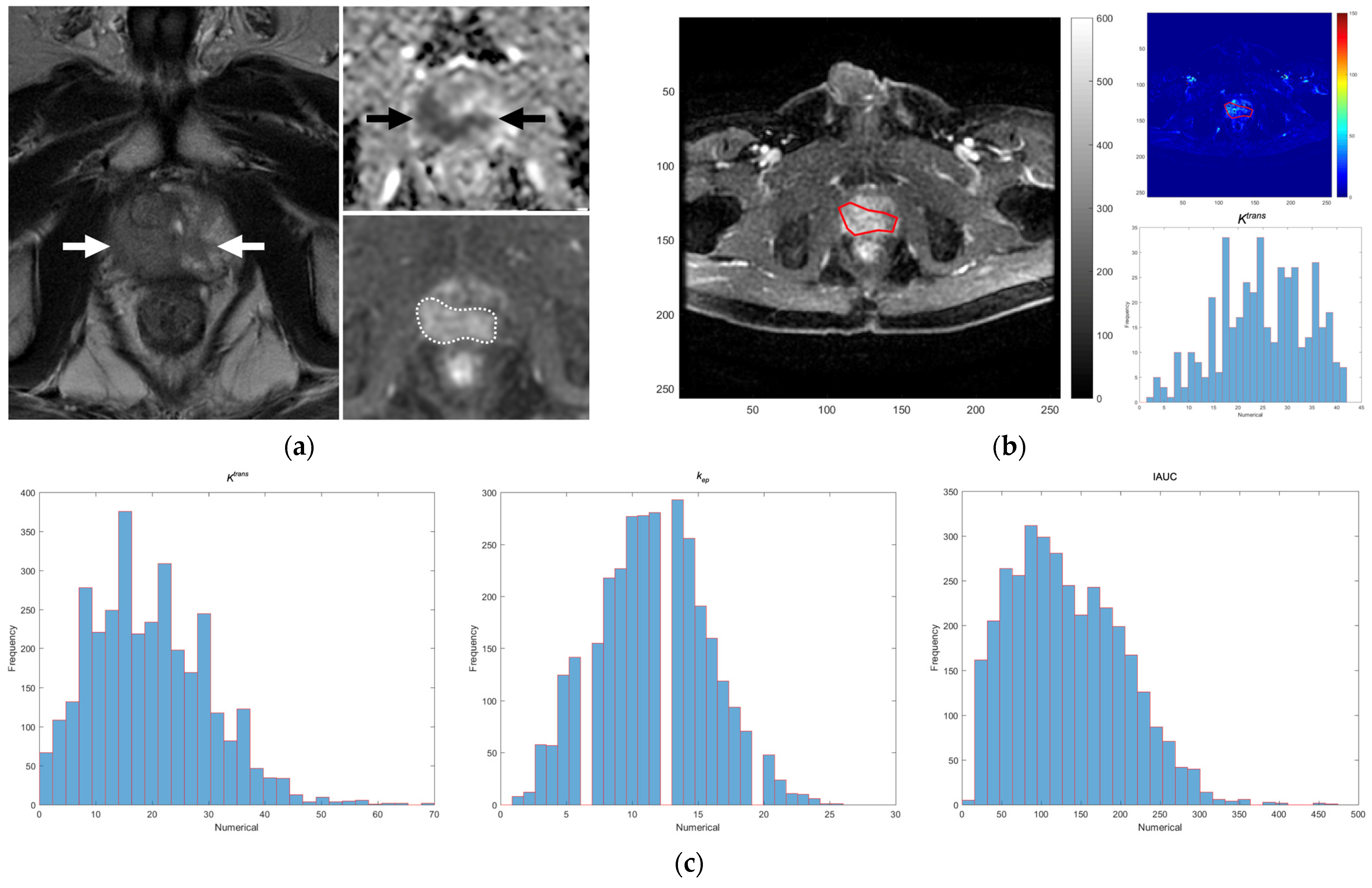
2.2. MRI Technique and DCE Parameters on MRI
2.3. ISUP Risk Groups and Surgical Margins on the Pathological Specimens
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mucci, L.A.; Powolny, A.; Giovannucci, E.; Liao, Z.; Kenfield, S.A.; Shen, R.; Stampfer, M.J.; Clinton, S.K. Prospective study of prostate tumor angiogenesis and cancer-specific mortality in the health professionals follow-up study. J. Clin. Oncol. 2009, 27, 5627–5633. [Google Scholar] [CrossRef]
- Turkbey, B.; Pinto, P.A.; Mani, H.; Bernardo, M.; Pang, Y.; McKinney, Y.L.; Khurana, K.; Ravizzini, G.C.; Albert, P.S.; Merino, M.J.; et al. Prostate cancer: Value of multiparametric MR imaging at 3 T for detection—Histopathologic correlation. Radiology 2010, 255, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Sabach, A.; Babb, J.S.; Matza, B.W.; Taneja, S.S.; Deng, F.M. Prostate cancer: Comparison of dynamic contrast-enhanced MRI techniques for localization of peripheral zone tumor. AJR Am. J. Roentgenol. 2013, 201, W471–W478. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Paul Hobbs, B.; Wei, W.; Kundra, V. Dynamic Contrast-Enhanced MRI for the Detection of Prostate Cancer: Meta-Analysis. Am. J. Roentgenol. 2015, 204, W439–W448. [Google Scholar] [CrossRef] [PubMed]
- Beyhan, M.; Sade, R.; Koc, E.; Adanur, S.; Kantarci, M. The evaluation of prostate lesions with IVIM DWI and MR perfusion parameters at 3T MRI. Radiol. Med. 2019, 124, 87–93. [Google Scholar] [CrossRef]
- Cuenod, C.A.; Fournier, L.; Balvay, D.; Guinebretière, J.M. Tumor angiogenesis: Pathophysiology and implications for contrast-enhanced MRI and CT assessment. Abdom Imaging 2006, 31, 188–193. [Google Scholar] [CrossRef]
- Vos, E.K.; Litjens, G.J.; Kobus, T.; Hambrock, T.; Hulsbergen-van de Kaa, C.A.; Barentsz, J.O.; Huisman, H.J.; Scheenen, T.W. Assessment of prostate cancer aggressiveness using dynamic contrast-enhanced magnetic resonance imaging at 3 T. Eur. Urol. 2013, 64, 448–455. [Google Scholar] [CrossRef]
- Cho, E.; Chung, D.J.; Yeo, D.M.; Sohn, D.; Son, Y.; Kim, T.; Hahn, S.T. Optimal cut-off value of perfusion parameters for diagnosing prostate cancer and for assessing aggressiveness associated with Gleason score. Clin. Imaging 2015, 39, 834–840. [Google Scholar] [CrossRef]
- Baur, A.D.J.; Schwabe, J.; Rogasch, J.; Maxeiner, A.; Penzkofer, T.; Stephan, C.; Rudl, M.; Hamm, B.; Jung, E.M.; Fischer, T. A direct comparison of contrast-enhanced ultrasound and dynamic contrast-enhanced magnetic resonance imaging for prostate cancer detection and prediction of aggressiveness. Eur. Radiol. 2018, 28, 1949–1960. [Google Scholar] [CrossRef]
