Immobilization of Lipase B from Candida antarctica on Magnetic Nanoparticles Enhances Its Selectivity in Kinetic Resolutions of Chiral Amines with Several Acylating Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enzyme and Materials
2.2. Methods
2.2.1. Analytical Methods and Calculations
2.2.2. Preparation of Isopropyl 2-Ethoxyacetate 2C
2-Ethoxyacetic Acid
Isopropyl 2-Ethoxyacetate 2C
2.2.3. Preparation of CaLB-MNP Biocatalysts
2.2.4. Kinetic Resolution of the Racemic Amines (±)-1a–d in Batch Mode with Different Acylating Agents 2A–C and CaLB on Different Supports
2.2.5. Kinetic Resolution of the Racemic Amines (±)-1a,b,d with Isopropyl 2-Cyanoacetate 2B in Batch Mode Using CaLB-MNPs for Isolation of the New Amides (R)-3(a,b,d)B
2-Cyano-N-(2-heptanyl)acetamide (R)-3aB
2-Cyano-N-(1-methoxy-2-propanyl)acetamide (R)-3bB
2-Cyano-N-(4-phenyl-2-butanyl)acetamide (R)-3dB
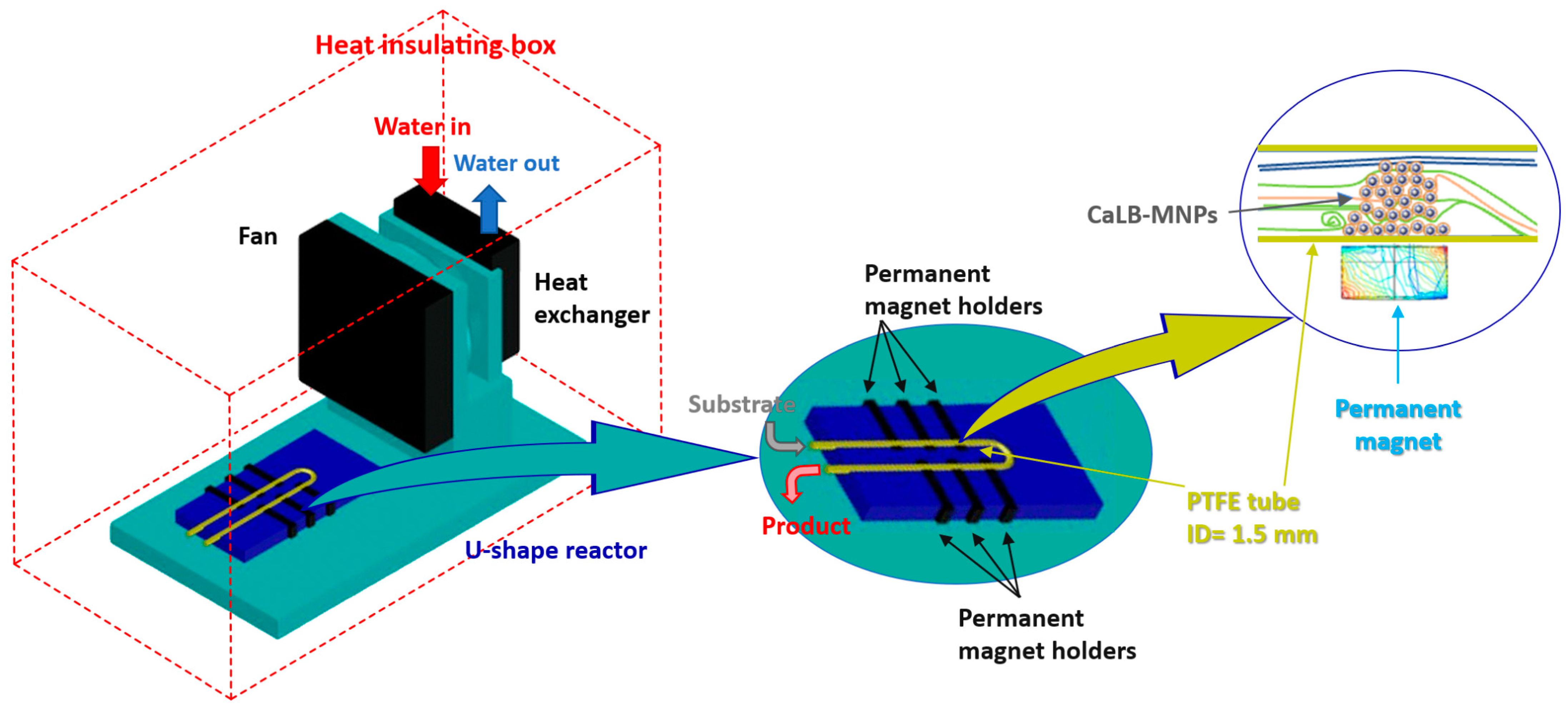
2.2.6. Design and Assembly of the Thermostatted U-Shape MNP Reactor
2.2.7. Kinetic Resolution of the Racemic Amines (±)-1b and (±)-1c with Isopropyl 2-Ethoxyacetate 2C Using CaLB-MNPs in Continuous-Flow U-Shape Reactor
3. Results and Discussion
3.1. Biocatalyst Characterization
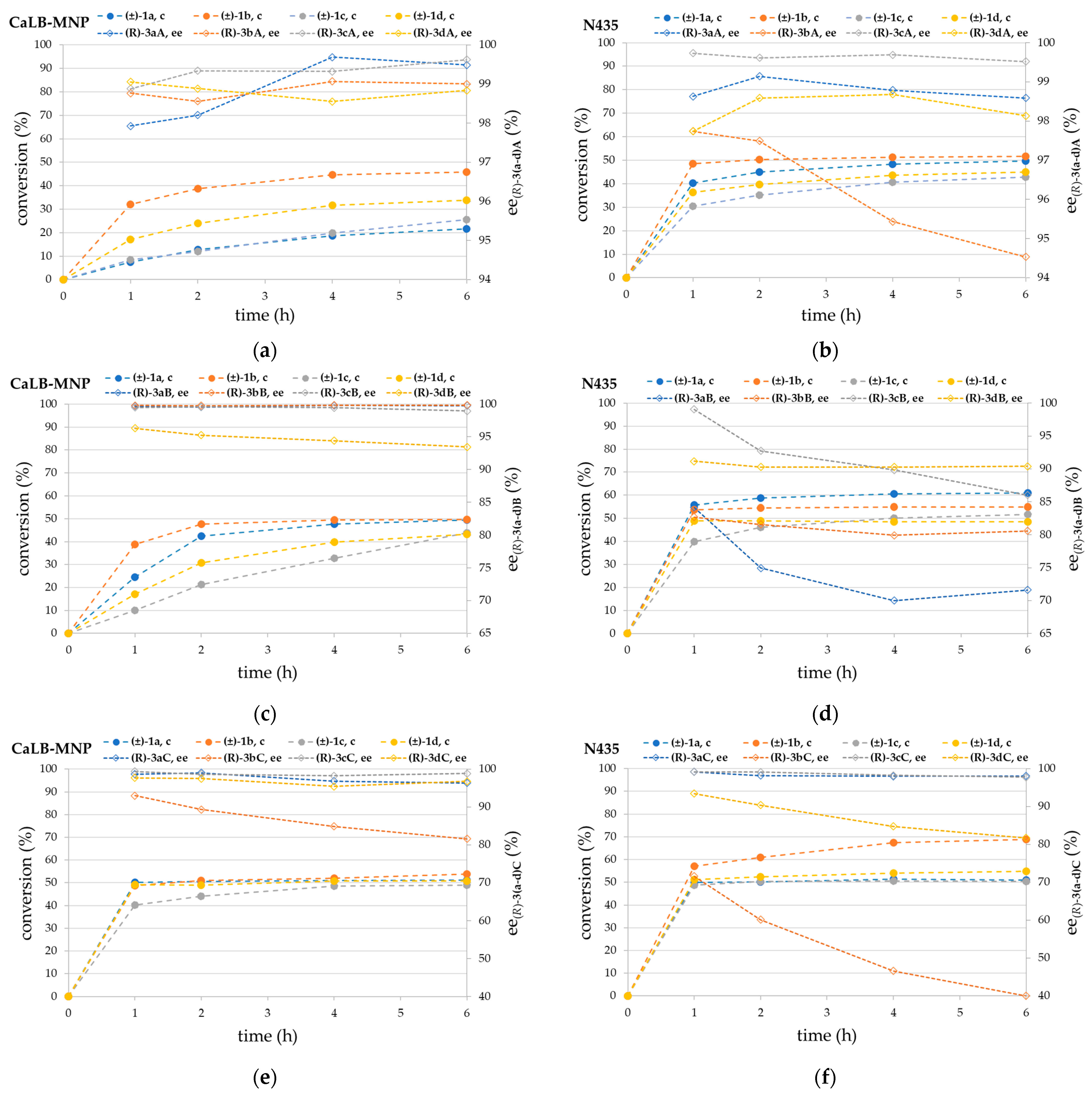
3.2. Kinetic Resolution of Chiral Amines (±)-1a–d with Different Acylating Agents (2A–C) in Batch Mode with CaLB-MNPs and N435
3.3. CaLB-MNP-Catalyzed Kinetic Resolution of Chiral Amines (±)-1b and (±)-1c with Isopropyl 2-Ethoxyacetate 2C in Thermostatted Continuous-Flow U-Shape Reactor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghislieri, D.; Turner, N.J. Biocatalytic Approaches to the Synthesis of Enantiomerically Pure Chiral Amines. Top. Catal. 2013, 57, 284–300. [Google Scholar] [CrossRef]
- Grogan, G. Synthesis of chiral amines using redox biocatalysis. Curr. Opin. Chem. Biol. 2018, 43, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.D.; Grogan, G.; Bommarius, A.; Yun, H. Recent Advances in ω-Transaminase-Mediated Biocatalysis for the Enantioselective Synthesis of Chiral Amines. Catalysts 2018, 8, 254. [Google Scholar] [CrossRef] [Green Version]
- Aguillón, A.R.; Miranda, A.S.; Junior, I.I.; Souza, R.O.M.A. Biocatalysis toward the Synthesis of Chiral Amines. In Synthetic Approaches to Nonaromatic Nitrogen Heterocycles; Faisca Philips, A.M.M.M., Ed.; Wiley: Hoboken, NJ, USA, 2020; Volume 2, pp. 667–697. [Google Scholar] [CrossRef]
