The Effects of the Combination of Rhein and Platelet-Rich Plasma on Human Articular Chondrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Isolation and Culture of Articular Chondrocytes
2.3. Rhein Solubility Determination
2.4. Determination of Rhein Working Concentration
2.5. Establishment of Experimental Groups
2.6. Cell Migration
2.7. Total RNA Extraction and Gene Expression Analysis
2.8. Quantification of Nitric Oxide Production
2.9. Quantification of Tumor Necrosis Factor-α
2.10. Statistical Analysis
3. Results
3.1. Determination of Rhein Working Concentration
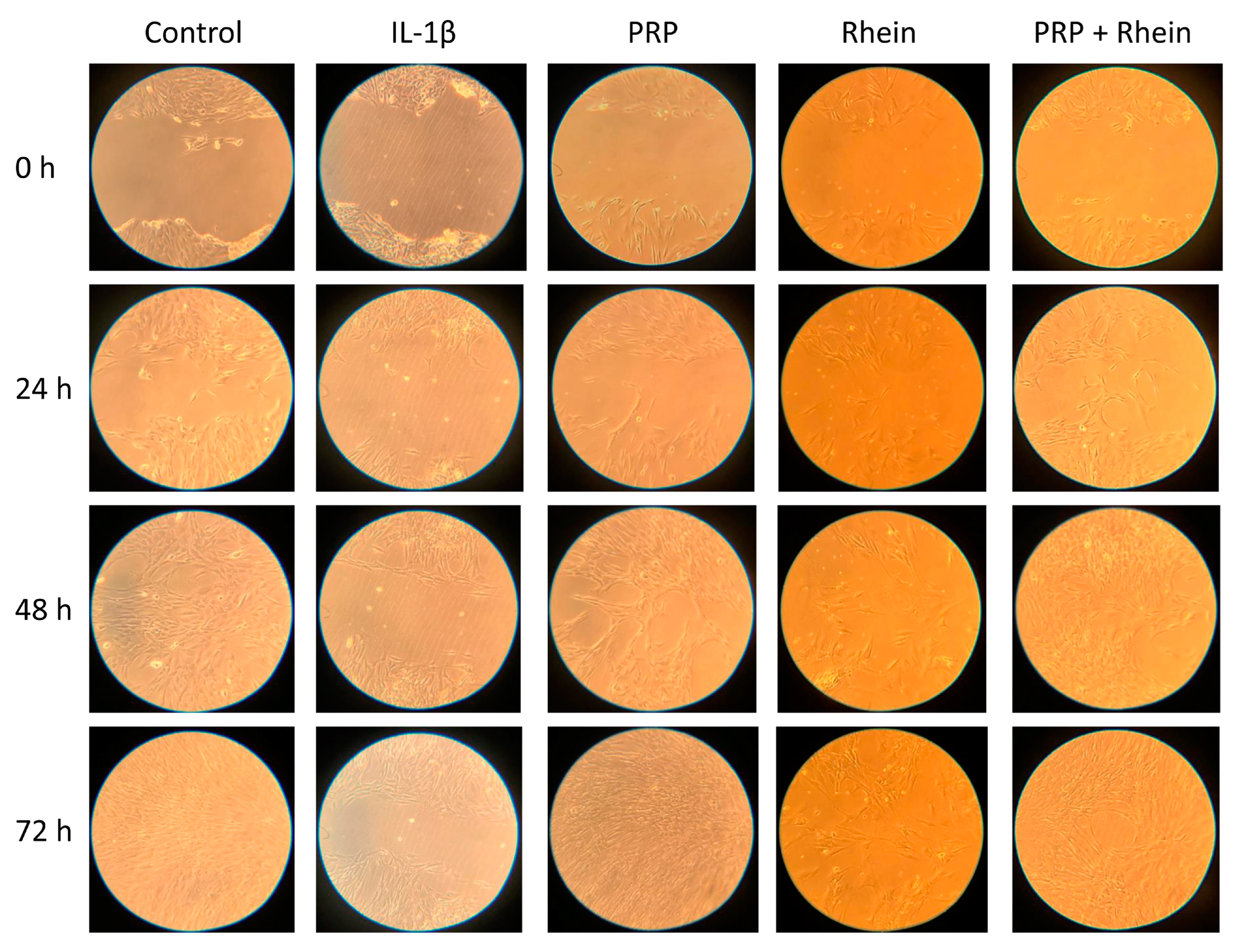
3.2. Effects of Rhein and PRP on Cell Migration
3.3. Effect of Rhein and PRP on Inflammation- and Matrix Degradation-Related Genes
3.4. Effect of Rhein and PRP on NO Production
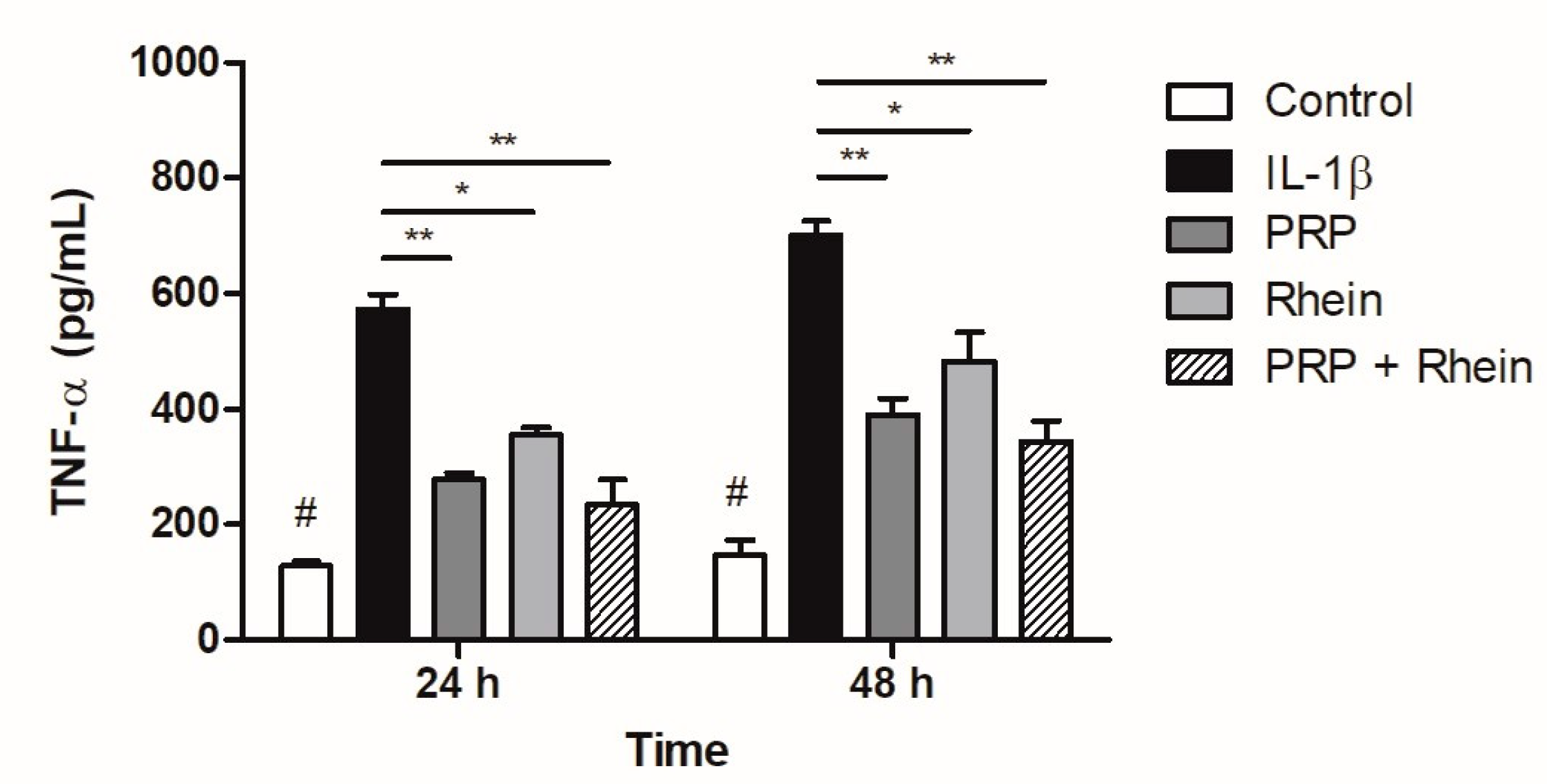
3.5. Effect of Rhein and PRP on TNF-α Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-Adjusted Life Years (DALYs) for 291 Diseases and Injuries in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.D.; Pfleger, B. Burden of Major Muskuloskeletal Conditions. Bull. World Health Organ 2003, 81, 646–656. Available online: https://apps.who.int/iris/handle/10665/269026 (accessed on 8 June 2022). [PubMed]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Losina, E.; Weinstein, A.M.; Reichmann, W.M.; Burbine, S.A.; Solomon, D.H.; Daigle, M.E.; Rome, B.N.; Chen, S.P.; Hunter, D.J.; Suter, L.G.; et al. Lifetime Risk and Age at Diagnosis of Symptomatic Knee Osteoarthritis in the US. Arthritis Care Res. 2013, 65, 703–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Overton, C.; Nelson, A.E.; Neogi, T. Osteoarthritis Treatment Guidelines from Six Professional Societies: Similarities and Differences. Rheum. Dis. Clin. N. Am. 2022, 48, 637–657. [Google Scholar] [CrossRef]
- Bruyère, O.; Cooper, C.; Pelletier, J.P.; Branco, J.; Luisa Brandi, M.; Guillemin, F.; Hochberg, M.C.; Kanis, J.A.; Kvien, T.K.; Martel-Pelletier, J.; et al. An Algorithm Recommendation for the Management of Knee Osteoarthritis in Europe and Internationally: A Report from a Task Force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin. Arthritis Rheum. 2014, 44, 253–263. [Google Scholar] [CrossRef]
- Panova, E.; Jones, G. Benefit-Risk Assessment of Diacerein in the Treatment of Osteoarthritis. Drug Saf. 2015, 38, 245–252. [Google Scholar] [CrossRef]
- Solignac, M. Mechanisms of Action of Diacerein, the First Inhibitor of Interleukin-1 in Osteoarthritis. Presse Med. 2004, 33, S10–S12. [Google Scholar]
