Changing Trends in the Incidence and Clinical Features of Pneumocystis jirovecii Pneumonia in Non-HIV Patients before and during the COVID-19 Era and Risk Factors for Mortality between 2016 and 2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Data Collection and Definitions
2.3. Statistical Analysis
3. Results
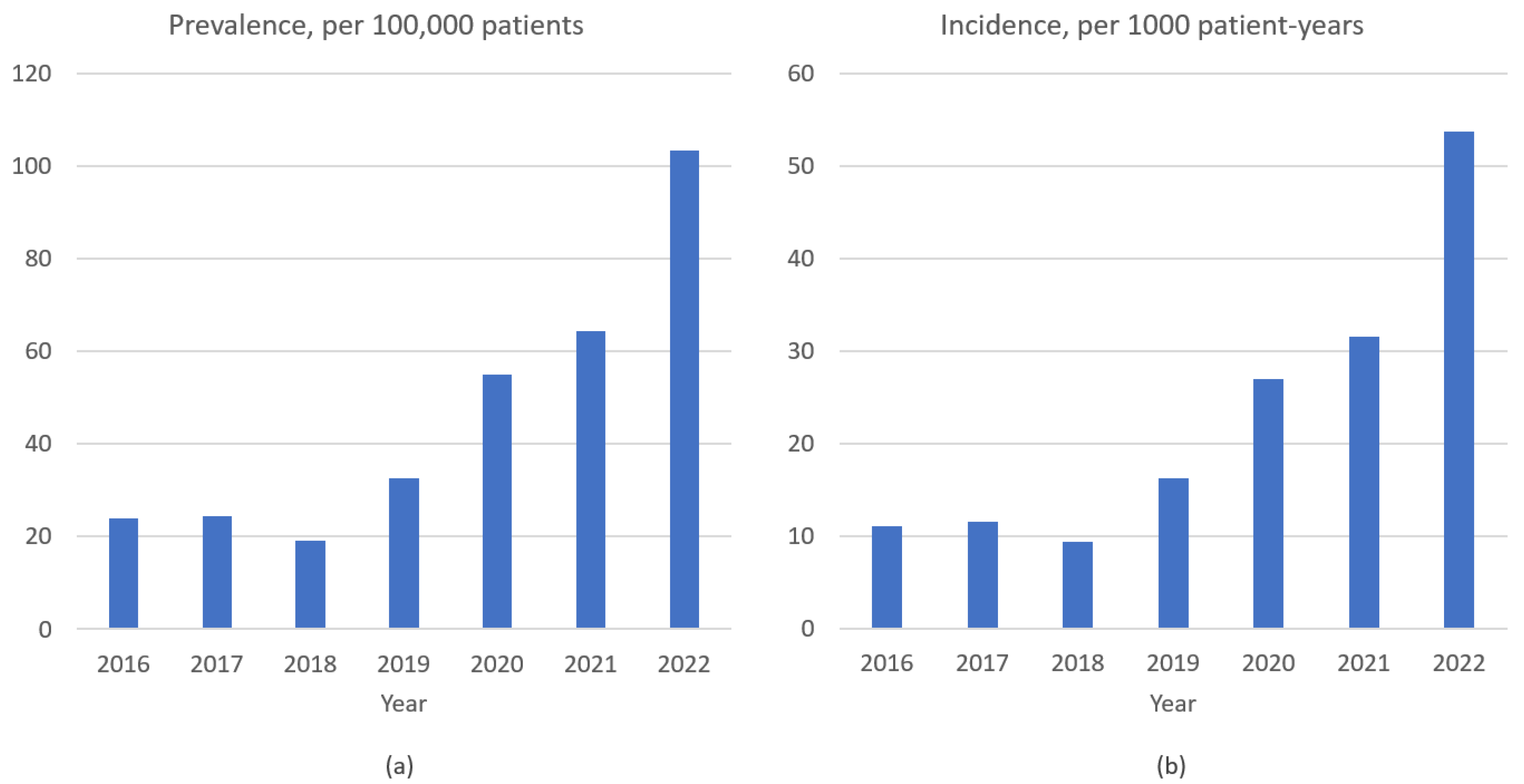
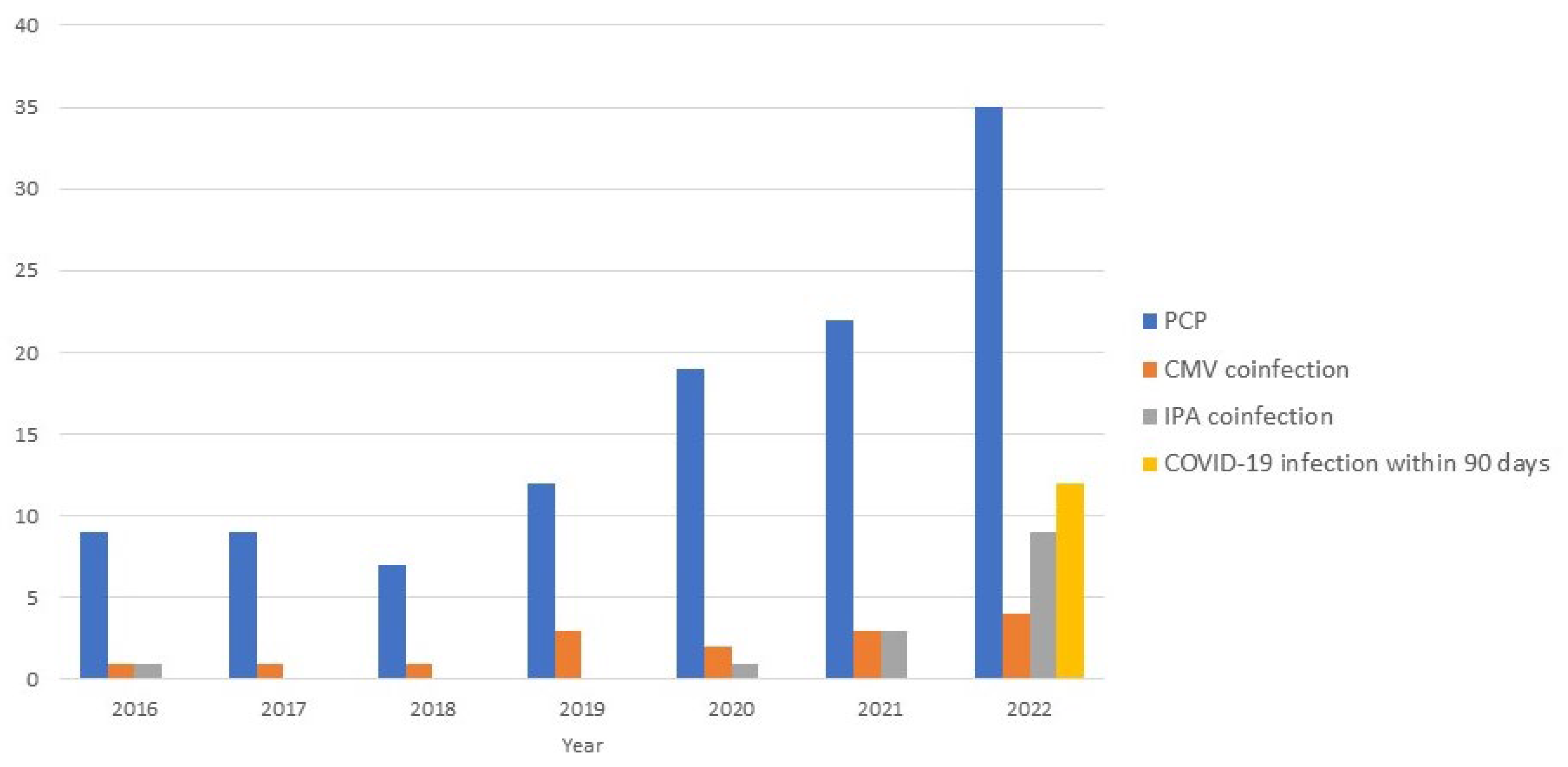
3.1. Prevalence, Incidence, and Clinical Features of P. jirovecii Pneumonia
3.2. Outcome of P. jirovecii Pneumonia
3.3. Risk Factors Associated with PCP-Related Mortality
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barber, R.M.; Sorensen, R.J.D.; Pigott, D.M.; Bisignano, C.; Carter, A.; Amlag, J.O.; Collins, J.K.; Abbafati, C.; Adolph, C.; Allorant, A.; et al. Estimating global, regional, and national daily and cumulative infections with SARS-CoV-2 through Nov 14, 2021: A statistical analysis. Lancet 2022, 399, 2351–2380. [Google Scholar] [CrossRef] [PubMed]
- Data on SARS-CoV-2 Variants in the EU/EE. European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/publications-data/data-virus-variants-covid-19-eueea (accessed on 12 April 2023).
