Plants as Biofactories for Therapeutic Proteins and Antiviral Compounds to Combat COVID-19
Abstract
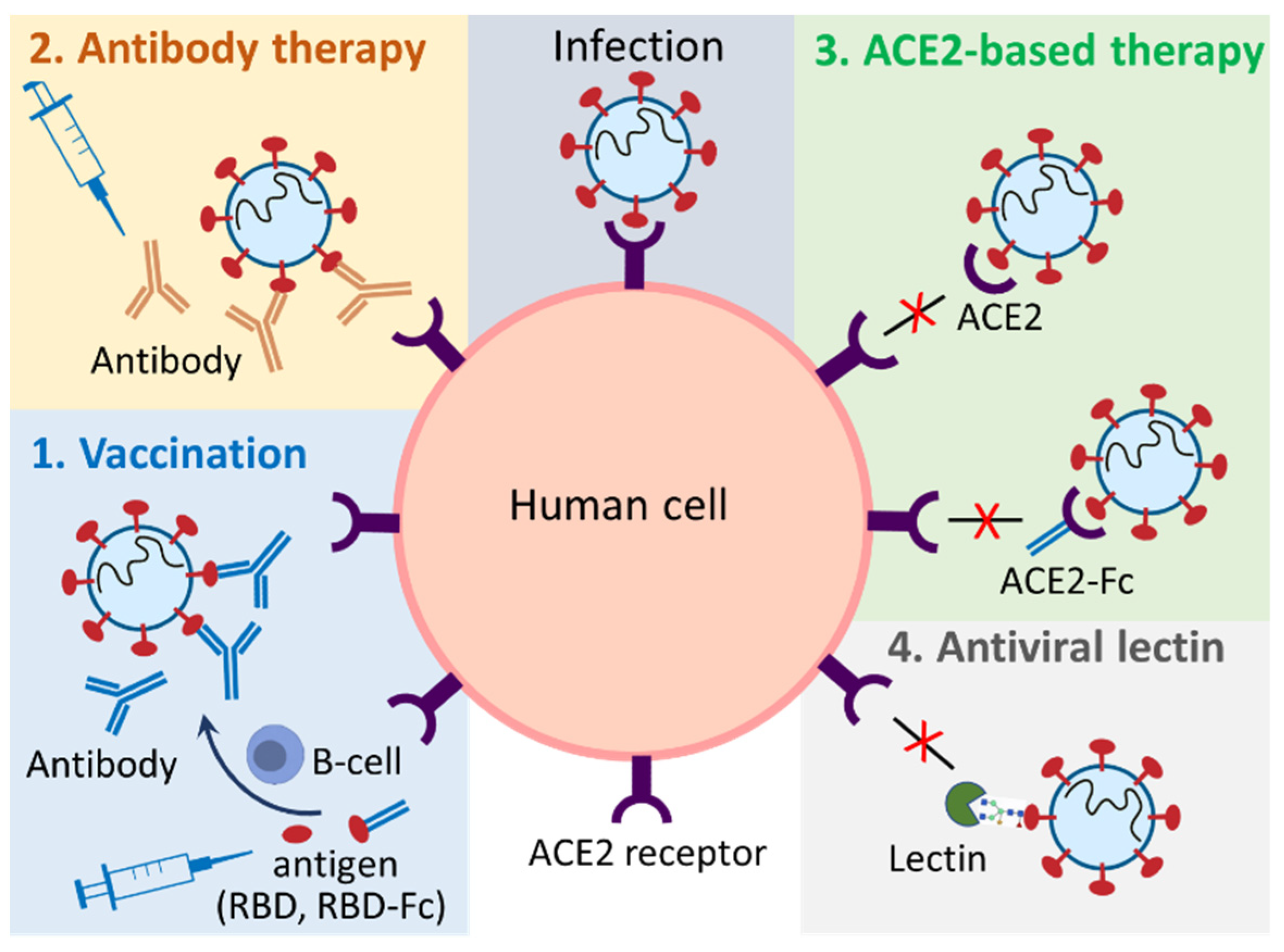
:1. Introduction
2. Plant Produced Biopharmaceuticals (Biologics) against SARS-CoV-2
2.1. Plant-Produced Vaccines
2.1.1. Plant-Produced Subunit Vaccines
2.1.2. Plant-Produced VLP Vaccines
2.2. Plant-Produced Antibodies
2.3. Plant-Produced ACE2-Based Biologics
2.3.1. Plant-Produced ACE2-Immunoadhesins
2.3.2. Plant-Produced ACE2 and ACE2-Based Chewing Gum
2.4. Plant Produced Antiviral Lectins
2.5. Challenges in Commercialization of Plant-Produced Biologics against SARS-CoV-2
3. Medicinal Plant-Produced Metabolites (Small Molecules) against SARS-CoV-2
3.1. Antiviral Mechanisms of PSMs
3.1.1. Inhibition of Viral Proteins
3.1.2. Intercalation of Nucleic Acids
3.1.3. Blocking of ACE2 Receptor
3.1.4. Immune Modulation
3.2. Major Classes of PSMs against SARS-CoV-2
3.2.1. Antiviral Alkaloids
3.2.2. Antiviral Polyphenols
3.2.3. Antiviral Terpenoids/Terpenes
3.3. Potential Anti-SARS-CoV-2 Compounds
3.3.1. Artemisinin
3.3.2. Hesperidin/Hesperetin
3.3.3. Emetine
3.3.4. Luteolin and Quercetin
3.3.5. Panduratin A
3.3.6. Tannins
3.4. Challenges in Clinical Applications of PSMs against SARS-CoV-2
4. Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Leung, K.; Lau, E.H.Y.; Wong, C.K.H.; Leung, G.M.; Wu, J.T. Estimating the transmission dynamics of SARS-CoV-2 Omicron BF.7 in Beijing after the adjustment of zero-COVID policy in November–December 2022. Nat. Med. 2023; ahead of print. [Google Scholar] [CrossRef]
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): An update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Lei, C.; Qian, K.; Li, T.; Zhang, S.; Fu, W.; Ding, M.; Hu, S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat. Commun. 2020, 11, 2070. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Flores, D.; Zepeda-Cervantes, J.; Cruz-Resendiz, A.; Aguirre-Sampieri, S.; Sampieri, A.; Vaca, L. SARS-CoV-2 vaccines based on the spike glycoprotein and implications of new viral variants. Front. Immunol. 2021, 12, 701501. [Google Scholar] [CrossRef]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martinez, D.R.; Raut, R.; Markmann, A.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y.; et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020, 5, eabc8413. [Google Scholar] [CrossRef]
- Abduljaleel, Z.; Shahzad, N.; Aziz, S.A.; Malik, S.M. Monoclonal antibody designed for SARS-nCoV-2 spike protein of receptor binding domain on antigenic targeted epitopes for inhibition to prevent viral entry. Mol. Divers. 2022; published online ahead of print. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Antonelli, M.; Pujol, J.C.; Spector, T.D.; Ourselin, S.; Steves, C.J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022, 399, 2263–2264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L.; Song, X.Q. Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase. Front. Immunol. 2022, 13, 1015355. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Ito, M.; Kiso, M.; Yamayoshi, S.; Uraki, R.; Fukushi, S.; Watanabe, S.; Suzuki, T.; Maeda, K.; Sakai-Tagawa, Y.; et al. Efficacy of antiviral agents against omicron subvariants BQ.1.1 and XBB. N. Engl. J. Med. 2023, 388, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Tomalka, J.A.; Suthar, M.S.; Deeks, S.G.; Sekaly, R.P. Fighting the SARS-CoV-2 pandemic requires a global approach to understanding the heterogeneity of vaccine responses. Nat. Immunol. 2022, 23, 360–370. [Google Scholar] [CrossRef]
- Galati, D.; Zanotta, S.; Capitelli, L.; Bocchino, M. A bird’s eye view on the role of dendritic cells in SARS-CoV-2 infection: Perspectives for immune-based vaccines. Allergy 2022, 77, 100–110. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. Broad-spectrum prodrugs with anti-SARS-CoV-2 activities: Strategies, benefits, and challenges. J. Med. Virol. 2022, 94, 1373–1390. [Google Scholar] [CrossRef]
- Jin, Y.H.; Jeon, S.; Lee, J.; Kim, S.; Jang, M.S.; Park, C.M.; Song, J.H.; Kim, H.R.; Kwon, S. Broad spectrum antiviral properties of cardiotonic steroids used as potential therapeutics for emerging coronavirus infections. Pharmaceutics 2021, 13, 1839. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Mendonca, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines 2021, 6, 97. [Google Scholar] [CrossRef]
- Larkin, H.D. Novavax COVID-19 vaccine booster authorized. JAMA 2022, 328, 2101. [Google Scholar] [CrossRef]
- Tan, C.Y.; Chiew, C.J.; Lee, V.J.; Ong, B.; Lye, D.C.; Tan, K.B. Comparative effectiveness of 3 or 4 doses of mRNA and inactivated whole-virus vaccines against COVID-19 infection, hospitalization and severe outcomes among elderly in Singapore. Lancet Reg. Health West. Pac. 2022, 29, 100654. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021, 21, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.P. Neutralizing monoclonal antibodies for COVID-19 treatment and prevention. Biomed. J. 2021, 44, 7–17. [Google Scholar] [CrossRef]
- Hentzien, M.; Autran, B.; Piroth, L.; Yazdanpanah, Y.; Calmy, A. A monoclonal antibody stands out against omicron subvariants: A call to action for a wider access to bebtelovimab. Lancet Infect. Dis. 2022, 22, 1278. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of COVID-19—Final report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Anonymous. An EUA for baricitinib (Olumiant) for COVID-19. Med. Lett. Drugs Ther. 2020, 62, 202–203. [Google Scholar]
- Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Zhou, X.; Wu, Q.; Zhang, X.; Feng, Z.; et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: A meta-analysis. Ann. Med. 2022, 54, 516–523. [Google Scholar] [CrossRef]