- Zhou, Y.X.; Xia, W.; Yue, W.; Peng, C.; Rahman, K.; Zhang, H. Rhein: A Review of Pharmacological Activities. Evid.-Based Complement. Altern. Med. 2015, 2015, 578107. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhang, Z.; Ye, Y.; Li, H.; Luo, H.; Tang, K.; Lai, Y. Efficacy, Residual Effectiveness and Safety of Diacerein in the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Placebo-Controlled Trials. Medicine 2022, 101, e31700. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, M.A.B.; Clegg, D.; Jansen, T.L.; Rasker, J.J. Emerging and New Treatment Options for Knee Osteoarthritis. Curr. Rheumatol. Rev. 2022, 18, 20–32. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C. Intra-Articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Review of Their Current Molecular Mechanisms of Action and Their Degree of Efficacy. Int. J. Mol. Sci. 2022, 23, 1301. [Google Scholar] [CrossRef] [PubMed]
- OECD Test No. 107: Partition Coefficient (n-Octanol/Water): Shake Flask Method; OECD Guidelines for the Testing of Chemicals, Section 1; OECD: Paris, France, 1995; ISBN 9789264069626.
- International Conference On Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Validation of Analytical Procedures: Text and Methodology Q2(R1). Available online: https://database.ich.org/sites/default/files/Q2%28R1%29 Guideline.pdf (accessed on 8 June 2022).
- Nicolas, P.; Tod, M.; Padoin, C.; Petitjean, O. Clinical Pharmacokinetics of Diacerein. Clin. Pharmacokinet. 1998, 35, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Mathy-hartert, M.; Deberg, M.A.; Ficheux, H.; Reginster, J.L.; Henrotin, Y.E. Effects of Rhein on Human Articular Chondrocytes in Alginate Beads. Biochem. Pharmacol. 2003, 65, 377–388. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, X.-M.; Li, X.-Y.; Wang, B.-X. Pharmacokinetic of Rhein in Healthy Male Volunteers Following Oral and Retention Enema Administration of Rhubarb Extract: A Single Dose Study. Am. J. Chin. Med. 2005, 33, 839–850. [Google Scholar] [CrossRef]
- Mandawgade, S.D.; Kulkarni, S.; Pal, A.; Srivastava, S.; Padhi, B.K.; Raghuvanshi, R.S. Development and Pharmacokinetic Evaluation of New Oral Formulations of Diacerein. Curr. Drug Deliv. 2016, 13, 83–89. [Google Scholar] [CrossRef]
- Sun, H.; Yin, Q.; Zhang, A.; Wang, X. UPLC-MS/MS Performing Pharmacokinetic and Biodistribution Studies of Rhein. J. Sep. Sci. 2012, 35, 2063–2068. [Google Scholar] [CrossRef]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L. V In Vitro Cell Migration and Invasion Assays. J. Vis. Exp. 2014, 88, e51046. [Google Scholar] [CrossRef] [Green Version]