- Fukunaga, T.; Tamada, T.; Kanomata, N.; Takeuchi, M.; Ueda, Y.; Miyaji, Y.; Kido, A.; Yamamoto, A.; Sone, T. Quantitative diffusion-weighted imaging and dynamic contrast-enhanced MR imaging for assessment of tumor aggressiveness in prostate cancer at 3T. Magn. Reson. Imaging 2021, 83, 152–159. [Google Scholar] [CrossRef]
- Pepe, P.; D’Urso, D.; Garufi, A.; Priolo, G.; Pennisi, M.; Russo, G.; Sabini, M.G.; Valastro, L.M.; Galia, A.; Fraggetta, F. Multiparametric MRI Apparent Diffusion Coefficient (ADC) Accuracy in Diagnosing Clinically Significant Prostate Cancer. In Vivo 2017, 31, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Urakami, A.; Arimura, H.; Takayama, Y.; Kinoshita, F.; Ninomiya, K.; Imada, K.; Watanabe, S.; Nishie, A.; Oda, Y.; Ishigami, K. Stratification of prostate cancer patients into low- and high-grade groups using multiparametric magnetic resonance radiomics with dynamic contrast-enhanced image joint histograms. Prostate 2022, 82, 330–344. [Google Scholar] [CrossRef]
- Martini, A.; Gandaglia, G.; Fossati, N.; Scuderi, S.; Bravi, C.A.; Mazzone, E.; Stabile, A.; Scarcella, S.; Robesti, D.; Barletta, F.; et al. Defining Clinically Meaningful Positive Surgical Margins in Patients Undergoing Radical Prostatectomy for Localised Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, E.; Matheu, R.; Muní, M.; Sureda, J.; García-Sorroche, M.; Ribal, M.J.; Alcaraz, A.; Vilaseca, A. The Effect of Adverse Surgical Margins on the Risk of Biochemical Recurrence after Robotic-Assisted Radical Prostatectomy. Biomedicines 2022, 10, 1911. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yoo, D.; Pyo, J.; Cho, W. Clinicopathological Significances of Positive Surgical Resection Margin after Radical Prostatectomy for Prostatic Cancers: A Meta-Analysis. Medicina 2022, 58, 1251. [Google Scholar] [CrossRef]
- Eastham, J.A.; Kattan, M.W.; Riedel, E.; Begg, C.B.; Wheeler, T.M.; Gerigk, C.; Gonen, M.; Reuter, V.; Scardino, P.T. Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens. J. Urol. 2003, 170, 2292–2295. [Google Scholar] [CrossRef]
- Yang, C.W.; Wang, H.H.; Hassouna, M.F.; Chand, M.; Huang, W.J.S.; Chung, H.J. Prediction of a positive surgical margin and biochemical recurrence after robot-assisted radical prostatectomy. Sci. Rep. 2021, 11, 14329. [Google Scholar] [CrossRef]
- Park, M.Y.; Park, K.J.; Kim, M.H.; Kim, J.K. Preoperative MRI-based estimation of risk for positive resection margin after radical prostatectomy in patients with prostate cancer: Development and validation of a simple scoring system. Eur. Radiol. 2021, 31, 4898–4907. [Google Scholar] [CrossRef]