- Musa, M.M. Enzymatic racemization of alcohols and amines: An approach for bi-enzymatic dynamic kinetic resolution. Chirality 2019, 32, 147–157. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.H. Lipase immobilization with support materials, preparation techniques, and applications: Present and future aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, Z.; Diao, Z.; Liang, S.; Han, S.; Zheng, S.; Lin, Y. Kinetic resolution of sec-alcohols catalysed by Candida antarctica lipase B displaying Pichia pastoris whole-cell biocatalyst. Enzym. Microb. Technol. 2018, 110, 8–13. [Google Scholar] [CrossRef]
- Imarah, A.O.; Silva, F.M.W.G.; Tuba, L.; Malta-Lakó, Á.; Szemes, J.; Sánta-Bell, E.; Poppe, L. A Convenient U-Shape Microreactor for Continuous Flow Biocatalysis with Enzyme-Coated Magnetic Nanoparticles-Lipase Catalyzed Enantiomer Selective Acylation of 4-(Morpholin-4-yl)butan-2-ol. Catalysts 2022, 12, 1065. [Google Scholar] [CrossRef]
- Silva, F.M.W.G.; Imarah, A.O.; Takács, O.; Tuba, L.; Poppe, L. Scalability of U-Shape Magnetic Nanoparticles-Based Microreactor–Lipase-Catalyzed Preparative Scale Kinetic Resolutions of Drug-like Fragments. Catalysts 2023, 13, 384. [Google Scholar] [CrossRef]
- Xing, X.; Jia, J.Q.; Zhang, J.F.; Zhou, Z.W.; Li, J.; Wang, N.; Yu, X.Q. CALB Immobilized onto Magnetic Nanoparticles for Efficient Kinetic Resolution of Racemic Secondary Alcohols: Long-Term Stability and Reusability. Molecules 2019, 24, 490. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.H.; Kou, B.S.; Tsai, S.W. CALB-catalyzed kinetic resolution of (RS)-3-benzoylthio-2-methylpropyl azolides: Kinetic and thermodynamic analysis. Biocatal. Biotransform. 2020, 38, 376–384. [Google Scholar] [CrossRef]
- Bäckvall, J.E.; Gustafson, K.; Görbe, T.; de Gonzalo, G.; Yang, N.; Schreiber, C.; Shchukarev, A.; Tai, C.-W.; Persson, I.; Zou, X. Chemoenzymatic Dynamic Kinetic Resolution of Primary Benzylic Amines using Pd(0)-CalB CLEA as a Biohybrid Catalyst. Chem. Eur. J. 2019, 25, 9174–9179. [Google Scholar] [CrossRef]
- El-Behairy, M.F.; Hassan, R.M.; Sundby, E. Enantioselective Chromatographic Separation and Lipase Catalyzed Asymmetric Resolution of Biologically Important Chiral Amines. Separations 2021, 8, 165. [Google Scholar] [CrossRef]