- Jonkman, J.E.N.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An Introduction to the Wound Healing Assay Using Live-Cell Microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Ebada, H.M.K.; Nasra, M.M.A.; Elnaggar, Y.S.R.; Nassra, R.A.; Solaiman, A.A.; Abdallah, O.Y. Novel Rhein Integrate Transphytosomes as Non-Invasive Local Therapy for Osteoarthritis to Ameliorate Cartilage Deterioration in MIA-Arthritic Rats. Colloids Surf. B Biointerfaces 2021, 202, 111713. [Google Scholar] [CrossRef]
- Chow, Y.Y.; Chin, K.-Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, N.A.; Murawski, C.D.; Fortier, L.A.; Cole, B.J.; Kennedy, J.G. Platelet-Rich Plasma in the Pathologic Processes of Cartilage: Review of Basic Science Evidence. Arthrosc.-J. Arthrosc. Relat. Surg. 2013, 29, 1399–1409. [Google Scholar] [CrossRef]
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Zabrzyński, J.; Gagat, M.; Mobasheri, A.; Jeka, S. The Effect of Platelet-Rich Plasma on the Intra-Articular Microenvironment in Knee Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5492. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.; Guo, A. Platelet-rich plasma protects rat chondrocytes from interleukin-1β-induced apoptosis. Mol. Med. Rep. 2016, 14, 4075–4082. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Scaranari, M.; Benelli, R.; Strada, P.; Reis, R.L.; Cancedda, R.; Gentili, C. Dual Effect of Platelet Lysate on Human Articular Cartilage: A Maintenance of Chondrogenic Potential and a Transient pro-Inflammatory Activity Followed by an Inflammation Resolution. Tissue Eng. Part A 2013, 19, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Legendre, F.; Heuze, A.; Boukerrouche, K.; Leclercq, S.; Boumediene, K.; Galera, P.; Domagala, F.; Pujol, J.P.; Ficheux, H. Rhein, the Metabolite of Diacerhein, Reduces the Proliferation of Osteoarthritic Chondrocytes and Synoviocytes without Inducing Apoptosis. Scand. J. Rheumatol. 2009, 38, 104–111. [Google Scholar] [CrossRef]
- Legendre, F.; Bogdanowicz, P.; Martin, G.; Domagala, F.; Leclercq, S.; Pujol, J.P.; Ficheux, H. Rhein, a Diacerhein-Derived Metabolite, Modulates the Expression of Matrix Degrading Enzymes and the Cell Proliferation of Articular Chondrocytes by Inhibiting ERK and JNK-AP-1 Dependent Pathways. Clin. Exp. Rheumatol. 2007, 25, 546–555. [Google Scholar] [PubMed]