- McEvoy, S.H.; Raeside, M.C.; Chaim, J.; Ehdaie, B.; Akin, O. Preoperative Prostate MRI: A Road Map for Surgery. Am. J. Roentgenol. 2018, 211, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Quentin, M.; Schimmöller, L.; Ullrich, T.; Valentin, B.; Demetrescu, D.; Al-Monajjed, R.; Mally, D.; Esposito, I.; Albers, P.; Antoch, G.; et al. Pre-operative magnetic resonance imaging can predict prostate cancer with risk for positive surgical margins. Abdom. Radiol. (N. Y.) 2022, 47, 2486–2493. [Google Scholar] [CrossRef]
- Yang, D.M.; Kim, H.C.; Kim, S.W.; Jahng, G.H.; Won, K.Y.; Lim, S.J.; Oh, J.H. Prostate cancer: Correlation of intravoxel incoherent motion MR parameters with Gleason score. Clin. Imaging 2016, 40, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Chen, X.; Liu, K.; Zeng, M.; Zhou, J. Prostate cancer aggressive prediction: Preponderant diagnostic performances of intravoxel incoherent motion (IVIM) imaging and diffusion kurtosis imaging (DKI) beyond ADC at 3.0 T scanner with gleason score at final pathology. Abdom. Radiol. (N. Y.) 2019, 44, 3441–3452. [Google Scholar] [CrossRef]
- Kety, S.S. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol. Rev. 1951, 3, 1–41. [Google Scholar] [PubMed]
- Tofts, P.S. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J. Magn. Reson. Imaging 1997, 7, 91–101. [Google Scholar] [CrossRef]
- Tofts, P.S.; Brix, G.; Buckley, D.L.; Evelhoch, J.L.; Henderson, E.; Knopp, M.V.; Larsson, H.B.; Lee, T.Y.; Mayr, N.A.; Parker, G.J.; et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J. Magn. Reson. Imaging 1999, 10, 223–232. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chan, T.H.; Chi, C.Y.; Ng, S.H.; Liu, H.L.; Wei, K.C.; Wai, Y.Y.; Wang, C.C.; Wang, J.J. Blind estimation of the arterial input function in dynamic contrast-enhanced MRI using purity maximization. Magn. Reson. Med. 2012, 68, 1439–1449. [Google Scholar] [CrossRef]
- Ng, S.H.; Liao, C.T.; Lin, C.Y.; Chan, S.C.; Lin, Y.C.; Yen, T.C.; Chang, J.T.; Ko, S.F.; Fan, K.H.; Wang, H.M.; et al. Dynamic contrast-enhanced MRI, diffusion-weighted MRI and (18)F-FDG PET/CT for the prediction of survival in oropharyngeal or hypopharyngeal squamous cell carcinoma treated with chemoradiation. Eur. Radiol. 2016, 26, 4162–4172. [Google Scholar] [CrossRef]
- Eastham, J.A.; Kuroiwa, K.; Ohori, M.; Serio, A.M.; Gorbonos, A.; Maru, N.; Vickers, A.J.; Slawin, K.M.; Wheeler, T.M.; Reuter, V.E.; et al. Prognostic Significance of Location of Positive Margins in Radical Prostatectomy Specimens. Urology 2007, 70, 965–969. [Google Scholar] [CrossRef]