- Yao, J.; Weng, Y.; Dickey, A.; Wang, K.Y. Plants as factories for human pharmaceuticals: Applications and challenges. Int. J. Mol. Sci. 2015, 16, 28549–28565. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Hassan, A.; Abd El-Aziz, T.M.; Stockand, J.D.; Arafa, R.K. Marine brominated tyrosine alkaloids as promising inhibitors of SARS-CoV-2. Molecules 2021, 26, 6171. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. Chinese herbal medicine: Fighting SARS-CoV-2 infection on all fronts. J. Ethnopharmacol. 2021, 270, 113869. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Fuloria, S.; Swain, S.S.; Panda, S.K.; Sekar, M.; Subramaniyan, V.; Panda, M.; Jena, A.K.; Sathasivam, K.V.; Fuloria, N.K. Potential of marine terpenoids against SARS-CoV-2: An in silico drug development approach. Biomedicines 2021, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Gaudreault, R.; Sasseville, G.; Nguyen, P.T.; Wiebe, H.; Van De Ven, T.; Bourgault, S.; Mousseau, N.; Ramassamy, C. Molecular interactions of tannic acid with proteins associated with SARS-CoV-2 infectivity. Int. J. Mol. Sci. 2022, 23, 2643. [Google Scholar] [CrossRef] [PubMed]
- Merarchi, M.; Dudha, N.; Das, B.C.; Garg, M. Natural products and phytochemicals as potential anti-SARS-CoV-2 drugs. Phytother. Res. 2021, 35, 5384–5396. [Google Scholar] [CrossRef]
- Bhuiyan, F.R.; Howlader, S.; Raihan, T.; Hasan, M. Plants metabolites: Possibility of natural therapeutics against the COVID-19 pandemic. Front. Med. 2020, 7, 444. [Google Scholar] [CrossRef]
- Schillberg, S.; Finnern, R. Plant molecular farming for the production of valuable proteins—Critical evaluation of achievements and future challenges. J. Plant Physiol. 2021, 258–259, 153359. [Google Scholar] [CrossRef]
- Fischer, R.; Buyel, J.F. Molecular farming—The slope of enlightenment. Biotechnol Adv. 2020, 40, 107519. [Google Scholar] [CrossRef]
- Hager, K.J.; Perez Marc, G.; Gobeil, P.; Diaz, R.S.; Heizer, G.; Llapur, C.; Makarkov, A.I.; Vasconcellos, E.; Pillet, S.; Riera, F.; et al. Efficacy and safety of a recombinant plant-based adjuvanted COVID-19 vaccine. N. Engl. J. Med. 2022, 386, 2084–2096. [Google Scholar] [CrossRef]
- Tuse, D.; Nandi, S.; McDonald, K.A.; Buyel, J.F. The Emergency Response Capacity of Plant-based biopharmaceutical manufacturing-what it is and what it could be. Front. Plant Sci. 2020, 11, 594019. [Google Scholar] [CrossRef]
- Capell, T.; Twyman, R.M.; Armario-Najera, V.; Ma, J.K.; Schillberg, S.; Christou, P. Potential applications of plant biotechnology against SARS-CoV-2. Trends Plant Sci. 2020, 25, 635–643. [Google Scholar] [CrossRef]
- Mahmood, N.; Nasir, S.B.; Hefferon, K. Plant-based drugs and vaccines for COVID-19. Vaccines 2020, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Berlanga, B.; Pniewski, T. Plant-based vaccines in combat against coronavirus diseases. Vaccines 2022, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Towler, M.; Weathers, P.J. Platforms for plant-based protein production. In Bioprocessing of Plant In Vitro Systems, 1st ed.; Pavlov, A., Bley, T., Eds.; Springer International Publishing AG: Midtown Manhattan, NY, USA, 2016; pp. 1–40. [Google Scholar] [CrossRef] [Green Version]
- Jugler, C.; Sun, H.; Nguyen, K.; Palt, R.; Felder, M.; Steinkellner, H.; Chen, Q. A novel plant-made monoclonal antibody enhances the synergetic potency of an antibody cocktail against the SARS-CoV-2 Omicron variant. Plant Biotechnol. J. 2022; published online ahead of print. [Google Scholar] [CrossRef]
- Ruocco, V.; Strasser, R. Transient expression of glycosylated SARS-CoV-2 antigens in Nicotiana benthamiana. Plants 2022, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Schillberg, S.; Raven, N.; Spiegel, H.; Rasche, S.; Buntru, M. Critical Analysis of the Commercial potential of plants for the production of recombinant proteins. Front. Plant Sci. 2019, 10, 720. [Google Scholar] [CrossRef] [Green Version]
- Tekoah, Y.; Shulman, A.; Kizhner, T.; Ruderfer, I.; Fux, L.; Nataf, Y.; Bartfeld, D.; Ariel, T.; Gingis-Velitski, S.; Hanania, U.; et al. Large-scale production of pharmaceutical proteins in plant cell culture-the protalix experience. Plant Biotechnol. J. 2015, 13, 1199–1208. [Google Scholar] [CrossRef]
- Ward, B.J.; Makarkov, A.; Seguin, A.; Pillet, S.; Trepanier, S.; Dhaliwall, J.; Libman, M.D.; Vesikari, T.; Landry, N. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18–64 years) and older adults (>/=65 years): Two multicentre, randomised phase 3 trials. Lancet 2020, 396, 1491–1503. [Google Scholar] [CrossRef]
- Siriwattananon, K.; Manopwisedjaroen, S.; Shanmugaraj, B.; Rattanapisit, K.; Phumiamorn, S.; Sapsutthipas, S.; Trisiriwanich, S.; Prompetchara, E.; Ketloy, C.; Buranapraditkun, S.; et al. Plant-produced receptor-binding domain of SARS-CoV-2 elicits potent neutralizing responses in mice and non-human primates. Front. Plant Sci. 2021, 12, 682953. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Shen, C.; Peng, W.; Li, D.; Zhao, C.; Li, Z.; Li, S.; Bi, Y.; Yang, Y.; et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020, 368, 1274–1278. [Google Scholar] [CrossRef]
- Mamedov, T.; Gurbuzaslan, I.; Yuksel, D.; Ilgin, M.; Mammadova, G.; Ozkul, A.; Hasanova, G. Soluble human angiotensin- converting enzyme 2 as a potential therapeutic tool for COVID-19 is produced at high levels in Nicotiana benthamiana plant with potent anti-SARS-CoV-2 activity. Front. Plant Sci. 2021, 12, 742875. [Google Scholar] [CrossRef]
- Alfaleh, M.A.; Zawawi, A.; Al-Amri, S.S.; Hashem, A.M. David versus goliath: ACE2-Fc receptor traps as potential SARS-CoV-2 inhibitors. MAbs 2022, 14, 2057832. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, W.; Gu, C.; Cai, X.; Qu, D.; Lu, L.; Xie, Y.; Jiang, S. Griffithsin with a broad-spectrum antiviral activity by binding glycans in viral glycoprotein exhibits strong synergistic effect in combination with a pan-coronavirus fusion inhibitor targeting SARS-CoV-2 spike S2 subunit. Virol. Sin. 2020, 35, 857–860. [Google Scholar] [CrossRef]
- Heidary, M.; Kaviar, V.H.; Shirani, M.; Ghanavati, R.; Motahar, M.; Sholeh, M.; Ghahramanpour, H.; Khoshnood, S. A comprehensive review of the protein subunit vaccines against COVID-19. Front. Microbiol. 2022, 13, 927306. [Google Scholar] [CrossRef]