- Yang, F.; Hu, H.; Yin, W.; Li, G.; Yuan, T.; Xie, X.; Zhang, C. Autophagy Is Independent of the Chondroprotection Induced by Platelet-Rich Plasma Releasate. BioMed Res. Int. 2018, 2018, 9726703. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.H.; Lo, W.C.; Hsu, W.C.; Wei, H.J.; Liu, H.Y.; Lee, C.H.; Tina Chen, S.Y.; Shieh, Y.H.; Williams, D.F.; Deng, W.P. Synergistic Anabolic Actions of Hyaluronic Acid and Platelet-Rich Plasma on Cartilage Regeneration in Osteoarthritis Therapy. Biomaterials 2014, 35, 9599–9607. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Nurden, A.T.; Zalduendo, M.M.; De la Fuente, M.; Azofra, J.; Andía, I. Platelet-Released Growth Factors Enhance the Secretion of Hyaluronic Acid and Induce Hepatocyte Growth Factor Production by Synovial Fibroblasts from Arthritic Patients. Rheumatology 2007, 46, 1769–1772. [Google Scholar] [CrossRef] [Green Version]
- Van Buul, G.M.; Koevoet, W.L.M.M.; Kops, N.; Bos, P.K.; Verhaar, J.A.N.N.; Weinans, H.; Bernsen, M.R.; Van Osch, G.J.V.M.V.M. Platelet-Rich Plasma Releasate Inhibits Inflammatory Processes in Osteoarthritic Chondrocytes. Am. J. Sports Med. 2011, 39, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Woodell-May, J.; Matuska, A.; Oyster, M.; Welch, Z.; O’Shaughnessey, K.; Hoeppner, J. Autologous Protein Solution Inhibits MMP-13 Production by IL-1β and TNFα-Stimulated Human Articular Chondrocytes. J. Orthop. Res. 2011, 29, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Lee, C.-H.H.; Peng, Y.J.; Salter, D.M.; Lee, H.S. Platelet-Rich Plasma Attenuates 30-KDa Fibronectin Fragment-Induced Chemokine and Matrix Metalloproteinase Expression by Meniscocytes and Articular Chondrocytes. Am. J. Sports Med. 2015, 43, 2481–2489. [Google Scholar] [CrossRef]
- Wu, C.C.; Chen, W.H.; Zao, B.; Lai, P.L.; Lin, T.C.; Lo, H.Y.; Shieh, Y.H.; Wu, C.H.; Deng, W.P. Regenerative Potentials of Platelet-Rich Plasma Enhanced by Collagen in Retrieving pro-Inflammatory Cytokine-Inhibited Chondrogenesis. Biomaterials 2011, 32, 5847–5854. [Google Scholar] [CrossRef]
- Tamura, T.; Kosaka, N.; Ishiwa, J.; Sato, T.; Nagase, H.; Ito, A. Rhein, an Active Metabolite of Diacerein, down-Regulates the Production of pro-Matrix Metalloproteinases-1, -3, -9 and -13 and up-Regulates the Production of Tissue Inhibitor of Metalloproteinase-1 in Cultured Rabbit Articular Chondrocytes. Osteoarthr. Cartil. 2001, 9, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman-Blas, J.A.; Jimenez, S.A. NF-KappaB as a Potential Therapeutic Target in Osteoarthritis and Rheumatoid Arthritis. Osteoarthr. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, F.; Li, J.; Wang, Y.H.; Li, M.; Song, F.F.; Liu, Y.L.; Tao, Z.Y. Platelet-Rich Plasma Inhibits Inflammation, Apoptosis, and the NLRP3/Caspase-1 Pathway and Induces Matrix Metalloproteinases and Proliferation of IL-1β-Induced Articular Chondrocytes by Downregulating T-Box Transcription Factor 3. Eur. J. Inflamm. 2022, 20, 1–10. [Google Scholar] [CrossRef]