- Szemes, J.; Malta-Lakó, Á.; Tóth, R.E.; Poppe, L. Diisopropyl Malonate as Acylating Agent in Kinetic Resolution of Chiral Amines with Lipase B from Candida antarctica. Period. Polytech. Chem. Eng. 2022, 66, 458–464. [Google Scholar] [CrossRef]
- Csuka, P.; Boros, Z.; Őrfi, L.; Dobos, J.; Poppe, L.; Hornyánszky, G. Chemoenzymatic route to Tyrphostins involving lipase-catalyzed kinetic resolution of 1-phenylethanamine with alkyl cyanoacetates as novel acylating agents. Tetrahedron Asymmetry 2015, 26, 644–649. [Google Scholar] [CrossRef]
- Oláh, M.; Boros, Z.; Hornyánszky, G.; Poppe, L. Isopropyl 2-ethoxyacetate—An efficient acylating agent for lipase-catalyzed kinetic resolution of amines in batch and continuous-flow modes. Tetrahedron 2016, 72, 7249–7255. [Google Scholar] [CrossRef]
- Oláh, M.; Kovács, D.; Katona, G.; Hornyánszky, G.; Poppe, L. Optimization of 2-alkoxyacetates as acylating agent for enzymatic kinetic resolution of chiral amines. Tetrahedron 2018, 74, 3663–3670. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Overview of Immobilized Enzymes’ Applications in Pharmaceutical, Chemical, and Food Industry. In Immobilization of Enzymes and Cells. Methods in Molecular Biology; Guisan, J., Bolivar, J., López-Gallego, F., Rocha-Martín, J., Eds.; Humana: New York, NY, USA, 2020; Volume 2100. [Google Scholar] [CrossRef]
- Heckmann, C.M.; Paradisi, F. Looking Back: A Short History of the Discovery of Enzymes and How They Became Powerful Chemical Tools. ChemCatChem 2020, 12, 6082–6102. [Google Scholar] [CrossRef]
- Falus, P.; Boros, Z.; Hornyánszky, G.; Nagy, J.; Ürge, L.; Darvas, F.; Poppe, L. Synthesis and Lipase Catalysed Kinetic Resolution of Racemic Amines. Stud. Univ. Babes-Bolyai Ser. Chem. 2010, 4, 289–296. [Google Scholar]
- Boros, Z.; Falus, P.; Márkus, M.; Weiser, D.; Oláh, M.; Hornyánszky, G.; Nagy, J.; Poppe, L. How the mode of Candida antarctica lipase B immobilization affects the continuous-flow kinetic resolution of racemic amines at various temperatures. J. Mol. Catal. B Enzym. 2013, 85–86, 119–125. [Google Scholar] [CrossRef]
- Päiviö, M.; Perkiö, P.; Kanerva, L.T. Solvent-free kinetic resolution of primary amines catalyzed by Candida antarctica lipase B: Effect of immobilization and recycling stability. Tetrahedron Asymmetry 2012, 23, 230–236. [Google Scholar] [CrossRef]