- Tariq, H.; Batool, S.; Asif, S.; Ali, M.; Abbasi, B.H. Virus-like particles: Revolutionary platforms for developing vaccines against emerging infectious diseases. Front. Microbiol. 2021, 12, 790121. [Google Scholar] [CrossRef]
- Moyle, P.M.; Toth, I. Modern subunit vaccines: Development, components, and research opportunities. ChemMedChem 2013, 8, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H.; Steele, J.F.C.; Jung, J.W.; Thuenemann, E.C.; Meshcheriakova, Y.; Lomonossoff, G.P. Producing vaccines against enveloped viruses in plants: Making the impossible, difficult. Vaccines 2021, 9, 780. [Google Scholar] [CrossRef] [PubMed]
- El Jaddaoui, I.; Al Idrissi, N.; Hamdi, S.; Wakrim, L.; Nejjari, C.; Amzazi, S.; Elouahabi, A.; Bakri, Y.; Ghazal, H. Plant-based vaccines against COVID-19 for massive vaccination in Africa. Front. Drug Deliv. 2022, 2, 909958. [Google Scholar] [CrossRef]
- Medicago Covifenz COVID-19 Vaccine. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines/medicago.html#a4 (accessed on 1 December 2022).
- COVID-19 Vaccine Development and Approvals Tracker. Available online: https://covid19.trackvaccines.org (accessed on 2 December 2022).
- KBP-201 COVID-19 Vaccine Trial in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT04473690 (accessed on 1 December 2022).
- Demarco, J.K.; Royal, J.M.; Severson, W.E.; Gabbard, J.D.; Hume, S.; Morton, J.; Swope, K.; Simpson, C.A.; Shepherd, J.W.; Bratcher, B.; et al. CoV-RBD121-NP vaccine candidate protects against symptomatic disease following SARS-CoV-2 challenge in K18-hACE2 mice and induces protective responses that prevent COVID-19-associated immunopathology. Vaccines 2021, 9, 1346. [Google Scholar] [CrossRef] [PubMed]
- Royal, J.M.; Simpson, C.A.; McCormick, A.A.; Phillips, A.; Hume, S.; Morton, J.; Shepherd, J.; Oh, Y.; Swope, K.; De Beauchamp, J.L.; et al. Development of a SARS-CoV-2 vaccine candidate using plant-based manufacturing and a tobacco mosaic virus-like nano-particle. Vaccines 2021, 9, 1347. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, P.M.; Choe, S. Plant-based COVID-19 vaccines: Current status, design, and development strategies of candidate vaccines. Vaccines 2021, 9, 992. [Google Scholar] [CrossRef]
- Uthaya Kumar, A.; Kadiresen, K.; Gan, W.C.; Ling, A.P.K. Current updates and research on plant-based vaccines for coronavirus disease 2019. Clin. Exp. Vaccine Res. 2021, 10, 13. [Google Scholar] [CrossRef]
- Balieu, J.; Jung, J.W.; Chan, P.; Lomonossoff, G.P.; Lerouge, P.; Bardor, M. Investigation of the N-glycosylation of the SARS-CoV-2 S protein contained in VLPs produced in Nicotiana benthamiana. Molecules 2022, 27, 5119. [Google Scholar] [CrossRef]
- Shanmugaraj, B.; Khorattanakulchai, N.; Panapitakkul, C.; Malla, A.; Im-Erbsin, R.; Inthawong, M.; Sunyakumthorn, P.; Hunsawong, T.; Klungthong, C.; Reed, M.C.; et al. Preclinical evaluation of a plant-derived SARS-CoV-2 subunit vaccine: Protective efficacy, immunogenicity, safety, and toxicity. Vaccine 2022, 40, 4440–4452. [Google Scholar] [CrossRef]
- Mamedov, T.; Yuksel, D.; Ilgin, M.; Gurbuzaslan, I.; Gulec, B.; Yetiskin, H.; Uygut, M.A.; Islam Pavel, S.T.; Ozdarendeli, A.; Mammadova, G.; et al. Plant-produced glycosylated and in vivo deglycosylated receptor binding domain proteins of SARS-CoV-2 Induce potent neutralizing responses in mice. Viruses 2021, 13, 1595. [Google Scholar] [CrossRef]
- Rattanapisit, K.; Shanmugaraj, B.; Manopwisedjaroen, S.; Purwono, P.B.; Siriwattananon, K.; Khorattanakulchai, N.; Hanittinan, O.; Boonyayothin, W.; Thitithanyanont, A.; Smith, D.R.; et al. Rapid production of SARS-CoV-2 receptor binding domain (RBD) and spike specific monoclonal antibody CR3022 in Nicotiana benthamiana. Sci. Rep. 2020, 10, 17698. [Google Scholar] [CrossRef]
- Demone, J.; Maltseva, M.; Nourimand, M.; Nasr-Sharif, M.; Galipeau, Y.; Alarcon, E.I.; Langlois, M.A.; MacLean, A.M. Scalable agroinfiltration-based production of SARS-CoV-2 antigens for use in diagnostic assays and subunit vaccines. PLoS ONE 2022, 17, e0277668. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, B.A.; Folgado, A.; Ferreira, A.C.; Abranches, R. Production of the SARS-CoV-2 spike protein and its receptor binding domain in plant cell suspension cultures. Front. Plant Sci. 2022, 13, 995429. [Google Scholar] [CrossRef] [PubMed]
- Granwehr, B.P. In adults who had not had COVID-19, Novavax vaccine had 90% efficacy at >/=7 d after the second dose. Ann. Intern. Med. 2022, 175, JC52. [Google Scholar] [CrossRef] [PubMed]
- Marabotti, C. Efficacy and effectiveness of COVID-19 vaccine—Absolute vs. relative risk reduction. Expert Rev. Vaccines 2022, 21, 873–875. [Google Scholar] [CrossRef]
- Mardanova, E.S.; Kotlyarov, R.Y.; Ravin, N.V. High-yield production of receptor binding domain of SARS-CoV-2 linked to bacterial flagellin in plants using self-replicating viral vector pEff. Plants 2021, 10, 2682. [Google Scholar] [CrossRef] [PubMed]
- Khorattanakulchai, N.; Srisutthisamphan, K.; Shanmugaraj, B.; Manopwisedjaroen, S.; Rattanapisit, K.; Panapitakkul, C.; Kemthong, T.; Suttisan, N.; Malaivijitnond, S.; Thitithanyanont, A.; et al. A recombinant subunit vaccine candidate produced in plants elicits neutralizing antibodies against SARS-CoV-2 variants in macaques. Front. Plant Sci. 2022, 13, 901978. [Google Scholar] [CrossRef] [PubMed]
- Khorattanakulchai, N.; Manopwisedjaroen, S.; Rattanapisit, K.; Panapitakkul, C.; Kemthong, T.; Suttisan, N.; Srisutthisamphan, K.; Malaivijitnond, S.; Thitithanyanont, A.; Jongkaewwattana, A.; et al. Receptor binding domain proteins of SARS-CoV-2 variants produced in Nicotiana benthamiana elicit neutralizing antibodies against variants of concern. J. Med. Virol. 2022, 94, 4265–4276. [Google Scholar] [CrossRef]
- Phoolcharoen, W.; (Chulalongkorn University, Bangkok, Thailand). Personal communication, 2022.