- Verma, S.; Turkbey, B.; Muradyan, N.; Rajesh, A.; Cornud, F.; Haider, M.A.; Choyke, P.L.; Harisinghani, M. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am. J. Roentgenol. 2012, 198, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Oto, A.; Yang, C.; Kayhan, A.; Tretiakova, M.; Antic, T.; Schmid-Tannwald, C.; Eggener, S.; Karczmar, G.S.; Stadler, W.M. Diffusion-weighted and dynamic contrast-enhanced MRI of prostate cancer: Correlation of quantitative MR parameters with Gleason score and tumor angiogenesis. AJR Am. J. Roentgenol. 2011, 197, 1382–1390. [Google Scholar] [CrossRef]
- Oikawa, M.; Tanaka, T.; Narita, T.; Noro, D.; Iwamura, H.; Tobisawa, Y.; Yoneyama, T.; Kodama, H.; Hashimoto, Y.; Koie, T.; et al. Impact of the Proportion of Biopsy Positive Core in Predicting Biochemical Recurrence in Patients with Pathological Pt2 and Negative Resection Margin Status after Radical Prostatectomy. Pathol. Oncol. Res. 2020, 26, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Berney, D.M.; Finnegan, K.; Chu, K.; Fine, S.W.; Varma, M.; Cuzick, J.; Beltran, L. Measuring cancer burden in prostatic needle core biopsies: Simplified assessments outperform complex measurements in assessing outcome: Evidence to assist pathologist efficiency and minimize datasets. Histopathology 2023, 82, 1021–1028. [Google Scholar] [CrossRef]
- van der Slot, M.A.; Remmers, S.; Kweldam, C.F.; den Bakker, M.A.; Nieboer, D.; Busstra, M.B.; Gan, M.; Klaver, S.; Rietbergen, J.B.W.; van Leenders, G. Biopsy prostate cancer perineural invasion and tumour load are associated with positive posterolateral margins at radical prostatectomy: Implications for planning of nerve-sparing surgery. Histopathology 2023. [Google Scholar] [CrossRef]
- Yamashita, S.; Kohjimoto, Y.; Sato, H.; Kikkawa, K.; Sonomura, T.; Hara, I. PI-RADS v2 Findings of MRI and Positive Biopsy Core Percentage would Predict Pathological Extraprostatic Extension in Patients who Underwent Robot Assisted Radical Prostatectomy: A Retrospective Study. Urol. J. 2022, 19, 438–444. [Google Scholar] [CrossRef]
- Belue, M.J.; Yilmaz, E.C.; Daryanani, A.; Turkbey, B. Current Status of Biparametric MRI in Prostate Cancer Diagnosis: Literature Analysis. Life 2022, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Tamada, T.; Kido, A.; Yamamoto, A.; Takeuchi, M.; Miyaji, Y.; Moriya, T.; Sone, T. Comparison of Biparametric and Multiparametric MRI for Clinically Significant Prostate Cancer Detection With PI-RADS Version 2.1. J. Magn. Reson. Imaging 2021, 53, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Iacob, R.; Stoicescu, E.R.; Cerbu, S.; Manolescu, D.L.; Bardan, R.; Cumpănaş, A. Could Biparametric MRI Replace Multiparametric MRI in the Management of Prostate Cancer? Life 2023, 13, 465. [Google Scholar] [CrossRef]