- Joubioux, F.L.; Achour, O.; Bridiau, N.; Graber, M.; Maugard, T.J. Kinetic study of 2-butanol O-acylation and sec-butylamine N-acylation catalyzed by Candida antarctica lipase B. J. Mol. Catal. B Enzym. 2011, 70, 108–113. [Google Scholar] [CrossRef]
- Hietanen, A.; Saloranta, T.; Leino, R.; Kanerva, L.T. Lipase catalysis in the preparation of 3-(1-amino-3-butenyl)pyridine enantiomers. Tetrahedron Asymmetry 2012, 23, 1629–1632. [Google Scholar] [CrossRef]
- Cavicchioli, M.; Pevarello, P.; Salom, B.; Vulpetti, A. Aminoisoxazole Derivatives Active as Kinase Inhibitors. International Patent Application WO 03/013517 A1, 20 February 2003. [Google Scholar]
- Viñambres, M.; Filice, M.; Marciello, M. Modulation of the Catalytic Properties of Lipase B from Candida antarctica by Immobilization on Tailor-Made Magnetic Iron Oxide Nanoparticles: The Key Role of Nanocarrier Surface Engineering. Polymers 2018, 10, 615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engstrom, K.; Johnston, E.V.; Verho, O.; Gustafson, K.P.J.; Shakeri, M.; Tai, C.W.; Backvall, J.E. Co-immobilization of an enzyme and a metal into the compartments of mesoporous silica for cooperative tandem catalysis: An artificial metalloenzyme. Angew. Chem. 2013, 52, 14006–14010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafson, K.P.J.; Lihammar, R.; Verho, O.; Engström, K.; Bäckvall, J.E. Chemoenzymatic Dynamic Kinetic Resolution of Primary Amines Using a Recyclable Palladium Nanoparticle Catalyst Together with Lipases. J. Org. Chem. 2014, 79, 3747–3751. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Wang, M.; Feng, B.; Han, X.; Lan, Z.; Gu, H.; Li, H.; Li, H. Dynamic kinetic resolution of amines by using palladium nanoparticles confined inside the cages of amine-modified MIL-101 and lipase. J. Catal. 2018, 363, 9–17. [Google Scholar] [CrossRef]
- Li, P.; Zhu, J.; Zhang, H.; Wang, L.; Wang, S.; Zhang, M.; Wu, J.; Yang, L.; Xu, G. Preparation of Coupling Catalyst HamZIF-90@Pd@CALB with Tunable Hollow Structure for Efficient Dynamic Kinetic Resolution of 1-Phenylethylamine. Molecules 2023, 28, 922. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Mohammadlou, M.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol. Lett. 2015, 38, 223–233. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef]