- Variants of the Virus. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/index.html (accessed on 25 March 2023).
- Jeong, G.H.; Lee, H.J.; Lee, J.; Lee, J.Y.; Lee, K.H.; Han, Y.J.; Yoon, S.; Ryu, S.; Kim, D.K.; Park, M.B.; et al. Effective Control of COVID-19 in South Korea: Cross-Sectional Study of Epidemiological Data. J. Med. Internet Res. 2020, 22, e22103. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Shrestha, S.; Radnaabaatar, M.; Park, H.; Jung, J. Optimal Social Distancing Policy for COVID-19 Control in Korea: A Model-Based Analysis. J. Korean Med. Sci. 2022, 37, e189. [Google Scholar] [CrossRef]
- Coronavirus (COVID-19), Republic of Korea. Central Disaster Management Headquarters. Available online: https://ncov.kdca.go.kr/ (accessed on 20 March 2023).
- World Health Organization COVID-19 Dashboard. Available online: https://covid19.who.int/region/wpro/country/kr (accessed on 25 April 2023).
- Post-COVID Conditions: Information for Healthcare Providers. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html (accessed on 20 March 2023).
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Long COVID or Post-COVID Conditions. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 15 March 2023).
- Global Burden of Disease Long COVID Collaborators; Hanson, S.W.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; et al. Estimated Global Proportions of Individuals with Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar] [CrossRef]
- Jakubec, P.; Fišerová, K.; Genzor, S.; Kolář, M. Pulmonary Complications after COVID-19. Life 2022, 12, 357. [Google Scholar] [CrossRef]
- Wong, L.-Y.R.; Perlman, S. Immune dysregulation and immunopathology induced by SARS-CoV-2 and related coronaviruses—Are we our own worst enemy? Nat. Rev. Immunol. 2022, 22, 47–56. [Google Scholar] [CrossRef]
- Davitt, E.; Davitt, C.; Mazer, M.B.; Areti, S.S.; Hotchkiss, R.S.; Remy, K.E. COVID-19 disease and immune dysregulation. Best Pract. Res. Clin. Haematol. 2022, 35, 101401. [Google Scholar] [CrossRef]
- Chong, W.H.; Saha, B.K.; Ramani, A.; Chopra, A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection 2021, 49, 591–605. [Google Scholar] [CrossRef]
- Kurra, N.; Woodard, P.I.; Gandrakota, N.; Gandhi, H.; Polisetty, S.R.; Ang, S.P.; Patel, K.P.; Chitimalla, V.; Baig, M.M.A.; Samudrala, G. Opportunistic Infections in COVID-19: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e23687. [Google Scholar] [CrossRef] [PubMed]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef] [PubMed]
- Sreenath, K.; Batra, P.; Vinayaraj, E.V.; Bhatia, R.; SaiKiran, K.; Singh, V.; Singh, S.; Verma, N.; Singh, U.B.; Mohan, A.; et al. Coinfections with Other Respiratory Pathogens among Patients with COVID-19. Microbiol. Spectr. 2021, 9, e0016321. [Google Scholar] [CrossRef] [PubMed]
- Casalini, G.; Giacomelli, A.; Ridolfo, A.; Gervasoni, C.; Antinori, S. Invasive Fungal Infections Complicating COVID-19: A Narrative Review. J. Fungi 2021, 7, 921. [Google Scholar] [CrossRef]