- Yang, J.; Guo, A.; Li, Q.; Wu, J. Platelet-Rich Plasma Attenuates Interleukin-1β-Induced Apoptosis and Inflammation in Chondrocytes through Targeting Hypoxia-Inducible Factor-2α. Tissue Cell 2021, 73, 101646. [Google Scholar] [CrossRef]
- Moussa, M.; Lajeunesse, D.; Hilal, G.; El Atat, O.; Haykal, G.; Serhal, R.; Chalhoub, A.; Khalil, C.; Alaaeddine, N. Platelet Rich Plasma (PRP) Induces Chondroprotection via Increasing Autophagy, Anti-Inflammatory Markers, and Decreasing Apoptosis in Human Osteoarthritic Cartilage. Exp. Cell Res. 2017, 352, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Yaron, M.; Shirazi, I.; Yaron, I. Anti-Interleukin-1 Effects of Diacerein and Rhein in Human Osteoarthritic Synovial Tissue and Cartilage Cultures. Osteoarthr. Cartil. 1999, 7, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, J.P.; Mineau, F.; Fernandes, J.C.; Duval, N.; Martel-Pelletier, J. Diacerhein and Rhein Reduce the Interleukin 1beta Stimulated Inducible Nitric Oxide Synthesis Level and Activity While Stimulating Cyclooxygenase-2 Synthesis in Human Osteoarthritic Chondrocytes. J. Rheumatol. 1998, 25, 2417–2424. [Google Scholar] [PubMed]
- Eitner, A.; Müller, S.; König, C.; Wilharm, A.; Raab, R.; Hofmann, G.O.; Kamradt, T.; Schaible, H.-G. Inhibition of Inducible Nitric Oxide Synthase Prevents IL-1β-Induced Mitochondrial Dysfunction in Human Chondrocytes. Int. J. Mol. Sci. 2021, 22, 2477. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.B. Osteoarthritis and Nitric Oxide. Osteoarthr. Cartil. 2008, 16, S15–S20. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.; Xu, H.; Sheng, J.; Xu, Z.; Xie, X.; Zhang, C. Comparative Evaluation of the Effects of Platelet-Rich Plasma Formulations on Extracellular Matrix Formation and the NF-ΚB Signaling Pathway in Human Articular Chondrocytes. Mol. Med. Rep. 2017, 15, 2940–2948. [Google Scholar] [CrossRef] [Green Version]
- Lo, Y.Y.; Conquer, J.A.; Grinstein, S.; Cruz, T.F. Interleukin-1 Beta Induction of c-Fos and Collagenase Expression in Articular Chondrocytes: Involvement of Reactive Oxygen Species. J. Cell. Biochem. 1998, 69, 19–29. [Google Scholar] [CrossRef]
- Gómez-Gaete, C.; Retamal, M.; Chávez, C.; Bustos, P.; Godoy, R.; Torres-Vergara, P. Development, Characterization and in Vitro Evaluation of Biodegradable Rhein-Loaded Microparticles for Treatment of Osteoarthritis. Eur. J. Pharm. Sci. 2017, 96, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, F.; Pelletier, J.P.; Jolicoeur, F.C.; Cloutier, J.M.; Martel-Pelletier, J. Diacerhein and Rhein Reduce the ICE-Induced IL-1beta and IL-18 Activation in Human Osteoarthritic Cartilage. Osteoarthr. Cartil. 