- Reynolds, H.M.; Tadimalla, S.; Wang, Y.F.; Montazerolghaem, M.; Sun, Y.; Williams, S.; Mitchell, C.; Finnegan, M.E.; Murphy, D.G.; Haworth, A. Semi-quantitative and quantitative dynamic contrast-enhanced (DCE) MRI parameters as prostate cancer imaging biomarkers for biologically targeted radiation therapy. Cancer Imaging 2022, 22, 71. [Google Scholar] [CrossRef]
- Chan, T.H.; Haworth, A.; Wang, A.; Osanlouy, M.; Williams, S.; Mitchell, C.; Hofman, M.S.; Hicks, R.J.; Murphy, D.G.; Reynolds, H.M. Detecting localised prostate cancer using radiomic features in PSMA PET and multiparametric MRI for biologically targeted radiation therapy. EJNMMI Res. 2023, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Weinreb, J.; Rosenkrantz, A.B.; Villeirs, G.; Turkbey, B.; Barentsz, J. Prostate Imaging-Reporting and Data System Steering Committee: PI-RADS v2 Status Update and Future Directions. Eur. Urol. 2019, 75, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Borkowetz, A.; Platzek, I.; Toma, M.; Renner, T.; Herout, R.; Baunacke, M.; Laniado, M.; Baretton, G.B.; Froehner, M.; Zastrow, S.; et al. Evaluation of Prostate Imaging Reporting and Data System Classification in the Prediction of Tumor Aggressiveness in Targeted Magnetic Resonance Imaging/Ultrasound-Fusion Biopsy. Urol. Int. 2017, 99, 177–185. [Google Scholar] [CrossRef]
- Kızılay, F.; Çelik, S.; Sözen, S.; Özveren, B.; Eskiçorapçı, S.; Özgen, M.; Özen, H.; Akdoğan, B.; Aslan, G.; Narter, F.; et al. Correlation of Prostate-Imaging Reporting and Data Scoring System scoring on multiparametric prostate magnetic resonance imaging with histopathological factors in radical prostatectomy material in Turkish prostate cancer patients: A multicenter study of the Urooncology Association. Prostate Int. 2020, 8, 10–15. [Google Scholar] [CrossRef]
- Ageeli, W.; Wei, C.; Zhang, X.; Szewcyk-Bieda, M.; Wilson, J.; Li, C.; Nabi, G. Quantitative ultrasound shear wave elastography (USWE)-measured tissue stiffness correlates with PIRADS scoring of MRI and Gleason score on whole-mount histopathology of prostate cancer: Implications for ultrasound image-guided targeting approach. Insights Into Imaging 2021, 12, 96. [Google Scholar] [CrossRef]
- Afshari Mirak, S.; Mohammadian Bajgiran, A.; Sung, K.; Asvadi, N.H.; Markovic, D.; Felker, E.R.; Lu, D.; Sisk, A.; Reiter, R.E.; Raman, S.S. Dynamic contrast-enhanced (DCE) MR imaging: The role of qualitative and quantitative parameters for evaluating prostate tumors stratified by Gleason score and PI-RADS v2. Abdom. Radiol. (N. Y.) 2020, 45, 2225–2234. [Google Scholar] [CrossRef]