- Abdoli, A.; Falahi, S.; Kenarkoohi, A. COVID-19-associated opportunistic infections: A snapshot on the current reports. Clin. Exp. Med. 2022, 22, 327–346. [Google Scholar] [CrossRef]
- Chong, W.H.; Saha, B.K.; Chopra, A. Narrative review of the relationship between COVID-19 and PJP: Does it represent coinfection or colonization? Infection 2021, 49, 1079–1090. [Google Scholar] [CrossRef]
- Niamatullah, H.; Nasir, N.; Jabeen, K.; Rattani, S.; Farooqi, J.; Ghanchi, N.; Irfan, M. Post-COVID-19 Pneumocystis pneumonia cases from Pakistan: An observational study. Access Microbiol. 2023, 5, 000406. [Google Scholar] [CrossRef]
- Takahashi, T.; Saito, A.; Kuronuma, K.; Nishikiori, H.; Chiba, H. Pneumocystis jirovecii Pneumonia Associated with COVID-19 in Patients with Interstitial Pneumonia. Medicina 2022, 58, 1151. [Google Scholar] [CrossRef]
- Cattaneo, L.; Buonomo, A.R.; Iacovazzo, C.; Giaccone, A.; Scotto, R.; Viceconte, G.; Mercinelli, S.; Vargas, M.; Roscetto, E.; Cacciatore, F.; et al. Invasive Fungal Infections in Hospitalized Patients with COVID-19: A Non-Intensive Care Single-Centre Experience during the First Pandemic Waves. J. Fungi 2023, 9, 86. [Google Scholar] [CrossRef]
- Gentile, I.; Viceconte, G.; Lanzardo, A.; Zotta, I.; Zappulo, E.; Pinchera, B.; Scotto, R.; Moriello, N.S.; Foggia, M.; Giaccone, A.; et al. Pneumocystis jirovecii Pneumonia in Non-HIV Patients Recovering from COVID-19: A Single-Center Experience. Int. J. Environ. Res. Public Health 2021, 18, 11399. [Google Scholar] [CrossRef]
- Cillóniz, C.; Dominedò, C.; Álvarez-Martínez, M.J.; Moreno, A.; García, F.; Torres, A.; Miro, J.M. Pneumocystis pneumonia in the twenty-first century: HIV-infected versus HIV-uninfected patients. Expert Rev. Anti-Infect. Ther. 2019, 17, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Salzer, H.J.; Schäfer, G.; Hoenigl, M.; Günther, G.; Hoffmann, C.; Kalsdorf, B.; Alanio, A.; Lange, C. Clinical, Diagnostic, and Treatment Disparities between HIV-Infected and Non-HIV-Infected Immunocompromised Patients with Pneumocystis jirovecii Pneumonia. Respiration 2018, 96, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Avino, L.J.; Naylor, S.M.; Roecker, A.M. Pneumocystis jirovecii Pneumonia in the Non–HIV-Infected Population. Ann. Pharmacother. 2016, 50, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, J.; Cho, Y.-J.; Park, Y.S.; Lee, C.-H.; Yoon, H.I.; Lee, S.-M.; Yim, J.-J.; Lee, J.H.; Yoo, C.-G.; et al. Prognostic factors of Pneumocystis jirovecii pneumonia in patients without HIV infection. J. Infect. 2014, 69, 88–95. [Google Scholar] [CrossRef]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin. Infect. Dis. 2017, 64, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Park, K.H.; Jung, C.-Y.; Jeong, W.; Lee, G.; Yang, J.S.; Nam, C.M.; Kim, H.W.; Kim, B.S. Nationwide Implementation of Nonpharmaceutical Interventions During the Coronavirus Disease 2019 Pandemic Is Associated with Decreased Incidence of Pneumocystis jirovecii Pneumonia in Kidney Transplant Recipients. Open Forum Infect. Dis. 2022, 9, ofac076. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.B.; Jeon, S.; Kim, S.; Lee, K.H.; Lee, H.S.; Han, S.H. No Change of Pneumocystis jirovecii Pneumonia after the COVID-19 Pandemic: Multicenter Time-Series Analyses. J. Fungi 2021, 7, 990. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, X.; Saimi, M.; Huang, X.; Sun, T.; Fan, G.; Zhan, Q. Risk Factors of Mortality from Pneumocystis Pneumonia in Non-HIV Patients: A Meta-Analysis. Front. Public Health 2021, 9, 680108. [Google Scholar] [CrossRef]
- Liu, Y.; Su, L.; Jiang, S.-J.; Qu, H. Risk factors for mortality from pneumocystis carinii pneumonia (PCP) in non-HIV patients: A meta-analysis. Oncotarget 2017, 8, 59729–59739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kageyama, T.; Furuta, S.; Ikeda, K.; Kagami, S.-I.; Kashiwakuma, D.; Sugiyama, T.; Umibe, T.; Watanabe, N.; Yamagata, M.; Nakajima, H. Prognostic factors of Pneumocystis pneumonia in patients with systemic autoimmune diseases. PLoS ONE 2019, 14, e0214324. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Jia, P.; Su, L.; Zhao, H.; Que, C. Outcomes and prognostic factors of non-HIV patients with pneumocystis jirovecii pneumonia and pulmonary CMV co-infection: A Retrospective Cohort Study. BMC Infect. Dis. 2017, 17, 392. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Ji, T.; Qin, D.; Cheng, D. Clinical characteristics and risk factors of in-hospital mortality in patients coinfected with Pneumocystis jirovecii and Aspergillus. J. Med. Mycol. 2023, 33, 101330. [Google Scholar] [CrossRef]
- Townsend, L.; Martin-Loeches, I. Invasive Aspergillosis in the Intensive Care Unit. Diagnostics 2022, 12, 2712. [Google Scholar] [CrossRef] [PubMed]
- Ghez, D.; Calleja, A.; Protin, C.; Baron, M.; LeDoux, M.-P.; Damaj, G.; Dupont, M.; Dreyfus, B.; Ferrant, E.; Herbaux, C.; et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood 2018, 131, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Yu, W.-L. COVID-19 associated with pulmonary aspergillosis: A literature review. J. Microbiol. Immunol. Infect. 2021, 54, 46–53. [Google Scholar] [CrossRef]
- Tasaka, S.; Hasegawa, N.; Kobayashi, S.; Yamada, W.; Nishimura, T.; Takeuchi, T.; Ishizaka, A. Serum Indicators for the Diagnosis of Pneumocystis Pneumonia. Chest 2007, 131, 1173–1180. [Google Scholar] [CrossRef]
- Del Corpo, O.; Butler-Laporte, G.; Sheppard, D.C.; Cheng, M.P.; McDonald, E.G.; Lee, T.C. Diagnostic accuracy of serum (1-3)-β-D-glucan for Pneumocystis jirovecii pneumonia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Bergeron, A.; Chevret, S.; Bele, N.; Schlemmer, B.; Menotti, J. Polymerase Chain Reaction for Diagnosing Pneumocystis Pneumonia in Non-HIV Immunocompromised Patients with Pulmonary Infiltrates. Chest 2009, 135, 655–661. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall (n = 113) | Pre-COVID-19 Era (n = 42) | COVID-19 Era (n = 71) | p |
|---|---|---|---|---|
| Prevalence (/100,000 patients) | 45.0 | 27.6 | 72.7 | 0.012 |