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83. [Google Scholar] [CrossRef]
- Schwarz, B.; Uchida, M.; Douglas, T. Biomedical and Catalytic Opportunities of Virus-Like Particles in Nanotechnology. Adv. Virus Res. 2017, 97, 1–60. [Google Scholar] [CrossRef] [Green Version]
- Ward, B.J.; Gobeil, P.; Séguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.Y.; Couture, M.; D’Aoust, M.A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021, 27, 1071–1078. [Google Scholar] [CrossRef]
- iBio Reports Preliminary Unaudited Fiscal Year 2022 Financial Results and Provides Corporate Update. Available online: https://www.globenewswire.com/news-release/2022/09/27/2523812/0/en/iBio-Reports-Preliminary-Unaudited-Fiscal-Year-2022-Financial-Results-and-Provides-Corporate-Update.html#:~:text=Preliminary%20Unaudited%20Financial%20Results%3A,comparable%20period%20in%20fiscal%202021 (accessed on 1 December 2022).
- Pipeline Therapeutic Candidates. Available online: https://ibioinc.com/pipeline/ (accessed on 2 December 2022).
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Jugler, C.; Sun, H.; Grill, F.; Kibler, K.; Esqueda, A.; Lai, H.; Li, Y.; Lake, D.; Chen, Q. Potential for a plant-made SARS-CoV-2 neutralizing monoclonal antibody as a synergetic cocktail component. Vaccines 2022, 10, 772. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q. Development of plant-made monoclonal antibodies against viral infections. Curr. Opin. Virol. 2022, 52, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.K.; Drossard, J.; Lewis, D.; Altmann, F.; Boyle, J.; Christou, P.; Cole, T.; Dale, P.; van Dolleweerd, C.J.; Isitt, V.; et al. Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol. J. 2015, 13, 1106–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugaraj, B.; Rattanapisit, K.; Manopwisedjaroen, S.; Thitithanyanont, A.; Phoolcharoen, W. Monoclonal antibodies B38 and H4 produced in Nicotiana benthamiana neutralize SARS-CoV-2 in vitro. Front. Plant Sci. 2020, 11, 589995. [Google Scholar] [CrossRef] [PubMed]
- Kallolimath, S.; Sun, L.; Palt, R.; Stiasny, K.; Mayrhofer, P.; Gruber, C.; Kogelmann, B.; Chen, Q.; Steinkellner, H. Highly active engineered IgG3 antibodies against SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2021, 118, e2107249118. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Zahmanova, G.; Minkov, I.; Lomonossoff, G.P. Plant-based expression and characterization of SARS-CoV-2 virus-like particles presenting a native spike protein. Plant Biotechnol. J. 2022, 20, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- VanBlargan, L.A.; Errico, J.M.; Halfmann, P.J.; Zost, S.J.; Crowe, J.E., Jr.; Purcell, L.A.; Kawaoka, Y.; Corti, D.; Fremont, D.H.; Diamond, M.S. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 2022, 28, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Dvashi, H.; Weinstein, J.; Katz, M.; Eilon-Ashkenazy, M.; Mor, Y.; Shimon, A.; Achdout, H.; Tamir, H.; Israely, T.; Strobelt, R.; et al. Anti-SARS-CoV-2 immunoadhesin remains effective against Omicron and other emerging variants of concern. iScience 2022, 25, 105193. [Google Scholar] [CrossRef] [PubMed]
- Chamow, S.M.; Ashkenazi, A. Immunoadhesins: Principles and applications. Trends Biotechnol. 1996, 14, 52–60. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, L.; Ullah, I.; Beaudoin-Bussieres, G.; Anand, S.P.; Hederman, A.P.; Tolbert, W.D.; Sherburn, R.; Nguyen, D.N.; Marchitto, L.; et al. Engineered ACE2-Fc counters murine lethal SARS-CoV-2 infection through direct neutralization and Fc-effector activities. Sci. Adv. 2022, 8, eabn4188. [Google Scholar] [CrossRef]
- Yasui, F.; Kohara, M.; Kitabatake, M.; Nishiwaki, T.; Fujii, H.; Tateno, C.; Yoneda, M.; Morita, K.; Matsushima, K.; Koyasu, S.; et al. Phagocytic cells contribute to the antibody-mediated elimination of pulmonary-infected SARS coronavirus. Virology 2014, 454–455, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Siriwattananon, K.; Manopwisedjaroen, S.; Kanjanasirirat, P.; Budi Purwono, P.; Rattanapisit, K.; Shanmugaraj, B.; Smith, D.R.; Borwornpinyo, S.; Thitithanyanont, A.; Phoolcharoen, W. Development of plant-produced recombinant ACE2-Fc fusion protein as a potential therapeutic agent against SARS-CoV-2. Front. Plant Sci. 2021, 11, 604663. [Google Scholar] [CrossRef]
- Glasgow, A.; Glasgow, J.; Limonta, D.; Solomon, P.; Lui, I.; Zhang, Y.; Nix, M.A.; Rettko, N.J.; Zha, S.; Yamin, R.; et al. Engineered ACE2 receptor traps potently neutralize SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 28046–28055. [Google Scholar] [CrossRef]
- Higuchi, Y.; Suzuki, T.; Arimori, T.; Ikemura, N.; Mihara, E.; Kirita, Y.; Ohgitani, E.; Mazda, O.; Motooka, D.; Nakamura, S.; et al. Engineered ACE2 receptor therapy overcomes mutational escape of SARS-CoV-2. Nat. Commun. 2021, 12, 3802. [Google Scholar] [CrossRef]
- Bernardi, A.; Huang, Y.; Harris, B.; Xiong, Y.; Nandi, S.; McDonald, K.A.; Faller, R. Development and simulation of fully glycosylated molecular models of ACE2-Fc fusion proteins and their interaction with the SARS-CoV-2 spike protein binding domain. PLoS ONE 2020, 15, e0237295. [Google Scholar] [CrossRef]
- Mou, H.; Quinlan, B.D.; Peng, H.; Liu, G.; Guo, Y.; Peng, S.; Zhang, L.; Davis-Gardner, M.E.; Gardner, M.R.; Crynen, G.; et al. Mutations derived from horseshoe bat ACE2 orthologs enhance ACE2-Fc neutralization of SARS-CoV-2. PLoS Pathog. 2021, 17, e1009501. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.; Dorosky, D.; Sharma, P.; Abbasi, S.A.; Dye, J.M.; Kranz, D.M.; Herbert, A.S.; Procko, E. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 2020, 369, 1261–1265. [Google Scholar] [CrossRef]
- Tada, T.; Fan, C.; Chen, J.S.; Kaur, R.; Stapleford, K.A.; Gristick, H.; Dcosta, B.M.; Wilen, C.B.; Nimigean, C.M.; Landau, N.R. An ACE2 microbody containing a single immunoglobulin Fc domain is a potent inhibitor of SARS-CoV-2. Cell Rep. 2020, 33, 108528. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.I.; Khalili, J.S.; Gilchrist, M.; Waight, A.B.; Cohen, D.; Zhuo, S.; Zhang, Y.; Ding, M.; Zhu, H.; Mak, A.N.; et al. ACE2-Fc fusion protein overcomes viral escape by potently neutralizing SARS-CoV-2 variants of concern. Antivir. Res. 2022, 199, 105271. [Google Scholar] [CrossRef] [PubMed]
- Castilho, A.; Schwestka, J.; Kienzl, N.F.; Vavra, U.; Grunwald-Gruber, C.; Izadi, S.; Hiremath, C.; Niederhofer, J.; Laurent, E.; Monteil, V.; et al. Generation of enzymatically competent SARS-CoV-2 decoy receptor ACE2-Fc in glycoengineered Nicotiana benthamiana. Biotechnol. J. 2021, 16, e2000566. [Google Scholar] [CrossRef]