- Pepe, P.; Pepe, L.; Tamburo, M.; Marletta, G.; Pennisi, M.; Fraggetta, F. Targeted prostate biopsy: 68Ga-PSMA PET/CT vs. mpMRI in the diagnosis of prostate cancer. Arch. Ital. Urol. Androl. 2022, 94, 274–277. [Google Scholar] [CrossRef]
- Pepe, P.; Pennisi, M. Targeted Biopsy in Men High Risk for Prostate Cancer: (68)Ga-PSMA PET/CT Versus mpMRI. In Clinical Genitourinary Cancer; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Pepe, P.; Pennisi, M. Should 68Ga-PSMA PET/CT Replace CT and Bone Scan in Clinical Staging of High-risk Prostate Cancer? Anticancer Res. 2022, 42, 1495–1498. [Google Scholar] [CrossRef]
- Gaustad, J.V.; Hauge, A.; Wegner, C.S.; Simonsen, T.G.; Lund, K.V.; Hansem, L.M.K.; Rofstad, E.K. DCE-MRI of Tumor Hypoxia and Hypoxia-Associated Aggressiveness. Cancers 2020, 12, 1979. [Google Scholar] [CrossRef]
| Variables | |
|---|---|
| Clinical parameters | |
| Age (years) | 66.000 (63.000–71.000) |
| PSA at diagnosis (ng/mL) | 14.180 (9.075–20.895) |
| PSA density (ng/mL/mL) | 0.414 (0.216–0.660) |
| Positive biopsy core (%) | 33.333 (8.330–50.000) |
| DCE parameters | |
| Ktrans-min (min−1) | 4.000 (2.000–7.000) |
| Ktrans-median (min−1) | 19.000 (13.500–28.000) |
| Ktrans-max (min−1) | 55.000 (34.500–78.000) |
| kep-min (min−1) | 2.000 (1.500–4.000) |
| kep-median (min−1) | 13.000 (9.250–14.000) |
| kep-max (min−1) | 25.000 (20.000–33.000) |
| IAUC-min (mM·s) | 39.000 (19.500–60.500) |
| IAUC-median (mM·s) | 137.000 (114.000–191.500) |
| IAUC-max (mM·s) | 286.000 (210.500–411.000) |
| Outcomes from radical prostatectomy specimens | |
| ISUP risk group | |
| Low risk (I–II) | 22 (48.889%) |
| High risk (III–V) | 23 (51.111%) |
| Surgical margins | |
| Positive | 15 (33.333%) |
| Negative | 30 (66.667%) |
| Variables | ISUP Risk Group | p | Surgical Margin on the Pathological Specimens | p | ||
|---|---|---|---|---|---|---|
| Low Risk (n = 22) | High Risk (n = 23) | Negative (n = 30) | Positive (n = 15) | |||
| Clinical parameters | ||||||
| Age (years) | 65.500 (63.000–71.000) | 66.000 (61.000–71.000) | 0.838 | 66.500 (63.000–71.000) | 66.000 (60.000–69.000) | 0.405 |
| PSA at diagnosis (ng/mL) | 11.750 (8.260–17.390) | 14.510 (9.120–21.370) | 0.586 | 11.450 (8.260–15.540) | 16.180 (10.120–32.710) | 0.041 * |
| PSA density (ng/mL/mL) | 0.405 (0.201–0.555) | 0.471 (0.212–0.709) | 0.570 | 0.306 (0.186–0.498) | 0.562 (0.278–0.794) | 0.043 * |
| Positive biopsy cores (%) | 25.000 (8.330–54.165) | 33.330 (10.415–50.000) | 0.733 | 16.670 (8.330–33.330) | 45.835 (27.080–62.498) | 0.049 * |