- Gupta, A.R.; Rathod, V.K. Biodiesel synthesis from palm fatty acid distillate using enzyme immobilized on magnetic nanoparticles. SN Appl. Sci. 2020, 2, 1778. [Google Scholar] [CrossRef]
- Alikhani, N.; Shahedi, M.; Habibi, Z.; Yousefi, M.; Ghasemi, S.; Mohammadi, M. A multi-component approach for co-immobilization of lipases on silica-coated magnetic nanoparticles: Improving biodiesel production from waste cooking oil. Bioprocess Biosyst. Eng. 2022, 45, 2043–2060. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Wang, M.; He, W.; Wu, J.; Liu, X.; Guan, Y. Ultra-small magnetic Candida antarctica lipase B nanoreactors for enzyme synthesis of bixin-maltitol ester. Food Chem. 2023, 421, 136132. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, X.; Zheng, L. Engineering actively magnetic crosslinked inclusion bodies of Candida antarctica lipase B: An efficient and stable biocatalyst for enzyme-catalyzed reactions. Mol. Catal. 2021, 504, 111467. [Google Scholar] [CrossRef]
- Ferraz, C.A.; do Nascimento, M.A.; Almeida, R.F.O.; Sergio, G.G.; Junior, A.A.T.; Dalmônico, G.; Caraballo, R.; Finotelli, P.V.; Leão, R.A.C.; Wojcieszak, R.; et al. Synthesis and characterization of a magnetic hybrid catalyst containing lipase and palladium and its application on the dynamic kinetic resolution of amines. Mol. Catal. 2020, 493, 111106. [Google Scholar] [CrossRef]
- Baumann, M.; Moody, T.S.; Smyth, M.; Wharry, S. A Perspective on Continuous Flow Chemistry in the Pharmaceutical Industry. Org. Process Res. Dev. 2020, 24, 1802–1813. [Google Scholar] [CrossRef]
- Rakels, J.L.L.; Straathof, A.J.J.; Heijnen, J.J. A simple method to determine the enantiomeric ratio in enantioselective biocatalysis. Enzym. Microb. Technol. 1993, 15, 1051–1056. [Google Scholar] [CrossRef]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]
- Abaházi, E.; Lestál, D.; Boros, Z.; Poppe, L. Tailoring the Spacer Arm for Covalent Immobilization of Candida antarctica Lipase B–Thermal Stabilization by Bisepoxide-activated Aminoalkyl Resins in Continuous-flow Reactors. Molecules 2016, 21, 767. [Google Scholar] [CrossRef] [Green Version]
- Csuka, P.; Molnár, Z.; Tóth, V.; Imarah, A.O.; Balogh-Weiser, D.; Vértessy, B.G.; Poppe, L. Immobilization of the Aspartate Ammonia-lyase from Pseudomonas fluorescens R124 on Magnetic Nanoparticles—Characterization and Kinetics. ChemBioChem 2022, 23, e202100708. [Google Scholar] [CrossRef]