- Daniell, H.; Nair, S.K.; Esmaeili, N.; Wakade, G.; Shahid, N.; Ganesan, P.K.; Islam, M.R.; Shepley-McTaggart, A.; Feng, S.; Gary, E.N. Debulking SARS-CoV-2 in saliva using angiotensin converting enzyme 2 in chewing gum to decrease oral virus transmission and infection. Mol. Ther. 2022, 30, 1966–1978. [Google Scholar] [CrossRef]
- Daniell, H.; Nair, S.K.; Guan, H.; Guo, Y.; Kulchar, R.J.; Torres, M.D.; Shahed-Al-Mahmud, M.; Wakade, G.; Liu, Y.M.; Marques, A.D. Debulking different Corona (SARS-CoV-2 delta, omicron, OC43) and influenza (H1N1, H3N2) virus strains by plant viral trap proteins in chewing gums to decrease infection and transmission. Biomaterials 2022, 288, 121671. [Google Scholar] [CrossRef]
- Ganesan, P.K.; Kulchar, R.J.; Kaznica, P.; Montoya-Lopez, R.; Green, B.J.; Streatfield, S.J.; Daniell, H. Optimization of biomass and target protein yield for Phase III clinical trial to evaluate Angiotensin Converting Enzyme 2 expressed in lettuce chloroplasts to reduce SARS-CoV-2 infection and transmission. Plant Biotechnol. J. 2022; published online ahead of print. [Google Scholar] [CrossRef]
- Ghafoor, D.; Ahmed, S.; Zaman, N. Lectins; a hope of treatment for COVID-19. Am. J. Biomed. Sci. Res. 2021, 12, 280–282. [Google Scholar] [CrossRef]
- Ahmed, M.N.; Jahan, R.; Nissapatorn, V.; Wilairatana, P.; Rahmatullah, M. Plant lectins as prospective antiviral biomolecules in the search for COVID-19 eradication strategies. Biomed. Pharmacother. 2022, 146, 112507. [Google Scholar] [CrossRef]
- Naik, S.; Kumar, S. Lectins from plants and algae act as anti-viral against HIV, influenza and coronaviruses. Mol. Biol. Rep. 2022, 49, 12239–12246. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Van Damme, E.J.M.; Simplicien, M.; Le Poder, S.; Klonjkowski, B.; Benoist, H.; Peyrade, D.; Rouge, P. Man-Specific lectins from plants, fungi, algae and cyanobacteria, as potential blockers for SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) Coronaviruses: Biomedical perspectives. Cells 2021, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Nascimento da Silva, L.C.; Mendonca, J.S.P.; de Oliveira, W.F.; Batista, K.L.R.; Zagmignan, A.; Viana, I.F.T.; Dos Santos Correia, M.T. Exploring lectin-glycan interactions to combat COVID-19: Lessons acquired from other enveloped viruses. Glycobiology 2021, 31, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Oh, Y.J.; Yuan, S.; Chu, H.; Yeung, M.L.; Canena, D.; Chan, C.C.; Poon, V.K.; Chan, C.C.; Zhang, A.J.; et al. A molecularly engineered, broad-spectrum anti-coronavirus lectin inhibits SARS-CoV-2 and MERS-CoV infection in vivo. Cell Rep. Med. 2022, 3, 100774. [Google Scholar] [CrossRef]
- Liu, Y.M.; Shahed-Al-Mahmud, M.; Chen, X.; Chen, T.H.; Liao, K.S.; Lo, J.M.; Wu, Y.M.; Ho, M.C.; Wu, C.Y.; Wong, C.H.; et al. A carbohydrate-binding protein from the edible lablab beans effectively blocks the infections of influenza viruses and SARS-CoV-2. Cell Rep. 2020, 32, 108016. [Google Scholar] [CrossRef]
- Lee, C. Griffithsin, a highly potent broad-spectrum antiviral lectin from red algae: From discovery to clinical application. Mar. Drugs 2019, 17, 567. [Google Scholar] [CrossRef] [Green Version]
- Ahan, R.E.; Hanifehnezhad, A.; Kehribar, E.S.; Oguzoglu, T.C.; Foldes, K.; Ozcelik, C.E.; Filazi, N.; Oztop, S.; Palaz, F.; Onder, S.; et al. A highly potent SARS-CoV-2 blocking lectin protein. ACS Infect. Dis. 2022, 8, 1253–1264. [Google Scholar] [CrossRef]
- Lokhande, K.B.; Apte, G.R.; Shrivastava, A.; Singh, A.; Pal, J.K.; Swamy, K.V.; Gupta, R.K. Sensing the interactions between carbohydrate-binding agents and N-linked glycans of SARS-CoV-2 spike glycoprotein using molecular docking and simulation studies. J. Biomol. Struct. Dyn. 2022, 40, 3880–3898. [Google Scholar] [CrossRef]
- Nabi-Afjadi, M.; Heydari, M.; Zalpoor, H.; Arman, I.; Sadoughi, A.; Sahami, P.; Aghazadeh, S. Lectins and lectibodies: Potential promising antiviral agents. Cell Mol. Biol. Lett. 2022, 27, 37. [Google Scholar] [CrossRef]
- Gecchele, E.; Merlin, M.; Brozzetti, A.; Falorni, A.; Pezzotti, M.; Avesani, L. A comparative analysis of recombinant protein expression in different biofactories: Bacteria, insect cells and plant systems. J. Vis. Exp. 2015, 97, 52459. [Google Scholar] [CrossRef] [Green Version]
- Gomord, V.; Fitchette, A.C.; Menu-Bouaouiche, L.; Saint-Jore-Dupas, C.; Plasson, C.; Michaud, D.; Faye, L. Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol. J. 2010, 8, 564–587. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Fakhrany, O.M.; Elekhnawy, E.; Al-Gareeb, A.I.; Alorabi, M.; De Waard, M.; Albogami, S.M.; Batiha, G.E. Traditional herbs against COVID-19: Back to old weapons to combat the new pandemic. Eur. J. Med. Res. 2022, 27, 186. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Sarkar, C.; El-Kersh, D.M.; Jamaddar, S.; Uddin, S.J.; Shilpi, J.A.; Mubarak, M.S. Natural products and their derivatives against coronavirus: A review of the non-clinical and pre-clinical data. Phytother. Res. 2020, 34, 2471–2492. [Google Scholar] [CrossRef] [PubMed]
- Bagde, H.; Dhopte, A. Effects of plant metabolites on the growth of COVID-19 (Coronavirus Disease-19) including omicron strain. Cureus 2022, 14, e26549. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Dev, K.; Sourirajan, A. Antiviral activity of bioactive phytocompounds against coronavirus: An update. J. Virol. Methods 2021, 290, 114070. [Google Scholar] [CrossRef]
- Pawar, K.S.; Mastud, R.N.; Pawar, S.K.; Pawar, S.S.; Bhoite, R.R.; Bhoite, R.R.; Kulkarni, M.V.; Deshpande, A.R. Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: A randomized clinical trial. Front. Pharmacol. 2021, 12, 669362. [Google Scholar] [CrossRef]
- Bhar, A.; Jain, A.; Das, S. Natural therapeutics against SARS-CoV-2: The potentiality and challenges. Vegetos, 2022; published online ahead of print. [Google Scholar] [CrossRef]
- Holghoomi, R.; Abdolmaleki, A.; Asadi, A.; Kajkolah, M.; Gurushankar, K.; Bidarlord, M. Plant-derived metabolites as potent inhibitor and treatment for COVID-19. J. Pharm. Care 2022, 10, 84–93. [Google Scholar] [CrossRef]
- Lopes, A.J.O.; Calado, G.P.; Froes, Y.N.; Araujo, S.A.; Franca, L.M.; Paes, A.M.A.; Morais, S.V.; Rocha, C.Q.D.; Vasconcelos, C.C. Plant metabolites as SARS-CoV-2 inhibitors candidates: In silico and in vitro studies. Pharmaceuticals 2022, 15, 1045. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Efferth, T.; Das, B.; Kar, A.; Ghosh, S.; Singha, S.; Debnath, P.; Sharma, N.; Bhardwaj, P.K.; Haldar, P.K. Role of medicinal plants in inhibiting SARS-CoV-2 and in the management of post-COVID-19 complications. Phytomedicine 2022, 98, 153930. [Google Scholar] [CrossRef]