| Incidence (/1000 patient-years) | 22.1 | 13.1 | 37.0 | <0.001 |
| Age, years | 69 (60–75) | 62 (54–73) | 72 (69–76) | 0.002 |
| Male sex | 65 (57.5) | 23 (54.8) | 42 (59.2) | 0.648 |
| Underlying disease | ||||
| Connective tissue diseases | 23 (20.4) | 9 (21.4) | 14 (19.7) | 0.827 |
| Solid cancer | 43 (38.1) | 19 (45.2) | 24 (33.8) | 0.226 |
| Hematologic malignancy | 21 (18.6) | 5 (11.9) | 16 (22.5) | 0.160 |
| Hematopoietic stem cell transplantation | 5 (4.4) | 3 (7.1) | 2 (2.8) | 0.359 |
| Solid organ transplantation | 6 (5.3) | 2 (4.8) | 4 (5.6) | 1.000 |
| Immunosuppressive drugs | ||||
| Glucocorticoids | 59 (52.2) | 24 (57.1) | 35 (49.3) | 0.420 |
| Dose mg/day * | 5 (5–10) | 5 (5–30) | 5 (5–10) | 0.114 |
| Prednisolone ≥ 20 mg | 12 (10.6) | 8 (19.0) | 4 (5.6) | 0.025 |
| Chemotherapy | 53 (46.9) | 23 (54.8) | 30 (42.3) | 0.198 |
| Calcineurin Inhibitor | 10 (8.8) | 3 (7.1) | 7 (9.9) | 0.742 |
| Methotrexate | 21 (18.6) | 7 (16.7) | 14 (19.7) | 0.687 |
| Mycophenolate mofetil | 6 (5.3) | 3 (7.1) | 3 (4.2) | 0.669 |
| Biologic cytokine inhibitors | 23 (20.4) | 8 (19.0) | 15 (21.1) | 0.791 |
| Prophylactics for PCP | 15 (13.3) | 6 (14.3) | 9 (12.7) | 0.807 |
| COVID-19 infection within 90 days | 12 (10.6) | 0 (0) | 12 (16.9) | 0.003 |
| COVID-19 infection within 30 days | 5 (4.4) | 0 (0) | 5 (7.0) | 0.155 |
| Shock on initial visit | 26 (23.0) | 12 (28.6) | 14 (19.7) | 0.280 |
| Initial moderate-to-severe PCP ** | 88 (77.9) | 32 (76.2) | 56 (78.9) | 0.740 |
| ICU admission during PCP treatment | 38 (33.6) | 18 (42.9) | 20 (28.2) | 0.110 |
| Co-infection during PCP treatment | ||||
| Cytomegalovirus pneumonia | 15 (13.3) | 6 (14.3) | 9 (12.7) | 0.807 |
| Invasive pulmonary aspergillosis | 14 (12.4) | 1 (2.4) | 13 (18.3) | 0.013 |
| In-hospital mortality *** | 55 (49.1) | 20 (47.6) | 35 (50) | 0.807 |
| PCP-related mortality | 50 (44.6) | 19 (45.2) | 31 (44.3) | 0.922 |
| Underlying disease progression | 5 (4.5) | 1 (2.4) | 4 (5.7) | 0.649 |
| Characteristics | Survivor (n = 62) | Non-Survivor (n = 50) | p |
|---|---|---|---|
| Age, years | 74 (63–76) | 69 (65–74) | 0.128 |
| Male sex | 35 (56.5) | 30 (60.0) | 0.705 |
| Prophylactics for PCP | 6 (9.7) | 9 (18.0) | 0.199 |
| Immunosuppressive drugs | |||
| Glucocorticoids | 26 (41.9) | 32 (64.0) | 0.020 |
| Dose mg/day * | 5 (4–8) | 5 (5–20) | 0.186 |
| Prednisolone ≥20 mg | 5 (8.1) | 7 (14.0) | 0.313 |
| Chemotherapy | 31 (50.0) | 22 (44.0) | 0.527 |
| Calcineurin Inhibitor | 6 (9.7) | 3 (6.0) | 0.729 |
| Methotrexate | 9 (14.5) | 11 (22.0) | 0.304 |
| Mycophenolate mofetil | 4 (6.5) | 2 (4.0) | 0.690 |
| Biologic cytokine inhibitors | 16 (25.8) | 7 (14.0) | 0.124 |
| COVID-19 infection within 90 days | 9 (14.5) | 2 (4.0) | 0.108 |
| COVID-19 infection within 30 days | 2 (3.2) | 2 (4.0) | 1.000 |
| Blood examination at diagnosis of PCP | |||
| WBC/μL | 13,345 (11,000–14,970) | 11,540 (5985–16,630) | 0.086 |
| Lymphocyte count/μL | 916 (598–1373) | 787 (500–1089) | 0.212 |