| DCE parameters | ||||||
| Ktrans-min (min−1) | 5.000 (2.000–8.250) | 3.000 (2.000–7.000) | 0.490 | 5.000 (2.000–7.250) | 3.000 (2.000–7.000) | 0.379 |
| Ktrans-median (min−1) | 17.250 (13.000–26.000) | 22.000 (15.000–28.000) | 0.246 | 16.750 (13.000–27.000) | 25.000 (17.000–28.000) | 0.159 |
| Ktrans-max (min−1) | 43.000 (26.500–62.500) | 70.000 (39.000–83.000) | 0.028 * | 42.000 (31.750–64.750) | 79.000 (56.000–91.000) | 0.010 * |
| kep-min (min−1) | 2.000 (1.000–4.000) | 3.000 (2.000–5.000) | 0.508 | 2.000 (1.000–4.250) | 3.000 (2.000–4.000) | 0.530 |
| kep-median (min−1) | 11.000 (8.750–13.625) | 14.000 (10.000–15.000) | 0.019 * | 11.000 (9.000–14.000) | 14.000 (13.000–15.000) | 0.013 * |
| kep-max (min−1) | 21.500 (16.500–29.750) | 27.000 (22.000–37.000) | 0.033 * | 22.500 (20.000–27.500) | 36.000 (21.000–44.000) | 0.017 * |
| IAUC-min (mM·s) | 43.500 (25.500–77.500) | 32.000 (18.000–60.000) | 0.364 | 40.500 (23.000–60.250) | 32.000 (18.000–65.000) | 0.485 |
| IAUC-median (mM·s) | 129.500 (96.000–183.000) | 150.000 (115.000–234.000) | 0.433 | 124.750 (102.500–183.000) | 177.000 (128.000–234.000) | 0.075 |
| IAUC-max (mM·s) | 247.000 (187.750–373.750) | 150.000 (115.000–234.000) | 0.059 | 259.500 (204.250–368.750) | 366.000 (297.000–425.000) | 0.060 |
| Variables | Univariate | p | Multivariate | p | ||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | OR (95% CI) | B | S.E. | OR (95% CI) | |||
| Age (years) | −0.022 | 0.053 | 0.978 (0.881–1.087) | 0.684 | ||||
| PSA at diagnosis (ng/mL) | 0.016 | 0.028 | 1.016 (0.963–1.073) | 0.557 | ||||
| PSA density (ng/mL/mL) | 0.793 | 0.950 | 2.211 (0.344–14.227) | 0.404 | ||||
| Positive biopsy cores (%) | 0.001 | 0.013 | 1.001 (0.976–1.027) | 0.943 | ||||
| DCE parameters | ||||||||
| Ktrans-min (min−1) | −0.022 | 0.055 | 0.978 (0.879–1.089) | 0.686 | ||||
| Ktrans-median (min−1) | 0.039 | 0.035 | 1.040 (0.971–1.112) | 0.263 | ||||
| Ktrans-max (min−1) | 0.029 | 0.013 | 1.030 (1.004–1.056) | 0.026 * | 0.031 | 0.014 | 1.032 (1.005–1.060) | 0.021 * |
| kep-min (min−1) | 0.076 | 0.140 | 1.079 (0.820–1.419) | 0.588 | ||||
| kep-median (min−1) | 0.204 | 0.099 | 1.227 (1.011–1.488) | 0.038 * | ||||
| kep-max (min−1) | 0.035 | 0.027 | 1.035 (0.982–1.091) | 0.198 | ||||
| IAUC-min (mM·s) | −0.007 | 0.008 | 0.993 (0.977–1.009) | 0.396 | ||||
| IAUC-median (mM·s) | 0.004 | 0.005 | 1.004 (0.994–1.014) | 0.419 | ||||
| IAUC-max (mM·s) | 0.005 | 0.003 | 1.005 (1.000–1.011) | 0.060 | ||||
| Variables | Univariate | p | Multivariate | p | ||||
|---|---|---|---|---|---|---|---|---|