- Llivisaca-Contreras, S.A.; Naranjo-Moran, J.; Pino-Acosta, A.; Pieters, L.; Vanden Berghe, W.; Manzano, P.; Vargas-Perez, J.; Leon-Tamariz, F.; Cevallos-Cevallos, J.M. Plants and natural products with activity against various types of coronaviruses: A review with focus on SARS-CoV-2. Molecules 2021, 26, 4099. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Behl, T.; Upadhyay, T.; Chigurupati, S.; Bhatt, S.; Sehgal, A.; Bhatia, S.; Singh, S.; Sharma, N.; Vijayabalan, S.; et al. Targeting natural products against SARS-CoV-2. Environ. Sci Pollut. Res. Int. 2022, 29, 42404–42432. [Google Scholar] [CrossRef] [PubMed]
- Jamshidnia, M.; Sewell, R.D.E.; Rafieian-Kopaei, M. An update on promising agents against COVID-19: Secondary metabolites and mechanistic aspects. Curr. Pharm. Des. 2022, 28, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Zrig, A. The effect of phytocompounds of medicinal plants on coronavirus (2019-NCOV) infection. Pharm. Chem. J. 2022, 55, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Abbas, A.; Lehmann, C.; Rupasinghe, H.P.V. Antiviral and anti-inflammatory plant-derived bioactive compounds and their potential use in the treatment of COVID-19-related pathologies. J. Xenobiot. 2022, 12, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z. Natural products, alone or in combination with FDA-Approved drugs, to treat COVID-19 and lung cancer. Biomedicines 2021, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Khan, M.A.; Mashwani, Z.U.; Ullah, N.; Nadhman, A. Therapeutic potential of medicinal plants against COVID-19: The role of antiviral medicinal metabolites. Biocatal. Agric. Biotechnol. 2021, 31, 101890. [Google Scholar] [CrossRef] [PubMed]
- Remali, J.; Aizat, W.M. A Review on Plant Bioactive Compounds and Their Modes of Action Against Coronavirus Infection. Front. Pharmacol. 2020, 11, 589044. [Google Scholar] [CrossRef]
- Park, J.; Park, R.; Jang, M.; Park, Y.I.; Park, Y. Coronavirus enzyme inhibitors-experimentally proven natural compounds from plants. J. Microbiol. 2022, 60, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Chandel, V.; Raj, S.; Rathi, B. In silico identification of potent FDA approved drugs against Coronavirus COVID-19 main protease: A drug repurposing approach. Chem. Biol. Lett. 2020, 7, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Sk, M.F.; Sonawane, A.; Kar, P.; Sadhukhan, S. Plant-derived natural polyphenols as potential antiviral drugs against SARS-CoV-2 via RNA-dependent RNA polymerase (RdRp) inhibition: An in-silico analysis. J. Biomol. Struct. Dyn. 2021, 39, 6249–6264. [Google Scholar] [CrossRef]
- Jan, J.T.; Cheng, T.R.; Juang, Y.P.; Ma, H.H.; Wu, Y.T.; Yang, W.B.; Cheng, C.W.; Chen, X.; Chou, T.H.; Shie, J.J.; et al. Identification of existing pharmaceuticals and herbal medicines as inhibitors of SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2021579118. [Google Scholar] [CrossRef] [PubMed]
- Muchtaridi, M.; Fauzi, M.; Khairul Ikram, N.K.; Mohd Gazzali, A.; Wahab, H.A. Natural flavonoids as potential angiotensin-converting enzyme 2 inhibitors for anti-SARS-CoV-2. Molecules 2020, 25, 3980. [Google Scholar] [CrossRef]
- Liu, X.; Raghuvanshi, R.; Ceylan, F.D.; Bolling, B.W. Quercetin and Its Metabolites Inhibit Recombinant Human Angiotensin-Converting Enzyme 2 (ACE2) Activity. J. Agric. Food Chem. 2020, 68, 13982–13989. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, K.J.; Gokila Vani, M.; Wang, C.S.; Chen, C.C.; Chen, Y.C.; Lu, L.P.; Huang, C.H.; Lai, C.S.; Wang, S.Y. Geranium and lemon essential oils and their active compounds downregulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain, in epithelial cells. Plants 2020, 9, 770. [Google Scholar] [CrossRef]
- Lucas, K.; Frohlich-Nowoisky, J.; Oppitz, N.; Ackermann, M. Cinnamon and hop extracts as potential immunomodulators for severe COVID-19 cases. Front. Plant Sci. 2021, 12, 589783. [Google Scholar] [CrossRef] [PubMed]
- Gyebi, G.A.; Ogunro, O.B.; Adegunloye, A.P.; Ogunyemi, O.M.; Afolabi, S.O. Potential inhibitors of coronavirus 3-chymotrypsin-like protease (3CL(pro)): An in silico screening of alkaloids and terpenoids from African medicinal plants. J. Biomol. Struct. Dyn. 2021, 39, 3396–3408. [Google Scholar] [CrossRef]
- Wink, M. Potential of DNA intercalating alkaloids and other plant secondary metabolites against SARS-CoV-2 causing COVID-19. Diversity 2020, 12, 175. [Google Scholar] [CrossRef]
- Gonzalez, B.L.; de Oliveira, N.C.; Ritter, M.R.; Tonin, F.S.; Melo, E.B.; Sanches, A.C.C.; Fernandez-Llimos, F.; Petruco, M.V.; de Mello, J.C.P.; Chierrito, D.; et al. The naturally-derived alkaloids as a potential treatment for COVID-19: A scoping review. Phytother. Res. 2022, 36, 2686–2709. [Google Scholar] [CrossRef]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D’Ammen, E. An overview on dietary polyphenols and their biopharmaceutical classification system (BCS). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef]
- Tirado-Kulieva, V.A.; Hernandez-Martinez, E.; Choque-Rivera, T.J. Phenolic compounds versus SARS-CoV-2: An update on the main findings against COVID-19. Heliyon 2022, 8, e10702. [Google Scholar] [CrossRef]
- Annunziata, G.; Sanduzzi Zamparelli, M.; Santoro, C.; Ciampaglia, R.; Stornaiuolo, M.; Tenore, G.C.; Sanduzzi, A.; Novellino, E. May polyphenols have a role against coronavirus infection? An overview of in vitro evidence. Front. Med. 2020, 7, 240. [Google Scholar] [CrossRef]
- Abdelmageed, M.I.; Abdelmoneim, A.H.; Mustafa, M.I.; Elfadol, N.M.; Murshed, N.S.; Shantier, S.W.; Makhawi, A.M. Design of a multiepitope-based peptide vaccine against the e protein of human COVID-19: An immunoinformatics approach. Biomed. Res. Int. 2020, 2020, 2683286. [Google Scholar] [CrossRef] [PubMed]
- Owis, A.I.; El-Hawary, M.S.; El Amir, D.; Aly, O.M.; Abdelmohsen, U.R.; Kamel, M.S. Molecular docking reveals the potential of Salvadora persica flavonoids to inhibit COVID-19 virus main protease. RSC Adv. 2020, 10, 19570–19575. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Brestic, M.; Hajihashemi, S.; Skalicky, M.; Kubes, J.; Lamilla-Tamayo, L.; Ibrahimova, U.; Ibadullayeva, S.; Landi, M. COVID-19 prophylaxis efforts based on natural antiviral plant extracts and their compounds. Molecules 2021, 26, 727. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J. Plant-microbe interactions and secondary metabolites with antibacterial, antifungal and antiviral properties. In Annual Plant Reviews: Functions and Biotechnology of Plant Secondary Metabolites, 2nd ed.; Wink, M., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; Volume 39, pp. 214–347. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Mohamed, H.S.; Abd-Ellatief, R.B.; Gomaa, M.A.; Hammam, D.S. Advancing combination treatment with glycyrrhizin and boswellic acids for hospitalized patients with moderate COVID-19 infection: A randomized clinical trial. Inflammopharmacology 2022, 30, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.; Huang, Y.; Fidock, D.A.; Polyak, S.J.; Wagoner, J.; Towler, M.J.; Weathers, P.J. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. J. Ethnopharmacol. 2021, 274, 114016. [Google Scholar] [CrossRef]