- Hellner, G.; Boros, Z.; Tomin, A.; Poppe, L. Novel Sol-Gel Lipases by Designed Bioimprinting for Continuous-Flow Kinetic Resolutions. Adv. Synth. Catal. 2011, 353, 2481–2491. [Google Scholar] [CrossRef]
- Csajági, C.; Szatzker, G.; Tőke, E.R.; Ürge, L.; Darvas, F.; Poppe, L. Enantiomer selective acylation of racemic alcohols by lipases in continuous-flow bioreactors. Tetrahedron Asymmetry 2008, 19, 237–246. [Google Scholar] [CrossRef]
- Imarah, A.O.; Silva, F.M.W.G.; Bataa, N.; Decsi, B.; Balogh-Weiser, D.; Poppe, L. Magnetically Agitated Continuous Flow Tube Reactors with Aspartate Ammonia-Lyase Immobilized on Magnetic Nanoparticles. React. Chem. Eng. 2023, 8, 1250–1259. [Google Scholar] [CrossRef]


| Entry | Amine | AA a | Amount | CaLB | t | c | ee(R)-3(a–d)(A–C) | ee(S)-1a | E | UB | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine (mM) | AA (eq.) | Support | Amount (mg mL−1) | (h) | (%) | (%) | (%) | (U g−1) | |||||
| 1 | (±)-1a | 2A | 45 | 1 | MNPs | 50 | 6 | 21.6 | 99.5 | 29.0 | ≫200 | 0.5 | b |
| 2 | 2A | 45 | 1 | N435 | 50 | 1 | 40.4 | 98.6 | 63.7 | >200 | 6.1 | b | |
| 3 | 2A | 180 | 1 | N435 | 100 | 4 | 50.0 | 98.5 | 98.7 | ≫200 | - | [14] | |
| 4 | 2B | 45 | 1 | MNPs | 50 | 6 | 49.5 | 99.8 | 97.0 | ≫200 | 1.2 | b | |
| 5 | 2B | 45 | 1 | N435 | 50 | 1 | 55.7 | 84.2 | 99.0 | 59.7 | 8.4 | b | |
| 6 | 2C | 45 | 1 | MNPs | 50 | 1 | 50.5 | 99.1 | 97.7 | ≫200 | 7.6 | b | |
| 7 | 2C | 45 | 1 | N435 | 50 | 1 | 49.9 | 99.1 | 97.9 | ≫200 | 7.5 | b | |
| 8 | (±)-1b | 2A | 45 | 1 | MNPs | 50 | 6 | 45.9 | 99.0 | 83.9 | ≫200 | 1.2 | b |
| 9 | 2A | 45 | 1 | N435 | 50 | 1 | 48.6 | 97.7 | 93.0 | >200 | 7.3 | b | |
| 10 | 2A | 180 | 1 | N435 | 100 | 4 | 52.1 | 92.0 | 99.9 | >100 | - | [14] | |
| 11 | 2B | 45 | 1 | MNPs | 50 | 4 | 49.8 | 99.8 | 98.7 | ≫200 | 1.9 | b | |
| 12 | 2B | 45 | 1 | N435 | 50 | 1 | 53.7 | 82.7 | 96.1 | 41.2 | 8.1 | b | |
| 13 | 2C | 45 | 1 | MNPs | 50 | 1 | 48.7 | 93.0 | 89.1 | 83.0 | 7.3 | b | |
| 14 | 2C | 45 | 1 | N435 | 50 | 1 | 57.2 | 71.6 | 95.6 | 22.4 | 8.6 | b | |
| 15 | (±)-1c | 2A | 45 | 1 | MNPs | 50 | 6 | 25.6 | 99.6 | 34.4 | ≫200 | 0.6 | b |
| 16 | 2A | 45 | 1 | N435 | 50 | 1 | 30.5 | 99.7 | 43.8 | ≫200 | 4.6 | b | |
| 17 | 2A | 180 | 1 | N435 | 100 | 4 | 45.0 | 99.9 | 81.5 | ≫200 | - | [14] | |