| B | S.E. | OR (95% CI) | B | S.E. | OR (95% CI) | |||
| Age (years) | −0.052 | 0.057 | 0.949 (0.848–1.062) | 0.360 | ||||
| PSA at diagnosis (ng/mL) | 0.066 | 0.031 | 1.068 (1.004–1.135) | 0.036 * | ||||
| PSA density (ng/mL/mL) | 1.418 | 0.988 | 4.129 (0.596–28.606) | 0.151 | ||||
| Positive biopsy cores (%) | 0.030 | 0.015 | 1.030 (1.001–1.061) | 0.045 * | 0.034 | 0.016 | 1.035 (1.003–1.068) | 0.032 * |
| DCE parameters | ||||||||
| Ktrans-min (min−1) | −0.049 | 0.066 | 0.952 (0.837–1.083) | 0.453 | ||||
| Ktrans-median (min−1) | 0.033 | 0.035 | 1.034 (0.965–1.108) | 0.343 | ||||
| Ktrans-max (min−1) | 0.032 | 0.014 | 1.032 (1.005–1.060) | 0.019 * | ||||
| kep-min (min−1) | −0.007 | 0.146 | 0.993 (0.745–1.323) | 0.961 | ||||
| kep-median (min−1) | 0.033 | 0.035 | 1.034 (0.965–1.108) | 0.343 | ||||
| kep-max (min−1) | 0.074 | 0.031 | 1.076 (1.013–1.144) | 0.018 * | 0.075 | 0.032 | 1.078 (1.012–1.148) | 0.020 * |
| IAUC-min (mM·s) | −0.007 | 0.009 | 0.993 (0.976–1.012) | 0.475 | ||||
| IAUC-median (mM·s) | 0.008 | 0.005 | 1.008 (0.998–1.018) | 0.125 | ||||
| IAUC-max (mM·s) | 0.005 | 0.003 | 1.005 (1.000–1.010) | 0.065 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-B.; Lin, Y.-C.; Wong, Y.-C.; Lin, S.-N.; Lin, C.-Y.; Lin, Y.-H.; Sheng, T.-W.; Yang, L.-Y.; Wang, L.-J. Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Parameters Could Predict International Society of Urological Pathology Risk Groups of Prostate Cancers on Radical Prostatectomy. Life 2023, 13, 1944. https://doi.org/10.3390/life13091944
Chang C-B, Lin Y-C, Wong Y-C, Lin S-N, Lin C-Y, Lin Y-H, Sheng T-W, Yang L-Y, Wang L-J. Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Parameters Could Predict International Society of Urological Pathology Risk Groups of Prostate Cancers on Radical Prostatectomy. Life. 2023; 13(9):1944. https://doi.org/10.3390/life13091944
Chicago/Turabian StyleChang, Chun-Bi, Yu-Chun Lin, Yon-Cheong Wong, Shin-Nan Lin, Chien-Yuan Lin, Yu-Han Lin, Ting-Wen Sheng, Lan-Yan Yang, and Li-Jen Wang. 2023. "Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Parameters Could Predict International Society of Urological Pathology Risk Groups of Prostate Cancers on Radical Prostatectomy" Life 13, no. 9: 1944. https://doi.org/10.3390/life13091944
APA StyleChang, C.-B., Lin, Y.-C., Wong, Y.-C., Lin, S.-N., Lin, C.-Y., Lin, Y.-H., Sheng, T.-W., Yang, L.-Y., & Wang, L.-J. (2023). Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Parameters Could Predict International Society of Urological Pathology Risk Groups of Prostate Cancers on Radical Prostatectomy. Life, 13(9), 1944. https://doi.org/10.3390/life13091944