- Nair, M.S.; Huang, Y.; Fidock, D.A.; Towler, M.J.; Weathers, P.J. Artemisia annua L. hot-water extracts show potent activity in vitro against COVID-19 variants including delta. J. Ethnopharmacol. 2022, 284, 114797. [Google Scholar] [CrossRef]
- Zhou, Y.; Gilmore, K.; Ramirez, S.; Settels, E.; Gammeltoft, K.A.; Pham, L.V.; Fahnoe, U.; Feng, S.; Offersgaard, A.; Trimpert, J.; et al. In vitro efficacy of artemisinin-based treatments against SARS-CoV-2. Sci. Rep. 2021, 11, 14571. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, J.Y.; Kwon, H.C.; Jeon, S.; Lee, S.J.; Jung, H.; Kim, S.; Jang, D.S.; Lee, C.J. Astersaponin I from Aster koraiensis is a natural viral fusion blocker that inhibits the infection of SARS-CoV-2 variants and syncytium formation. Antivir. Res. 2022, 208, 105428. [Google Scholar] [CrossRef]
- Nag, A.; Banerjee, R.; Paul, S.; Kundu, R. Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study. Comput. Biol. Med. 2022, 146, 105552. [Google Scholar] [CrossRef]
- Valipour, M. Different aspects of emetine’s capabilities as a highly potent SARS-CoV-2 inhibitor against COVID-19. ACS Pharmacol. Transl. Sci. 2022, 5, 387–399. [Google Scholar] [CrossRef]
- Cheng, F.J.; Huynh, T.K.; Yang, C.S.; Hu, D.W.; Shen, Y.C.; Tu, C.Y.; Wu, Y.C.; Tang, C.H.; Huang, W.C.; Chen, Y.; et al. Hesperidin is a potential inhibitor against SARS-CoV-2 infection. Nutrients 2021, 13, 2800. [Google Scholar] [CrossRef]
- Delcanale, P.; Uriati, E.; Mariangeli, M.; Mussini, A.; Moreno, A.; Lelli, D.; Cavanna, L.; Bianchini, P.; Diaspro, A.; Abbruzzetti, S.; et al. The interaction of hypericin with SARS-CoV-2 reveals a multimodal antiviral activity. ACS Appl. Mater. Interfaces 2022, 14, 14025–14032. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Li, J.; Lai, X.; Zhang, M.; Kuang, Y.; Bao, Y.O.; Yu, R.; Hong, W.; Muturi, E.; Xue, H.; et al. Natural triterpenoids from licorice potently inhibit SARS-CoV-2 infection. J. Adv. Res. 2022, 36, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Munafo, F.; Donati, E.; Brindani, N.; Ottonello, G.; Armirotti, A.; De Vivo, M. Quercetin and luteolin are single-digit micromolar inhibitors of the SARS-CoV-2 RNA-dependent RNA polymerase. Sci. Rep. 2022, 12, 10571. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Cui, M.; Zheng, C.; Wang, M.; Sun, R.; Gao, D.; Bao, J.; Ren, S.; Yang, B.; Lin, J.; et al. Myricetin inhibits SARS-CoV-2 viral replication by targeting M(pro) and ameliorates pulmonary inflammation. Front. Pharmacol. 2021, 12, 669642. [Google Scholar] [CrossRef]
- Baeshen, N.A.; Albeshri, A.O.; Baeshen, N.N.; Attar, R.; Karkashan, A.; Abbas, B.; Bouback, T.A.; Aljaddawi, A.A.; Refai, M.Y.; Abdelkader, H.S.; et al. In silico screening of some compounds derived from the desert medicinal plant Rhazya stricta for the potential treatment of COVID-19. Sci. Rep. 2022, 12, 11120. [Google Scholar] [CrossRef]
- Kanjanasirirat, P.; Suksatu, A.; Manopwisedjaroen, S.; Munyoo, B.; Tuchinda, P.; Jearawuttanakul, K.; Seemakhan, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Rangkasenee, N.; et al. High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents. Sci. Rep. 2020, 10, 19963. [Google Scholar] [CrossRef]
- Furukawa, R.; Kitabatake, M.; Ouji-Sageshima, N.; Suzuki, Y.; Nakano, A.; Matsumura, Y.; Nakano, R.; Kasahara, K.; Kubo, K.; Kayano, S.I.; et al. Persimmon-derived tannin has antiviral effects and reduces the severity of infection and transmission of SARS-CoV-2 in a Syrian hamster model. Sci. Rep. 2021, 11, 23695. [Google Scholar] [CrossRef]
- Shekunov, E.V.; Efimova, S.S.; Yudintceva, N.M.; Muryleva, A.A.; Zarubaev, V.V.; Slita, A.V.; Ostroumova, O.S. Plant alkaloids inhibit membrane fusion mediated by calcium and fragments of MERS-CoV and SARS-CoV/SARS-CoV-2 fusion peptides. Biomedicines 2021, 9, 1434. [Google Scholar] [CrossRef]
- Shaban, M.S.; Mayr-Buro, C.; Meier-Soelch, J.; Albert, B.V.; Schmitz, M.L.; Ziebuhr, J.; Kracht, M. Thapsigargin: Key to new host-directed coronavirus antivirals? Trends Pharmacol. Sci. 2022, 43, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Saggam, A.; Limgaokar, K.; Borse, S.; Chavan-Gautam, P.; Dixit, S.; Tillu, G.; Patwardhan, B. Withania somnifera (L.) Dunal: Opportunity for Clinical Repurposing in COVID-19 Management. Front. Pharmacol. 2021, 12, 623795. [Google Scholar] [CrossRef]
- Farmanpour-Kalalagh, K.; Beyraghdar Kashkooli, A.; Babaei, A.; Rezaei, A.; van der Krol, A.R. Artemisinins in combating viral infections like SARS-CoV-2, inflammation and cancers and options to meet increased global demand. Front. Plant Sci. 2022, 13, 780257. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. Turning the tide: Natural products and natural-product-inspired chemicals as potential counters to SARS-CoV-2 infection. Front. Pharmacol. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Wang, A.; Sun, Y.; Liu, Q.; Wu, H.; Liu, J.; He, J.; Yu, J.; Chen, Q.Q.; Ge, Y.; Zhang, Z.; et al. Low dose of emetine as potential anti-SARS-CoV-2 virus therapy: Preclinical in vitro inhibition and in vivo pharmacokinetic evidences. Mol. Biomed. 2020, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.X.; Shang, W.J.; Yin, W.C.; Ge, H.; Wang, L.; Zhang, X.L.; Li, B.Q.; Li, H.L.; Xu, Y.C.; Xu, E.H.; et al. A multi-targeting drug design strategy for identifying potent anti-SARS-CoV-2 inhibitors. Acta Pharmacol. Sin. 2022, 43, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Afsar, M.; Khandelwal, N.; Chander, Y.; Riyesh, T.; Dedar, R.K.; Gulati, B.R.; Pal, Y.; Barua, S.; Tripathi, B.N.; et al. Emetine suppresses SARS-CoV-2 replication by inhibiting interaction of viral mRNA with eIF4E. Antivir. Res. 2021, 189, 105056. [Google Scholar] [CrossRef] [PubMed]
- Morales-Paredes, C.A.; Rodriguez-Diaz, J.M.; Boluda-Botella, N. Pharmaceutical compounds used in the COVID-19 pandemic: A review of their presence in water and treatment techniques for their elimination. Sci. Total Environ. 2022, 814, 152691. [Google Scholar] [CrossRef]