| 18 | 2B | 45 | 1 | MNPs | 50 | 6 | 43.9 | 98.9 | 74.5 | >200 | 1.1 | b | |
| 19 | 2B | 45 | 1 | N435 | 50 | 1 | 39.9 | 99.0 | 65.6 | >200 | 6.0 | b | |
| 20 | 2B* c | 200 | 0.5 | N435 | 50 | 24 | 31.7 c | 99.9 | 46.4 | - | 0.9 | [15] | |
| 21 | 2B* c | 200 | 1 | N435 | 50 | 24 | 50.1 c | 98.2 | 98.5 | - | 1.4 | [15] | |
| 22 | 2B* c | 82.5 | 0.5 | T2-150 | 100 | 24 | 20.0 c | 91.0 | 22.7 | - | - | [15] | |
| 23 | 2B* c | 82.5 | 0.5 | G250P | 100 | 24 | 17.1 c | 99.3 | 20.7 | - | - | [15] | |
| 24 | 2C | 45 | 1 | MNPs | 50 | 1 | 40.2 | 99.4 | 63.1 | ≫200 | 6.0 | b | |
| 25 | 2C | 45 | 1 | N435 | 50 | 1 | 48.8 | 99.1 | 94.1 | ≫200 | 7.3 | b | |
| 26 | 2C | 385 | 0.6 | G250P | 25 | 1 | 33.8 | >99.9 | - | ≫200 | - | [16] | |
| 27 | (±)-1d | 2A | 45 | 1 | MNPs | 50 | 6 | 33.8 | 98.8 | 51.2 | >200 | 0.8 | b |
| 28 | 2A | 45 | 1 | N435 | 50 | 1 | 36.3 | 97.7 | 55.3 | >100 | 5.5 | b | |
| 29 | 2A | 180 | 1 | N435 | 100 | 4 | 47.0 | 98.5 | 87.5 | >200 | - | [14] | |
| 30 | 2B | 45 | 1 | MNPs | 50 | 6 | 43.3 | 93.5 | 70.6 | 62.6 | 1.1 | b | |
| 31 | 2B | 45 | 1 | N435 | 50 | 1 | 49.0 | 91.2 | 83.4 | 56.6 | 7.3 | b | |
| 32 | 2C | 45 | 1 | MNPs | 50 | 1 | 49.1 | 97.7 | 93.4 | ≫200 | 7.4 | b | |
| 33 | 2C | 45 | 1 | N435 | 50 | 1 | 51.2 | 93.4 | 99.3 | >100 | 7.7 | b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.M.W.G.; Szemes, J.; Mustashev, A.; Takács, O.; Imarah, A.O.; Poppe, L. Immobilization of Lipase B from Candida antarctica on Magnetic Nanoparticles Enhances Its Selectivity in Kinetic Resolutions of Chiral Amines with Several Acylating Agents. Life 2023, 13, 1560. https://doi.org/10.3390/life13071560
Silva FMWG, Szemes J, Mustashev A, Takács O, Imarah AO, Poppe L. Immobilization of Lipase B from Candida antarctica on Magnetic Nanoparticles Enhances Its Selectivity in Kinetic Resolutions of Chiral Amines with Several Acylating Agents. Life. 2023; 13(7):1560. https://doi.org/10.3390/life13071560
Chicago/Turabian StyleSilva, Fausto M. W. G., József Szemes, Akan Mustashev, Orsolya Takács, Ali O. Imarah, and László Poppe. 2023. "Immobilization of Lipase B from Candida antarctica on Magnetic Nanoparticles Enhances Its Selectivity in Kinetic Resolutions of Chiral Amines with Several Acylating Agents" Life 13, no. 7: 1560. https://doi.org/10.3390/life13071560