| Platelet, ×103/μL | 223 (154–245) | 166 (77–247) | 0.144 |
| Albumin, g/dL | 2.8 (2.6–2.9) | 2.9 (2.7–3.3) | 0.256 |
| Lactate dehydrogenase, U/L | 485 (366–556) | 618 (407–366) | 0.005 |
| CRP mg/dL | 25.1 (20.5–32.7) | 10.8 (6.1–19.1) | 0.233 |
| Shock on initial visit | 12 (19.4) | 13 (26.0) | 0.401 |
| Initial moderate-to-severe PCP ** | 41 (66.1) | 46 (92.0) | 0.001 |
| ICU admission during PCP treatment | 11 (17.7) | 27 (54.0) | <0.001 |
| APACH II score | 19 (11–26) | 18 (13–22) | 0.446 |
| Co-infection during PCP treatment | |||
| Cytomegalovirus pneumonia | 3 (4.8) | 12 (24.0) | 0.003 |
| Invasive pulmonary aspergillosis | 4 (6.5) | 10 (20.0) | 0.031 |
| Ventilator | 9 (14.5) | 25 (50.0) | <0.001 |
| Invasive pulmonary drainage | 4 (6.5) | 11 (22.0) | 0.016 |
| Combined acute kidney injury | 10 (16.1) | 26 (52.0) | <0.001 |
| Pneumothorax occurrence | 2 (3.2) | 5 (10.0) | 0.239 |
| Risk Factors | OR (95% CI) | p | Adjusted OR (95% CI) | p |
|---|---|---|---|---|
| Previous glucocorticoids use | 2.5 (1.1–5.3) | 0.021 | 3.0 (1.1–8.1) | 0.029 |
| Initial moderate-to-severe PCP * | 5.9 (1.9–18.6) | 0.002 | 7.9 (1.8–34.3) | 0.006 |
| ICU admission | 5.4 (2.3–12.8) | <0.001 | ||
| Ventilator | 5.9 (2.4–14.5) | <0.001 | ||
| Invasive pulmonary drainage | 4.1 (1.2–13.8) | 0.023 | 4.4 (0.9–22.4) | 0.077 |
| Acute kidney injury | 5.6 (2.3–13.5) | <0.001 | 6.0 (2.1–16.8) | 0.001 |
| CMV co-infection | 6.2 (1.6–23.5) | 0.007 | 3.3 (0.8–14.3) | 0.102 |
| IPA co-infection | 3.6 (1.1–12.4) | 0.040 | 7.2 (1.4–36.8) | 0.019 |
| Lactate dehydrogenase ≥500, U/L | 2.9 (1.3–6.3) | 0.008 | ||
| CRP ≥ 5, mg/dL | 2.7 (1.1–6.8) | 0.035 |
| Risk Factors | OR (95% CI) | p | Adjusted OR (95% CI) | p |
|---|---|---|---|---|
| Previous use of tyrosine kinase inhibitor | 16.2 (1.4–191.9) | 0.027 | 36.7 (2.4–568.9) | 0.010 |
| COVID-19 infection within 30 days | 8.0 (1.0–62.1) | 0.047 | 19.4 (1.9–198.6) | 0.013 |
| Initial leucopenia (WBC < 1000/μL) | 16.2 (1.4–191.9) | 0.027 | 19.5 (1.3–296.9) | 0.032 |
| ICU admission | 4.3 (1.3–13.9) | 0.015 | 5.9 (1.4–24.6) | 0.016 |
| Ventilator | 3.7 (1.2–11.7) | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.S. Changing Trends in the Incidence and Clinical Features of Pneumocystis jirovecii Pneumonia in Non-HIV Patients before and during the COVID-19 Era and Risk Factors for Mortality between 2016 and 2022. Life 2023, 13, 1335. https://doi.org/10.3390/life13061335
Kang JS. Changing Trends in the Incidence and Clinical Features of Pneumocystis jirovecii Pneumonia in Non-HIV Patients before and during the COVID-19 Era and Risk Factors for Mortality between 2016 and 2022. Life. 2023; 13(6):1335. https://doi.org/10.3390/life13061335
Chicago/Turabian StyleKang, Jin Suk. 2023. "Changing Trends in the Incidence and Clinical Features of Pneumocystis jirovecii Pneumonia in Non-HIV Patients before and during the COVID-19 Era and Risk Factors for Mortality between 2016 and 2022" Life 13, no. 6: 1335. https://doi.org/10.3390/life13061335