- Weidenbacher, P.A.; Sanyal, M.; Friedland, N.; Tang, S.; Arunachalam, P.S.; Hu, M.; Kumru, O.S.; Morris, M.K.; Fontenot, J.; Shirreff, L.; et al. A ferritin-based COVID-19 nanoparticle vaccine that elicits robust, durable, broad-spectrum neutralizing antisera in non-human primates. bioRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Hameed, S.A.; Paul, S.; Dellosa, G.K.Y.; Jaraquemada, D.; Bello, M.B. Towards the future exploration of mucosal mRNA vaccines against emerging viral diseases; lessons from existing next-generation mucosal vaccine strategies. NPJ Vaccines 2022, 7, 71. [Google Scholar] [CrossRef]
- Waltz, E. How nasal-spray vaccines could change the pandemic. Nature 2022, 609, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Waltz, E. China and India approve nasal COVID vaccines—Are they a game changer? Nature 2022, 609, 450. [Google Scholar] [CrossRef]
- Chan, H.T.; Daniell, H. Plant-made oral vaccines against human infectious diseases-Are we there yet? Plant Biotechnol. J. 2015, 13, 1056–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Dolan, M.C.; Medrano, G.; Cramer, C.L.; Weathers, P.J. Green factory: Plants as bioproduction platforms for recombinant proteins. Biotechnol. Adv. 2012, 30, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, N. On the way to commercializing plant cell culture platform for biopharmaceuticals: Present status and prospect. Pharm. Bioprocess. 2014, 2, 499–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karki, U.; Fang, H.; Guo, W.; Unnold-Cofre, C.; Xu, J. Cellular engineering of plant cells for improved therapeutic protein production. Plant Cell Rep. 2021, 40, 1087–1099. [Google Scholar] [CrossRef]
- Mlozi, S.H. The role of natural products from medicinal plants against COVID-19: Traditional medicine practice in Tanzania. Heliyon 2022, 8, e09739. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Halitschke, R.; Baldwin, I.T.; Gaquerel, E. Information theory tests critical predictions of plant defense theory for specialized metabolism. Sci. Adv. 2020, 6, eaaz0381. [Google Scholar] [CrossRef]



| Table | Trade Name | Antigen | Plant | Manufacturer | Efficacy | Commercialization Progress | Source |
|---|---|---|---|---|---|---|---|
| Virus-like particles | Covifenz | S protein | N. benthamiana | Medicago | 69.5% to 78.8% (Phase III) | Approved: Canada Phase III Trials: Argentina, Brazil, United Kingdom, USA | [40,61,62] |
| KBP-201 | RBD | N. benthamiana | Kentucky Bioprocessing | 100% (K18-hACE2 mice) | Phase I/II Trials: USA | [62,63,64,65] | |
| IBIO-200, IBIO-201, and IBIO-202 | S protein | N. benthamiana | iBio, Inc. | n.d. | Pre-clinical trials | [44,66,67] | |
| n/a | S protein | N. benthamiana | n/a | n.d. | no | [68] | |
| Subunit | Baiya SARS-CoV-2 Vax 1 | RBD-Fc | N. benthamiana | Baiya Phytopharm | 100% (K18-hACE2 mice) | Phase I Trials: Thailand | [62,69] |
| Baiya SARS-CoV-2 Vax 2 | RBD-Fc | N. benthamiana | Baiya Phytopharm | Unknown | Phase I Trials: Thailand | [62,69] | |
| n/a | RBD-Fc | N. benthamiana | n/a | n.d. | no | [51] | |
| n/a | RBD | N. benthamiana | n/a | n.d. | no | [70,71,72] | |
| n/a | S protein, RBD | Tobacco BY-2 and Medicago truncatula A17 cell | n/a | n.d. | no | [73] |
| Antibody Name | Plant | Affected Lineages | Neutralizing Capability (Neutralizing Titer *, IC50 † or NT100 ‡) | Source |
|---|---|---|---|---|
| CR3022 | N. benthamiana | Original strain | Fail to neutralize * | [71] |
| B38 | N. benthamiana | Unidentified | 640 at 0.492 µg/mL * | [90] |
| H4 | N. benthamiana | Unidentified | 40 at 5.45 µg/mL * | [90] |
| H4-IgG1-4 | N. benthamiana | Unidentified | 591 nM for H4-IgG3 ‡ | [91] |
| CA1 | N. benthamiana | Original strain, Delta | 9.29 nM: Original † 89.87 nM: Delta † | [87] |
| CB6 | N. benthamiana | Original strain, Delta | 0.93 nM: Original † 0.75 nM: Delta † | [87] |
| 11D7 | N. benthamiana | Original strain, Delta, Omicron | 25.37 µg/mL: Original † 59.52 µg/mL: Delta † 948.7 µg/mL: Omicron † | [46] |
| Major Active Compounds | Plant Species | Efficacy/Mechanism of Action * | References |
|---|---|---|---|
| Artemisinin, flavonoids, artesunate, artemether, nonidentified metabolites | Artemisia annua L. | Inhibiting viral replication | [159] |
| Inhibiting replication of five virus variants including Delta | [160] | ||
| Inhibiting viral infection | [161] | ||
| Astersaponin I (AI) | Aster koraiensis | Inhibiting virus entry pathways at plasma membrane and within endosomal compartments | [162] |
| Curcumin | Curcuma longa | Binding and inhibiting S protein of Omicron variant (in silico analysis) | [163] |
| Emetine | Carapichea ipecacuanha | Blocking viral entry into cells; inhibiting virus replication; anti-inflammation | [164] |
| Hesperidin (Hesperetin) | Various species | Reducing expression of TMPRSS2 and ACE2 (in silico analysis) | [165] |
| Hypericin | Hypericum perforatum | Binding viral envelope and reducing its infectivity | [166] |
| Licorice-saponin A3 (A3) and glycyrrhetinic acid (GA) | Glycyrrhiza uralensis | Inhibiting viral infection | [167] |
| Luteolin | Various species | Reducing viral replication by inhibition of RdRp | [168] |
| Myricetin | Various species | Inhibiting viral replication and transcription by inhibition of protease (Mpro); anti-inflammation (in vivo analysis) | [169] |
| Nonalkaloid compounds | Rhazya stricta | Binding key residues of S proteins and impeding viral infection (in silico analysis) | [170] |
| Panduratin A | Boesenbergia rotunda | Inhibiting viral replication | [171] |
| Persimmon-derived tannins | Diospyros kaki | Inhibiting virus replication; potential as a prophylactic agent | [172] |
| Piperine | Piper spp. | Inhibiting viral replication | [173] |
| Quercetin | Various species | Impeding viral replication by inhibition of RdRp | [168] |
| Thapsigargin | Thapsia garganica | Inhibiting viral replication by inducing stress in ER and increasing the viability of infected cells | [174] |
| Withaferin A, Withanone, Withanolide A | Withania somnifera (L.) | Inhibiting viral infection and replication; anti-inflammation and proinflammatory cytokines (in vivo analysis) | [175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
England, C.; TrejoMartinez, J.; PerezSanchez, P.; Karki, U.; Xu, J. Plants as Biofactories for Therapeutic Proteins and Antiviral Compounds to Combat COVID-19. Life 2023, 13, 617. https://doi.org/10.3390/life13030617
England C, TrejoMartinez J, PerezSanchez P, Karki U, Xu J. Plants as Biofactories for Therapeutic Proteins and Antiviral Compounds to Combat COVID-19. Life. 2023; 13(3):617. https://doi.org/10.3390/life13030617
Chicago/Turabian StyleEngland, Corbin, Jonathan TrejoMartinez, Paula PerezSanchez, Uddhab Karki, and Jianfeng Xu. 2023. "Plants as Biofactories for Therapeutic Proteins and Antiviral Compounds to Combat COVID-19" Life 13, no. 3: 617. https://doi.org/10.3390/life13030617