2000, 8, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, L.; Ma, C.; Wang, G.; Zhang, Y.; Sun, S. Exosomes Derived from Platelet-Rich Plasma Present a Novel Potential in Alleviating Knee Osteoarthritis by Promoting Proliferation and Inhibiting Apoptosis of Chondrocyte via Wnt/β-Catenin Signaling Pathway. J. Orthop. Surg. Res. 2019, 14, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domagala, F.; Martin, G.; Bogdanowicz, P.; Ficheux, H.; Pujol, J.-P. Inhibition of Interleukin-1beta-Induced Activation of MEK/ERK Pathway and DNA Binding of NF-KappaB and AP-1: Potential Mechanism for Diacerein Effects in Osteoarthritis. Biorheology 2006, 43, 577–587. [Google Scholar] [PubMed]
- Cavallo, C.; Filardo, G.; Mariani, E.; Kon, E.; Marcacci, M.; Pereira Ruiz, M.T.; Facchini, A.; Grigolo, B. Comparison of Platelet-Rich Plasma Formulations for Cartilage Healing: An in Vitro Study. J. Bone Jt. Surg. Am. 2014, 96, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Sakata, R.; McNary, S.M.; Miyatake, K.; Lee, C.A.; Van den Bogaerde, J.M.; Marder, R.A.; Reddi, A.H. Stimulation of the Superficial Zone Protein and Lubrication in the Articular Cartilage by Human Platelet-Rich Plasma. Am. J. Sports Med. 2015, 43, 1467–1473. [Google Scholar] [CrossRef] [Green Version]




| Gene | Symbol | Probe Assay ID 1 |
|---|---|---|
| Interleukin-1 β | IL-1β | Hs.PT.58.1518186 |
| Interleukin-6 | IL-6 | Hs.PT.58.40226675 |
| Matrix metallopeptidase 13 | MMP13 | Hs.PT.58.40735012 |
| ADAM metallopeptidase with thrombospondin type 1 motif 4 | ADAMTS4 | Hs.PT.58.4659383 |
| β-2-microglobulin | B2M | Hs.PT.58v.18759587 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simental-Mendía, M.; Lozano-Sepúlveda, S.A.; Garza-Tapia, M.; Lara-Arias, J.; Acosta-Olivo, C.A.; Vilchez-Cavazos, F.; Peña-Martínez, V.M. The Effects of the Combination of Rhein and Platelet-Rich Plasma on Human Articular Chondrocytes. Life 2023, 13, 1723. https://doi.org/10.3390/life13081723
Simental-Mendía M, Lozano-Sepúlveda SA, Garza-Tapia M, Lara-Arias J, Acosta-Olivo CA, Vilchez-Cavazos F, Peña-Martínez VM. The Effects of the Combination of Rhein and Platelet-Rich Plasma on Human Articular Chondrocytes. Life. 2023; 13(8):1723. https://doi.org/10.3390/life13081723
Chicago/Turabian StyleSimental-Mendía, Mario, Sonia Amelia Lozano-Sepúlveda, Marsela Garza-Tapia, Jorge Lara-Arias, Carlos Alberto Acosta-Olivo, Félix Vilchez-Cavazos, and Víctor Manuel Peña-Martínez. 2023. "The Effects of the Combination of Rhein and Platelet-Rich Plasma on Human Articular Chondrocytes" Life 13, no. 8: 1723. https://doi.org/10.3390/life13081723





